Abstract
The purpose of the current study was to examine the interrelationships between Autonomous Regulation (AR) and locus of control (LOC) and their prediction of Antiretroviral Therapy (ART) adherence among 189 HIV+ patients. Path analyses revealed that neither AR nor LOC directly predicted adherence although AR was indirectly related when mediated by self-efficacy. AR was positively related to internal and doctors LOC, but not related to chance or others LOC. Overall, results support Self Determination Theory’s conceptualization of AR and indicate that AR may be a more robust predictor of medication adherence than LOC variables.
Keywords: AR, Locus of Control, HIV, Adherence
Antiretroviral Therapy (ART) has been shown to greatly decrease morbidity and mortality associated with HIV (Palella et al., 1998), however its efficacy is dependent on strict adherence (Alfonso, Geller, Bermbach, Drummond, & Montaner, 2006). Failure to adhere to ART medications at ≥95% has been shown to increase morbidity and mortality in those with baseline CD4 counts of 200 to 350 cells/μL (Wood et al., 2003). Poor adherence can also lead to the development of drug resistant virus (Clavel & Hance, 2004; Deeks, 2003).
Despite its importance for health, many patients struggle with the high level of adherence required (Altice & Friedland, 1998; Singh et al., 1996). Reasons for non-adherence include forgetting, being away from home, being busy with other things, having a change in daily routine, or sleeping through dose times (Chesney et al., 2000). Psychosocial factors that influence adherence include depression, perceived stress, anxiety, positive affect, self-regulation, social support, and self-efficacy (Chesney, 2000; Johnson et al., 2003). In this study we focus on two constructs that have received limited attention in the literature, autonomous regulation (AR) and locus of control (LOC).
AR is part of Self-Determination Theory (SDT) (Ryan & Deci, 2000) which proposes that motivation to perform a specific behavior is enhanced when individuals perceive themselves to be competent to perform the behavior and choose of their own free will to engage in the behavior (Deci & Ryan, 1985; Williams et al., 2002). Williams and colleagues (2004) describe autonomously regulated behavior as that in which a person has a sense of choice or full volition, as opposed to controlled behavior where individuals feel coerced or pressured to perform.
A few studies have provided support for AR as a predictor of adherence behavior. Williams et al. (2005) conducted a longitudinal study that examined autonomy support (i.e., the extent to which patients perceived that their providers encouraged a feeling of autonomy), AR and perceived control as predictors of diabetes self-management, including medication adherence. Increases in AR predicted improvement in glycemic control over 12 months. Williams, Rodin, Ryan, Grolnick, and Deci (1998) examined adult outpatients with diverse diagnoses that were on medication for at least one month. Higher levels of AR explained better adherence over and above age, gender, autonomy support, and perceived barriers.
One study has examined AR as a predictor of ART adherence (Kennedy, Goggin, & Nollen, 2004) which was measured with a 3 day recall and verified by pharmacy refill logs. Results provided support for a mediation model in which greater AR predicted greater perceived competence, which in turn predicted better adherence.
The second construct examined in this study, LOC, is drawn from Rotter’s social learning theory that has also been shown to predict adherence. LOC is a type of control belief that concerns the locus, or place, where control over outcomes resides (Wallston, 2001). Those who have an external locus believe that events in their life occur because of luck, fate, powerful others, or other things outside of their own control, whereas those with an internal locus believe that events in their life occur due to their own behavior (Rotter, 1966). LOC is distinct from perceived competence or self-efficacy, which is another type of control belief related to whether one believes one can do a specific behavior. LOC differs from AR in that it concerns an individual’s beliefs regarding the locus of what determines his or her health status, whereas AR refers to the nature of a person’s motivation to engage in a (health) behavior (i.e., to what extent they perceive it to be freely chosen or fully volitional).
