Cell communication modulates numerous biological processes including proliferation, apoptosis, motility, invasion, and differentiation.[1-3] Correspondingly, there has been significant interest in the development of surface-display strategies for the presentation of signaling molecules to living cells.[3-13] This effort has primarily focused on naturally surface-bound ligands, such as extracellular-matrix components and cell membranes. Soluble ligands (e.g. growth factors and cytokines) play an important role in intercellular communications,[14] and their display in a surface-bound format would be of great utility in the design of array-based live-cell assays. Recently, several cell microarray systems that display cDNA, RNAi, or small molecules in a surface-array format were proven to be useful in accelerating high-throughput functional genetic studies and screening therapeutic agents.[15-17] These surface-display methods provide a flexible platform for the systematic, combinatorial investigation of genes and small molecules that affect cellular processes and phenotypes of interest. In an analogous sense, it would be an important advance if one could display soluble signaling ligands in a surface-assay format that allows for systematic, patterned presentation of soluble ligands to live cells. Such a technique would make it possible to examine cellular phenotypes of interest in a parallel format with soluble signaling ligands as one of the display parameters.
Herein we report a ligand-modified fluid supported-lipid bilayer (SLB)[5, 10, 18-22] assay system that can be used to functionally display soluble ligands to cells in situ (Figure 1 A). By displaying soluble ligands on a SLB surface, both solution behavior (the ability to become locally enriched by reaction–diffusion processes) and solid behavior (the ability to control the spatial location of the ligands in an open system) could be combined. The method reported herein benefits from the naturally fluid state of the supported membrane, which allows surface-linked ligands to diffuse freely in two dimensions. Ligands can become reorganized beneath cells, by reaction–diffusion processes, and may also adopt spatial configurations that reflect those of their cognate receptors on the cell surface (Figure 1 B). This provides a significant benefit over conventional cell-signaling and -culturing systems[6, 7, 11, 23] that present inflexible distributions of signaling molecules. In this study, we observe marked differences in the response of cells to membrane surface-displayed soluble ligands as a function of membrane fluidity. Tethering of soluble signaling molecules to fluid supported membranes opens up opportunities to use already developed membrane-fabrication technologies[5, 10, 18-22] to present soluble components within a surface-array format.
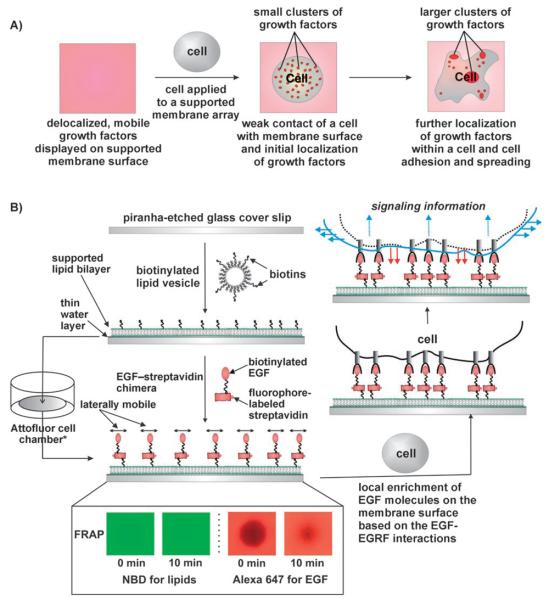
Figure 1.
A) Conceptual schematic of the fluid membrane-based soluble-ligand display strategy. B) The fluid membrane-tethered EGF-based cell assay and fluorescence recovery after photobleaching (FRAP) experiments to test the fluidity of both EGF molecules and lipids on a glass cover slide. See Experimental Section for details. *Attofluor cell chamber was used throughout the addition of EGF to the SLB, FRAP experiments, the addition and incubation of cells on the SLB, and imaging processes.