Studies have demonstrated that individuals are not entirely internal or external in their LOC, but rather that they consider different sources of control. The Multidimensional Health Locus of Control (MHLC) was developed to specifically tap different LOC beliefs in relation to health conditions (Wallston, Wallston, & DeVellis, 1978). It includes three orthogonal dimensions (viz., internal, chance, powerful others). A revised form of the MHLC further subdivides the powerful others scale into two separate scales: doctors and others (Wallston, Stein, & Smith, 1994).
Studies of LOC and adherence have produced mixed findings with some studies observing no relation between the LOC constructs and adherence (Bane, Hughes, & McElnay, 2006; Christensen, Wiebe, & Lawton, 1997). However, most studies that have used the MHLC have observed significant associations between adherence and high internal LOC (Hong, Oddone, Dudley, & Bosworth, 2006; O’Hea et al., 2005; Stanton, 1987; Voils, Steffens, Flint, & Bosworth, 2005). In addition high powerful others has also been shown to be independently related to better adherence (Myers & Myers, 1999; Sensky, Leger, & Gilmour, 1996).
Other research has found internal LOC to be predictive in interaction with other LOC subscales (Christiansen, Wiebe, Benotsch, & Lawton, 1996; O’Hea et al., 2005). For example, in a study of diabetic patients internal LOC was found to interact with chance LOC to predict adherence such that those who reported high internal LOC and high chance LOC had better adherence than those who reported low internal LOC and high chance LOC (O’Hea et al., 2005). In a study of renal dialysis patients internal LOC was found to interact with powerful others LOC and perceived health competence to predict adherence. Surprisingly, those who reported low perceived health competence, low internal LOC, and high powerful other LOC had the best adherence (Christiansen et al., 1996). One of the only studies conducted concerning LOC and HIV medication adherence found that high internal LOC was one of several variables that predicted greater adherence (Molassiotis et al., 2002).
Although both AR and LOC appear to be related to adherence, there is little research exploring their interrelationships and how they are collectively associated with adherence. SDT posits that AR is characterized by a more internal perceived LOC than external perceived LOC (Ryan & Deci, 2000). Williams et al. (1998) found among a sample of patients taking a variety of medications that all three sub-domains of LOC (internal, powerful others and chance) were not significantly predictive of adherence over and above AR (Williams et al., 1998). The study did not, however, examine the interrelationships between the sub-domains of LOC and AR. The study was also limited in that adherence was assessed via self-report and the nature of adherence was not comparable to HIV medication adherence because regimens were simple (i.e., medications only needed to be taken for a short period of time).
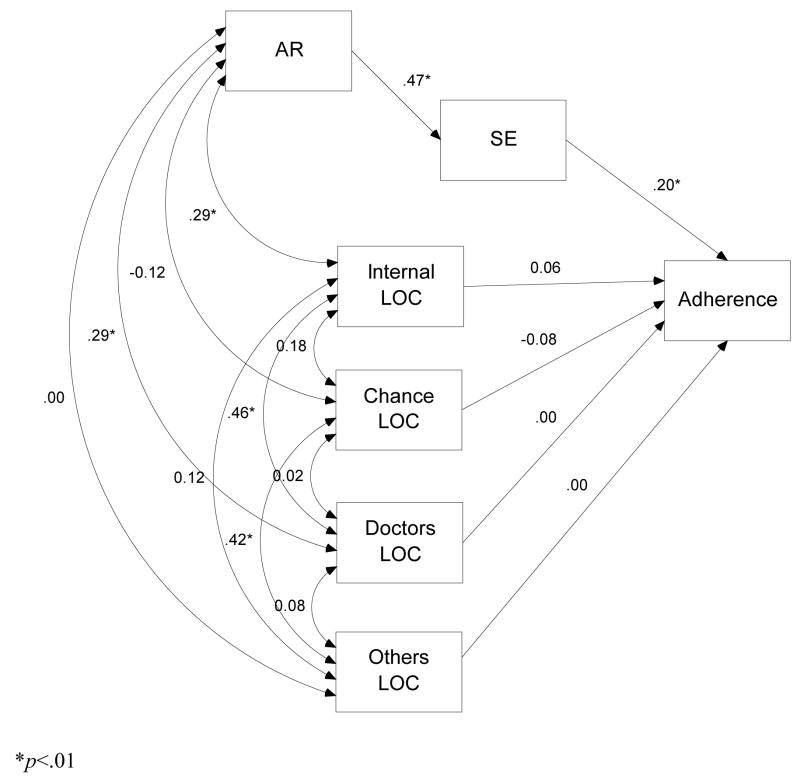
The purpose of this study was, therefore, to examine the interrelationships between all LOC constructs and AR and their relative association with medication adherence. Adherence was assessed using an electronic adherence monitoring device among patients receiving ART. Based on prior studies it was hypothesized that a model in which the LOC subscales and AR mediated by self-efficacy were predictors of adherence, would provide a good fit for the data (see Figure 1). In addition, based on theory it was hypothesized that AR would be positively related to internal LOC, but negatively related to doctors, others and chance LOC. With respect to adherence we hypothesized that internal LOC and AR would be related to adherence, but based on prior results that AR would be the best predictor of adherence.