We chose epidermal growth factor (EGF) and the EGF-receptor tyrosine kinase (EGFR) as a prototypical signaling system to evaluate the SLB platform. EGFR is a member of the type I (ErbB) receptor tyrosine kinases (RTKs) and is activated by a number of ligands from the EGF family.[24-26] This results in receptor dimerization and a cascade of signaling events culminating in a number of biologic end points, including proliferation.[26, 27] ErbB deregulation is a common event in human cancer in which EGFR and a second family member, ErbB2, have become targets for directed therapeutic interventions such as Tarceva, Herceptin, and Iressa.[28] It is clear that an understanding of EGFR and ErbB2 will yield translational insight and a more detailed understanding of the molecular interactions of these molecules might yield further clinical benefit. Recent insights into the molecular mechanisms of EGFR signaling suggest that localization of EGFR on the cell membrane enhances receptor dimerization/clustering, which is pre-requisite for ligand binding and activation of receptor-kinase activity.[26, 27] Applying the fluid membrane-tethered ligand-display method reported herein to the EGF-EGFR system has clear benefits. The system allows for fast local enrichment of EGF induced by the EGF-EGFR interactions, facile in situ monitoring of fluorescently labeled EGF, and temporal analysis of cellular phenotypes in a surface-assay format.
The design of an EGF-modified fluid SLB (EGF–SLB) assay is outlined in Figure 1 B. To measure the fluidity of lipid bilayers (DMOPC, 1,2-dimyristoleoyl-sn-glycero-3-phosphocholine) with and without substrate-bound EGF, a focal region of the membrane was photobleached, and fluorescence from Alexa Fluor 647 (labeling EGF-modified lipids) or 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD; labeling bare lipids) was monitored. Photobleached regions for both bare lipids and EGF-modified lipids recovered fluorescence; this indicates that they are fluid (Figure 1 B, inset). Interestingly, the EGF-modified lipids were slightly less fluid than the bare NBD-modified lipids; this suggests that EGF binding to the SLB alters the fluidity of the EGF-tethered lipids.
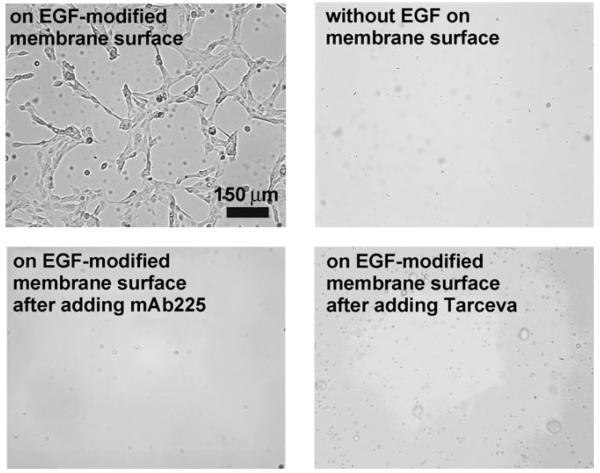
As a practical test of this system, we examined the EGF–EGFR interactions between the EGF–SLB and live cells. We chose the immortal, nontransformed human breast epithelial cell line MCF-10a for this purpose, as these cells express EGFR and are dependent on EGF signaling for proliferation and survival (all the cells in this paper refer to MCF-10a cells). We applied MCF-10a cells in serum-free, growth factor-free DMEM/F-12 media (∼300 000 cells per mL) to an EGF–SLB array and to a streptavidin-modified lipid membrane without EGF molecules. The cells were incubated at 37°C for 20 h, after which they were gently washed with DMEM/F-12 media and visualized by epifluorescence microscopy (TE300, Nikon, Inc.). Analysis of the membranes after washing revealed attachment of cells to the EGF–SLB array but not to the streptavidin-modified lipid membrane (Figure 2); this suggests EGF-dependent attachment of cells to the lipid surface. However, it was unclear whether direct ligand–receptor interactions alone were responsible for cell-membrane attachment, or whether EGFR signaling modulated cell attachment to the EGF–SLB through secondary mechanisms. To investigate whether the direct binding of EGF to EGFR facilitated attachment, we added a competing antibody for EGFR (mAb225) to the cells. The presence of 3ng mL−1 competing antibody reduced the number of cells attached to the membrane by 94% after 20 h (Figure 2, bottom left panel). This confirmed the specificity of the EGF–EGFR interaction and that it is required for cell-to-EGF–SLB attachment. EGF stimulation of EGFR kinase activity signaling activates a number of downstream pathways, some of which regulate cytoskeletal molecules, cell attachment and motility.[24-28] Therefore, we next tested whether EGFR kinase activity is required for attachment by treating cells with Tarceva, a specific kinase inhibitor of EGFR. When the assay was performed in the presence of Tarceva, there was a significant reduction in the number of cells attached to the membrane (Figure 2, bottom right panel); this confirmed that activation of EGFR kinase activity is required for cell attachment.