Figure 1.
Initial path analysis model predicting adherence
*p<.01.
Method
Overview
Data for this study were drawn from a randomized controlled trial of ART medication adherence. Data used were from the baseline session and adherence data collected at the one week visit. APA ethical standards were followed in the treatment of participants and approval of the study was obtained by the Institutional Review Board at the University of Missouri-Kansas City.
Participants
Participants were recruited from five clinics that provide medical services for patients with HIV. To be eligible patients had to be starting a new medication regimen or restarting a regimen after a significant break, or have self-reported or physician suspected adherence problems that were supported by clinical values (CD4 cells and viral load). In addition participants had to be at least 18 years old and not pregnant. Participants who were not independently responsible for their medications (i.e., prisoners, residents of assisted living facilities) or could not understand and give consent in English were excluded.
The sample for this study was the first 189 participants who completed one week of the study and for whom complete adherence data were available (see baseline characteristics in Table 1).
Table 1.
Demographics and descriptive characteristics
| Characteristic | % | Median |
|---|---|---|
| Male | 73 | |
| Ethnicity | ||
| African American | 57 | |
| Caucasian | 34 | |
| High school education or less | 56.9 | |
| Single | 57.9 | |
| Have children | 50 | |
| Income (below $1000/month) | 60.2 | |
| Viral load (undetectable) | 9.8 | |
| CD4 cells/μL | 229.5 | |
| Naive to HIV medication regime | 30.9 | |
Procedure
Providers at the various clinics referred potentially eligible participants to a study coordinator who described the study. Participants who were interested and eligible completed informed consent procedures and scheduled an enrollment visit. Upon enrollment, participants completed a set of baseline measures, including those used for this study. Participants were randomized into standard care or one of the two treatment arms (counseling or counseling with observed therapy). To measure adherence, participants received a Medication Event Monitoring System (MEMS) bottle (Aardex, 2006) accompanied with instructions on its use. One of their HIV medicines was placed in the MEMS bottle and participants were told to take their medication as prescribed. At the end of one week, data were downloaded from the MEMS bottle to use as the adherence measure. Data from the MEMS bottle were collected for the duration of the study, but for the current study, we analyzed only the first week of adherence data collected via the MEMS bottle.
Measures
Autonomous regulation
To assess AR the autonomous regulation subscale of the Treatment Self-Regulation Questionnaire (TSRQ) was used (Williams, Grow, Freedman, Ryan, & Deci, 1996). In past research, the TSRQ has been modified to address specific health behaviors such as diet, exercise, and smoking cessation. For the purposes of this study, scale items were modified to address ART adherence. Participants were asked to rate their level of agreement with 6 items using a 7-point Likert-type scale (1 = strongly disagree, 7 = strongly agree) where the stem of each item was “The reason I would take my HIV medications as they were prescribed to me is…” The alpha for the AR subscale was .84 which is in accordance with prior studies (Kennedy et al., 2004; Williams et al., 2004).
Self-efficacy
This 10-item self-report measure (Chesney et al., 2000) asks patients to indicate their level of confidence in performing specific adherence tasks using a 11-point Likert- type response format ranging from 1 (cannot do at all) to 6 (moderately certain I can do) to 11 (certain I can do). Sample items include: “stick to your medication schedule when it means changing your eating habits,” “stick exactly to your medication schedule,” and “continue even when you are feeling sick.” This measure has been used successfully in previous studies of AIDS adherence behavior (Chesney et al., 2000) and for this study had an alpha of .87.
Multi-dimensional health LOC
Health LOC was measured using the 18-item Form C version of the Multi-dimensional Health Locus of Control (MHLC) measure, developed by Wallston et al. (1994). This self-report instrument assesses the extent to which participants believe their condition (i.e., HIV disease) is due to: (1) their own behavior (internality); (2) the behavior of powerful others (which in form C is split into two subscales: doctors and others) or (3) chance, luck, or fate. Participants were asked to rate their agreement with each item using a 6-point Likert-type (1 = strongly disagree; 6 = strongly agree). Alphas for this study ranged from .52 to .76, which are within acceptable limits and similar to previous work in which adequate reliability and validity for this measure have been documented (Wallston et al., 1994).
Adherence
Adherence was assessed using a MEMS bottle in which the number of doses taken is recorded. A MEMS bottle records an event whenever the bottle is open for at least 5 seconds. Adherence was indexed by the number of doses taken divided by the number of doses prescribed, with the additional criterion that the dose must be taken within a 4 hour time window around their proscribed dose time. This method of collecting adherence to medications data is currently the “gold standard” (Chesney, 2000).
Analyses
Path analysis was performed using Amos 7. The initial model included paths from self-efficacy, internal LOC, chance LOC, doctors LOC and others LOC. These five variables were specified to directly predict adherence (see Figure 1). AR was specified to have an indirect influence on adherence through self-efficacy. In addition, intercorrelations between all of the exogenous variables were specified. We then tested a second model in which a direct path from AR to adherence was added.
The chi-square statistic assessed the overall fit of the model to the data. Although one would generally like to retain the null hypothesis, it is difficult to do so with the chi-square statistic, particularly with large samples (Ullman, 2001). Some researchers argue that the chi-square statistic to degrees of freedom ratio provides a fairer test of fit (Ullman, 2001). Generally, ratios less than 2.0 indicate acceptable model fit. We also report the root-mean-square-error of approximation (RMSEA), for which values less than .08 are generally seen as indicating acceptable fit, and the comparative fit index (CFI) for which values greater than .90 indicate acceptable fit (Ullman, 2001).
Results
Preliminary Analyses
As can be seen in Table 1 the sample was predominantly male and ethnically diverse. The sample was also primarily single, low income, and of lower educational attainment. An ANOVA with treatment group as the independent variable and adherence as the dependent variable was not significant, so treatment group was not used as a covariate. Bivariate associations between all variables were also examined (see Table 2) and only self-efficacy was associated with adherence.
Table 2.
Correlations between variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Adherence | - | ||||||
| 2. AR | .06 | - | |||||
| 3. SE | .23** | .47** | - | ||||
| 4. Internal LOC | .07 | .29** | .11 | - | |||
| 5. Chance LOC | −.11 | −.12 | −.18* | .18* | - | ||
| 6. Doctors LOC | .06 | .30** | .19** | .46** | .02 | - | |
| 7. Others LOC | −.03 | .00 | −.01 | .12 | .42** | .08 | - |
p<.05,
p<.01
Path Models
The initial model provided good fit, χ2 (5) = 6.66, p = .248, χ2/df = 1.33, RMSEA = .04, CFI = .99. With respect to the interrelationships between LOC variables and AR the model revealed that, as hypothesized, internal LOC was positively related to AR (β = .29, p < .01). Contrary to our hypotheses, neither chance LOC (β = −.12, p > .05) nor others LOC (β = −.01, p > .05) were related to AR. Similarly, while doctors LOC was significantly related to AR, the association was positive rather than negative (β = .30, p < .01).