Figure 2.
Bright-field images of cells on supported lipid bilayers.
To understand the temporal and spatial kinetics of the EGF–EGFR interaction, time-lapse experiments were employed to observe cell attachment to the EGF–SLB and subsequent EGF localization. This dynamic interaction was monitored by using bright-field microscopy to image cells and epifluorescent microscopy to image the EGF-coupled Alexa Fluor 647 (Figure 3). Cells were observed to weakly adhere to the surface as early as 80 min post plating.
Figure 3.
Cell attachment to the EGF-modified SLB, and EGF cluster formation. A) Bright field and fluorescence images were recorded over incubation time; fluorescence images show dynamic clustering of EGF within a cell. B) Bright field (left) and fluorescence (right) images of a cell on the EGF-modified SLB after 20 h of incubation at 37°C.
At this time, EGF was still randomly distributed across the surface. By 100 min, EGF molecules were observed to cluster into small focal points, which increased in size over time. These small clusters began to form larger clusters at around 150 min (Figure 3 A). After 20 h, a cell spread and adhered to the surface with many distinctive EGF clusters (Figure 3 B). These clusters are reminiscent of focal adhesions required for cell-attachment to substrata. Since these EGF clusters appear to lie partially out of the supported-membrane plane, as determined by focusing the microscope at different positions, we suspect that these clusters could be endocytosed EGFRs with bound EGFs and fluorophore labels. Since natural triggering of EGFR by EGF is followed by endocytosis, we interpret this observation as further support of the signaling functionality of membrane-tethered EGF. It should also be noted that cells cannot apply tensile forces to membrane adhesion sites; the fluid membrane will simply flow under such forces.[5] The stretched cell attachment phenotype (Figure 2 and Figure 3 B) clearly indicates the presence of tensile forces; this suggests that the cells are anchored to the underlying solid substrate through focal adhesion sites. Formation of these focal adhesions likely involves remodeling of the surface by secretion of extracellular matrix (ECM) proteins.
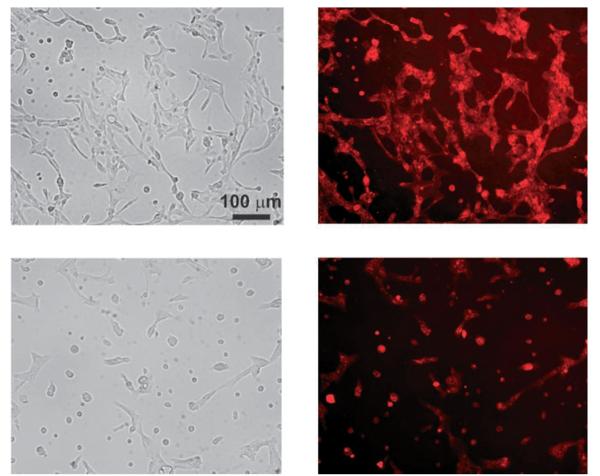
Clustering of EGFR on the cell surface is a prerequisite for signal activation by ligand binding and is dependent on ligand diffusion across the SLB.[26,27] Therefore, we hypothesized that increasing the fluidity of membrane-tethered EGF would facilitate this process. To test this hypothesis, we directly compared the DMOPC-based system to the DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine)-based system, as DMOPC is much more fluid than DPPC (at 37°C, the diffusion constant for DMOPC is 9 μm2 s−1 and the diffusion constant for DPPC is 0.1 μm2 s−1; Avanti Polar Lipids, Inc., Alabaster, AL).[29, 30] Cells membrane substrate. Cells adhered to the DMOPC–EGF–SLB exhibited increased cell spreading, indicative of a motile phenotype, compared to the DPPC–EGF–SLB. Cells attached to the DMOPC-based EGF–SLB surface displayed EGF clusters at the location where the cells were adhered. In contrast, fewer EGF clusters were found where cells were attached to the DPPC membrane arrays (Figure 4). These results suggest that supported membrane fluidity facilitates localized clustering of EGF, which is essential for its signaling functionality.
Figure 4.