With respect to the prediction of adherence using the LOC subscales, none of the subscales were significant predictors (β = −.08, p > .10 for chance; β = −.01, p > .10 for doctors; β = .01, p > .10 for others; and β = .07, p > .10 for internal).1
The second model, in which we added a direct path between AR and adherence, also had fairly good fit, χ2 (4)= 5.63, p = .228 ., χ2/df = 1.41, RMSEA = .05, CFI = .99. However, this model did not provide any better fit than the first, more parsimonious model, χ2 (1) = 1.03, p > .05. With respect to predicting adherence, we found support for our hypothesis that AR, mediated by self-efficacy, would be related to adherence. AR was not a direct predictor of adherence (β = −.08, p > .05), but the standardized indirect effect of AR was significant (β = .11, p < .05).
Discussion
The primary purpose of this study was to examine AR and LOC as predictors of adherence. The analyses examined the relationships between AR and LOC variables and adherence and found mixed support for the hypotheses. In bivariate analyses AR was not associated with adherence. However, in the path analysis model AR was indirectly associated with adherence, mediated by self-efficacy. This was consistent with our hypothesis, confirming the findings of Kennedy, Goggin and Nollen (2004), but extending prior work by demonstrating this effect using MEMS for adherence data, rather than self report. This is a significant result because it confirms the finding that AR is associated with adherence in an HIV+ sample consistent with many other previous studies outside of HIV adherence (Kennedy et al., 2004; Senecal, Nouwen, & White, 2000; Williams et al., 2004; Williams et al., 1998). This result also highlights the potential importance of clinicians fostering autonomous motivation among their HIV+ patients.
With respect to LOC variables and adherence, contrary to predictions, internal LOC and all of the other LOC subscales were not significantly related to adherence. Although internal LOC has been the most consistent predictor of adherence among the LOC subscales (Christiansen et al., 1996; Hong et al., 2006; O’Hea et al., 2005; Stanton, 1987; Voils et al., 2005), findings have nevertheless been mixed (Christensen et al., 1997; McDonald-Miszczak, Maki, & Gould, 2000; Myers & Myers, 1999; Sensky et al., 1996), suggesting the association is not robust.
The analysis of the interrelationships between AR and LOC revealed that AR was associated with two subscales of LOC: internal and doctors LOC. As predicted, those with a greater internal LOC were more autonomously or intrinsically motivated. This finding is consistent with the prediction of Self-determination theory (Ryan & Deci, 2000). While it was hypothesized that AR and doctors LOC would be negatively associated, the result is not inconsistent with more recent conceptualizations of LOC which recognize that the sub-scales are orthogonal. Thus individuals may be autonomously regulated and high on internal LOC yet vary in the extent to which they attribute control to doctors, others and chance. HIV+ individuals may be autonomously motivated to adhere, believe that their HIV disease is under their control, yet also believe that their physician and other uncontrollable factors (e.g., being infected with a resistant strain of HIV, availability of new medications) can play a role in the outcome of their disease.
Path analysis results revealed that neither LOC subscales nor AR were significant independent predictors of adherence. Furthermore, self-efficacy was the only significant direct predictor. These results suggest that there is significant overlap between chance LOC and AR in the prediction of adherence and that there is little advantage to including both variables in the same model of adherence. Given that the second path analysis model which included AR with paths both directly to adherence and mediated through self-efficacy still showed a significant indirect effect of AR, and given that in the literature AR appears to be a more reliable predictor of adherence (Kennedy et al., 2004; Senecal et al., 2000; Williams et al., 2004) than LOC (Bane et al., 2006; Christensen et al., 1997; Christiansen et al., 1996; Hong et al., 2006; O’Hea et al., 2005; Ricker, Delamater, & Hsu, 1998) it may be preferable to use AR rather than LOC when predicting adherence.
Although self-efficacy was the most powerful independent predictor of adherence, variables such as AR and LOC may nonetheless be important because of their role as precursors or predictors of a person’s self-efficacy. The finding of support for a mediation model in which self-efficacy mediated the effect of AR on adherence is consistent with this, however, it is worth noting that this analysis was not a causal one, and does not rule out the possibility that AR mediates the impact of self-efficacy on adherence. More theoretical work that can explore causal ordering of these variables is needed.
One limitation of this study is that the main measure of adherence was short term (i.e., only one week). Findings may have been different if adherence was examined over a longer period of time when challenges associated with maintaining behavior may have come into play. However, only 31% of participants were naive, or new to a medication regimen. So, for most, one week of adherence may be an accurate measure of their typical medication adherence behavior.
In spite of these limitations, the findings clarify the inter-relationships between AR, LOC variables, and adherence. Although neither AR nor LOC variables contributed uniquely to the prediction of adherence, results contribute to an emerging picture of AR as a more reliable predictor of adherence than LOC across different demographic groups and diseases, and suggest AR may contribute significantly to adherence through important mediators such as self-efficacy.
Acknowledgments
This research was supported by the National Institutes of Mental Health (RO1 MH68197) and made possible by the participants of Project MOTIV8. We gratefully acknowledge the contributions of the clinicians in our participating clinics: Kansas City Free Health clinic (Sally Neville, Brooke Patterson, Craig Dietz, Edie Toubes-Klingler), Truman Medical Center (James Stanford, David Bamberger, Maithe Enriquez, Sharon Kathrens, Alan Salkind, Rose Farnan), Department of Veterans Affairs Medical Center – Kansas City (Arundhati Desai, Vinutha Kumar), Kansas University Medical Center (Broderick Crawford, Himal Bajracharya, Lisa Clough, Dan Hinthorn, Michael Luchi, Stephen Waller), and Infectious Disease Associates of Kansas City (Michael Driks, David McKinsey, Joe McKinsey). We also acknowledge the extraordinary efforts of our MOTIV8 team (Jannette Berkley-Patton, Andrea Bradley-Ewing, Tara Carruth, Delwyn Catley, Kristine Clark, Mary Gerkovich, Kathy Goggin, Kirsten Kakolewski, Domonique Malomo Thomson, Karen Williams, Julie Wright, Megan Pinkston-Camp, David Martinez, Bradley Clark, Anthony Firner, Robin Liston).
Biographies
Ian Lynam is a doctoral student in the Clinical Psychology program at the University of Missouri-Kansas City. His research interests are in motivation for health behavior change and smoking cessation.
Dr. Delwyn Catley an Associate Professor and Director of the Smoking and Motivation Laboratory in the Department of Psychology at the University of Missouri Kansas City. His research interests are in motivation for health behavior change, Motivational Interviewing and smoking cessation.
Dr. Kathy Goggin is an Associate Professor and Director of the HIV Research Group in the Department of Psychology at the University of Missouri Kansas City. She is the Principal Investigator of the Motiv8 trial and has research interests in HIV prevention and disease management.
Dr. Joshua L. Rabinowitz is an Assistant Research Scientist at the Institute for Social Research at the University of Michigan. His research interests concern political psychology, intergroup relations, political attitudes, and structural equation modeling.
Dr. Mary Gerkovich is Research Associate Professor in the Department of Psychology at the University of Missouri Kansas City. Her research interests are in decision making associated with risk and health behaviors.
Dr. Karen Williams is Professor of Dental Hygiene and Professor of Dental Public Health and Behavioral Science at the University of Missouri Kansas City. Her interests are in clinical research.
Dr. Julie Wright is a professor in the School of Medicine at the University of Missouri–Kansas City. Her research interest is HIV.
Footnotes
Modified Social Learning Theory (Wallston, 1992) suggests that the interaction between internal LOC and self-efficacy may be the best predictor of health behavior. We conducted additional regression analyses to examine this effect as well as a 3 way interaction between internal LOC, self-efficacy and AR and found no significant effects.