Cells cultured at 37°C for 20 h on top: fluid (DMOPC) and bottom: nonfluid (DPPC) EGF–SLB surfaces. Left: Bright field; right: Alexa Fluor 647
In summary, we have demonstrated the utility of fluid SLBs for the presentation of soluble signaling ligands to cells in culture. We found that membrane-tethered EGF is sufficient to promote cell adhesion and the fluidity of membrane-tethered ligands enhances its efficacy. Dynamic local enrichment of EGF molecules by reaction–diffusion processes was observed. The stretched morphology of the cells and the existence of focal adhesions suggest that the underlying substrate has been locally remodeled by ECM secretion. This process, however, is triggered by membrane-displayed EGF. Through competition with inhibitory antibodies and EGFR kinase inhibitors, we demonstrated that this is an EGF–EGFR interaction-dependent phenotype and that kinase activation of the EGFR is also required. By studying the temporal adhesion of cells to EGF–SLB, it is clear that full adhesion takes several hours; this suggests that signaling through EGFR up-regulates a genetic program stimulating cell-adhesion.
This fluidity-based soluble ligand-display system offers an experimental environment in which one can monitor dynamic reorganization and endocytosis of soluble ligands on a planar platform in the absence of ligands in solution. By eliminating ligands in solution, improved observation of soluble signaling molecules is possible because background fluorescence intensity is minimal in this system.
The ligand-display strategy reported herein provides a new dimension to controlling soluble ligand exposure to cells in culture. Display of soluble signaling ligands in an array format allows for the utilization of developed membrane array technologies to present soluble ligands to cells in various configurations. We anticipate that this strategy will be useful in understanding the biology of ligand-receptor interactions as well as developing patterned soluble ligand–based high-throughput cell-screening assays for medical diagnostic and cell biological applications. This simple system is expected to be applicable to other soluble ligands, such as other growth factors, cytokines, and hormones as well as membrane-bound ligands (e.g. ephrins).
Experimental Section
EGF-modified SLBs were fabricated with by the following procedures. First, biotinylated lipid vesicles along with NBD-modified vesicles were prepared by existing methods.[18-20, 22] In short, the desired lipids were dissolved in chloroform, which was then evaporated off by using a rotary evaporator. The lipids were thoroughly dried under nitrogen gas and then hydrated with water (1 mL). The hydrated lipids were extruded through 100 nmd pore filters and stored at 4°C until needed. Then, the vesicles (3% biotin-modified DPPE, 2% NBD-modified PC, and 95% DMOPC, purchased from Avanti Polar Lipids, Inc., Alabaster, AL) were allowed to warm to room temperature. Next they were ruptured on a piranhaetched microscopic cover glass (Fisher Scientific, Pittsburgh, PA) in aqueous NaCl (25 mM). The resulting lipid-bilayered glass substrate, immersed in aqueous NaCl, was sealed in an Attofluor cell chamber (Invitrogen Corp., Carlsbad, CA). Subsequently, EGF molecules conjugated to streptavidin and Alexa Fluor 647 (150 μL at 100 μg mL−1) were applied to the biotinylated membrane-modified glass substrate for 45 min at room temperature (approximately one biotinylated EGF molecule was bound to each streptavidin-modified Alexa Fluor 647, leaving three binding sites for each streptavidin to bind to a membrane-bound biotin molecule; Invitrogen Corp., Carlsbad, CA). This allowed attachment of EGF molecules to the membrane through streptavidin-biotin interactions. The NaCl solution in which the SLB was immersed was then exchanged by washing the Attofluor cell chamber three times with DMEM/F-12 (GIBCO, Invitrogen Corp., Carlsbad, CA). This washing step served the dual purpose of removing unbound EGF-streptavidin-Alexa Fluor 647 molecules and immersing the SLB in a medium that was suitable for the desired cells to survive in, while still retaining membrane fluidity. At this point MCF-10a cells (1 mL, 3×105 cells per mL) were added to the Attofluor cell chamber. The chamber was then wrapped in parafilm, with holes to allow oxygen into the chamber, and the cells were incubated at 37°C for 20 h. After the incubation period, the Attofluor cell chamber was washed three times with DMEM/F-12 to remove any nonadhered MCF-10a cells. The cells were then imaged by using bright-field and epifluorescence microscopy.
FRAP experiments were conducted to verify the fluidity of the phospholipids in the bilayer, labeled with 2% NBD, and the lipidtethered EGF-streptavidin complex, labeled with Alexa Fluor 647, on a glass substrate. First, both fluorophores were photobleached for approximately 3 min. The photobleached area (the dark octagon in the center of the images) was then allowed to recover for 10 min, and epifluorescence images were taken (the bilayer was exposed to the excitation wavelengths for 3 s). The resulting images were then false-colored and processed by using Adobe Photoshop 7.0 (green for NBD and red for Alexa Fluor 647). Recovery of fluorescence for both NBD and Alexa Fluor 647 confirms that the DMOPC phospholipids in the bilayer, as well as the EGF bound to the streptavidin, were fluid under the experimental conditions.
For the studies with DPPC, the initial lipid concentrations of the vesicles were 3% biotin-modified DPPE, 2% NBD-modified PC, and 95% DPPC. After being extruded through 100 nm pore filters, the vesicles were extruded through 30 nm pore filters, so that they would be smaller and easier to rupture. Before being ruptured, the vesicles were heated to 50°C, as was the spreading solution and the NaCl solution. The piranha-etched microscopic cover glass was also heated above 50°C. All of these heating steps were required to ensure that the lipids were in the fluid phase while the bilayer was being formed. All other steps remained the same as when using DMOPC.
A human breast epithelial cell line, MCF-10a, was cultured in a serum-rich medium consisting of DMEM/F-12 (GIBCO, Invitrogen Corp., Carlsbad, CA), hydrocortisone (500 ng mL−1), horse serum (5% v/v), bovine insulin (0.01 mg mL−1), and EGF (20 ng mL−1). On the day of the experiments, the cells were treated with trypsin–EDTA, washed twice with 1x PBS, and centrifuged; then 3×105 of the cells were resuspended in serum-free medium lacking EGF (1 mL) for each experiment. These aliquots were then incubated at 37°C in a water bath until they were added to the EGF–SLB. For the studies with Tarceva and mAb225, the cells were incubated with either Tarceva or mAb225 for 45 min in a 37°C water bath before being added to the EGF-SLB. All other steps were as before.
For the studies to count cells adhered to EGF–SLBs, the initial lipid concentrations were as before, but with an additional 2% of the primary lipid constituent substituted for 2% NBD-PC (3% biotin-modified DPPE and 97% DMOPC or DPPC). After the 20-hour incubation of the cells on the EGF–SLBs, the chamber was washed three times with DMEM/F-12 as before, to remove nonadhered cells. Then the cells were stained with Hoechst 33342 (100 μL at 1 μg mL−1 for 10 min, and the chamber was washed four more times with DMEM/F-12 to remove any unbound Hoechst 33342. Then the cells were imaged by using bright-field and epifluorescence microscopy.
We used a TE300 Nikon inverted microscope with a mercury arc lamp for epifluorescence illumination and a 100 W halogen lamp for bright-field illumination. The image in Figure 3 A was taken with a Hamamatsu Orca CCD camera (Hamamatsu Corp., Hamamatsu City, Japan) and those in Figures 2, 3 B, and 4 were taken with a CoolSnap HQ CCD camera (Roper Scientific, Inc., Tucson, AZ). SimplePCI (Compix, Inc. Imaging Systems, Cranberry Township, PA) and MetaMorph (Molecular Devices Corp., Downington, PA) software was used to collect and analyze the images, which were then further processed by using Adobe Photoshop 7.0. Alexa Fluor 647 was imaged by using a Cy5 filter cube, and NBD was imaged by using an NBD/HPTS filter cube. For the cell-counting studies, Hoechst 33342 was imaged by using a DAPI/Hoechst/AMCA filter cube. All filter cubes were purchased from Chroma Technology Corp., Rockingham, VT.
Acknowledgements
This work was supported by DOE (Grant #: DE-AC03-76SF00098). J.-M.N. and P.M.N. would like to thank Nathan Clack and Bryan Jackson for helpful discussions and the initial experimental setup, respectively.
References
- 1.Feldmann M, Steinman L. Nature. 2005;435:612. doi: 10.1038/nature03727. [DOI] [PubMed] [Google Scholar]
- 2.Chen EH, Olson EN. Science. 2005;308:369. doi: 10.1126/science.1104799. [DOI] [PubMed] [Google Scholar]
- 3.Groves JT. Sci. STKE. 2005;301:pe45. doi: 10.1126/stke.3012005pe45. [DOI] [PubMed] [Google Scholar]
- 4.Irvine DJ, Hue K-A, Mayes AM, Griffith LG. Biophys. J. 2002;82:120. doi: 10.1016/S0006-3495(02)75379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groves JT, Mahal LK, Bertozzi CR. Langmuir. 2001;17:5129. [Google Scholar]
- 6.Kane RS, Takayama S, Ostuni E, Ingber DE. Biomaterials. 1999;20:2363. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 7.Schmeichel KL, Bissell MJ. J. Cell Sci. 2003;116:2377. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu M, Holowka D, Craighead HG, Baird B. Proc. Natl. Acad. Sci. USA. 2004;101:13798. doi: 10.1073/pnas.0403835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglass AD, Vale RD. Cell. 2005;121:937. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groves JT, Dustin ML. J. Immunol. Methods. 2003;278:19. doi: 10.1016/s0022-1759(03)00193-5. [DOI] [PubMed] [Google Scholar]
- 11.Tirrell M, Kokkoli E, Biesalski M. Surf. Sci. 2002;500:61. [Google Scholar]
- 12.Jensen TW, Hu B-H, Delatore SM, Garcia AS, Messersmith PB, Miller WM. J. Am. Chem. Soc. 2004;126:15223. doi: 10.1021/ja048684o. [DOI] [PubMed] [Google Scholar]
- 13.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Science. 1997;276:1425. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 14.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th ed. Garland Science; New York: 2002. pp. 883–884. [Google Scholar]
- 15.Ziauddin J, Sabatini DM. Nature. 2001;411:107. doi: 10.1038/35075114. [DOI] [PubMed] [Google Scholar]
- 16.Wheeler DB, Bailey SN, Guertin DA, Carpenter AE, Higgins CO, Sabatini DM. Nat. Methods. 2004;1:127. doi: 10.1038/nmeth711. [DOI] [PubMed] [Google Scholar]
- 17.Bailey SN, Sabatini DM, Stockwell BR. Proc. Natl. Acad. Sci. USA. 2004;101:16144. doi: 10.1073/pnas.0404425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sackmann E, Tanaka M. Trends Biotechnol. 2000;18:58. doi: 10.1016/s0167-7799(99)01412-2. [DOI] [PubMed] [Google Scholar]
- 19.Groves JT. Angew. Chem. 2005;117:3590. [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:3524. [Google Scholar]
- 20.Yee CK, Amweg ML, Parikh AN. J. Am. Chem. Soc. 2004;126:13962. doi: 10.1021/ja047714k. [DOI] [PubMed] [Google Scholar]
- 21.Holden MA, Jung S-Y, Yang T, Castellana ET, Cremer PS. J. Am. Chem. Soc. 2004;126:6512. doi: 10.1021/ja048504a. [DOI] [PubMed] [Google Scholar]
- 22.Kam L, Boxer SG. Langmuir. 2003;19:1624. [Google Scholar]
- 23.Zhang S. Nat. Biotechnol. 2004;22:151. doi: 10.1038/nbt0204-151. [DOI] [PubMed] [Google Scholar]
- 24.Yarden Y, Sliwkowski MX. Nat. Rev. Mol. Cell Biol. 2001;2:127. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 25.Olayioye MA, Neve RM, Lane HA, Hynes NE. EMBO J. 2000;19:3159. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawano A, Takayama S, Matsuda M, Myyawaki A. Dev. Cell. 2002;3:245. doi: 10.1016/s1534-5807(02)00224-1. [DOI] [PubMed] [Google Scholar]
- 27.Ichinose J, Murata M, Yanagida T, Sako Y. Biochem. Biophys. Res. Commun. 2004;324:1143. doi: 10.1016/j.bbrc.2004.09.173. [DOI] [PubMed] [Google Scholar]
- 28.Britten CD. Mol. Cancer Ther. 2004;3:1335. [PubMed] [Google Scholar]
- 29.Forstner MB, Yee CK, Parikh A, Groves JT. unpublished results.
- 30.Tocanne J-F, Dupou-Cczanne L, Lopez A. Prog. Lipid Res. 1994;33:203. doi: 10.1016/0163-7827(94)90027-2. [DOI] [PubMed] [Google Scholar]