References
- Aardex. Medication Event Monitoring System. Union City, CA: 2006. [Google Scholar]
- Alfonso V, Geller J, Bermbach N, Drummond A, Montaner JS. Becoming a “treatment success”: what helps and what hinders patients from achieving and sustaining undetectable viral loads. AIDS Patient Care and STDs. 2006;20(5):326–334. doi: 10.1089/apc.2006.20.326. [DOI] [PubMed] [Google Scholar]
- Altice FL, Friedland GH. The era of adherence to HIV therapy. Annals of Internal Medicine. 1998;129(6):503–505. doi: 10.7326/0003-4819-129-6-199809150-00015. [DOI] [PubMed] [Google Scholar]
- Bane C, Hughes CM, McElnay JC. The impact of depressive symptoms and psychosocial factors on medication adherence in cardiovascular disease. Patient Education and Counseling. 2006;60(2):187–193. doi: 10.1016/j.pec.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Chesney MA. Factors affecting adherence to antiretroviral therapy. Clinical Infectious Disease. 2000;30(Suppl 2):S171–176. doi: 10.1086/313849. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Christensen AJ, Wiebe JS, Lawton WJ. Cynical hostility, powerful others control expectancies, and patient adherence in hemodialysis. Psychosomatic Medicine. 1997;59(3):307–312. doi: 10.1097/00006842-199705000-00013. [DOI] [PubMed] [Google Scholar]
- Christiansen A, Wiebe J, Benotsch E, Lawton W. Perceived health competence, health locus of control, and patient adherence in renal dialysis. Cognitive Therapy and Research. 1996;20(4):411–421. [Google Scholar]
- Clavel F, Hance AJ. HIV drug resistance. New England Journal of Medicine. 2004;350(10):1023–1035. doi: 10.1056/NEJMra025195. [DOI] [PubMed] [Google Scholar]
- Deci EL, Ryan RM. Human Behavior. New York: Plenum; 1985. Intrinsic motivation and self-determination. [Google Scholar]
- Deeks SG. Treatment of antiretroviral-drug-resistant HIV-1 infection. Lancet. 2003;362(9400):2002–2011. doi: 10.1016/S0140-6736(03)15022-2. [DOI] [PubMed] [Google Scholar]
- Hong TB, Oddone EZ, Dudley TK, Bosworth HB. Medication barriers and anti-hypertensive medication adherence: The moderating role of locus of control. Psychology, Health & Medicine. 2006;11(1):20–28. doi: 10.1080/14786430500228580. [DOI] [PubMed] [Google Scholar]
- Johnson MO, Catz SL, Remien RH, Rotheram-Borus MJ, Morin SF, Charlebois E, et al. Theory-guided, empirically supported avenues for intervention on HIV medication nonadherence: findings from the Healthy Living Project. AIDS Patient Care and STDS. 2003;17(12):645–656. doi: 10.1089/108729103771928708. [DOI] [PubMed] [Google Scholar]
- Kennedy S, Goggin K, Nollen N. Adherence to HIV medications: Utility of the theory of self-determination. Cognitive Therapy and Research. 2004;28(5):611–628. [Google Scholar]
- McDonald-Miszczak L, Maki SA, Gould ON. Self-reported medication adherence and health status in late adulthood: the role of beliefs. Experimental Aging Research. 2000;26(3):189–207. doi: 10.1080/036107300404859. [DOI] [PubMed] [Google Scholar]
- Molassiotis A, Nahas-Lopez V, Chung WY, Lam SW, Li CK, Lau TF. Factors associated with adherence to antiretroviral medication in HIV-infected patients. International Journal of STD and AIDS. 2002;13(5):301–310. doi: 10.1258/0956462021925117. [DOI] [PubMed] [Google Scholar]
- Myers L, Myers F. The relationship between control beliefs and self-reported adherence in adults with cystic fibrosis. Psychology, Health & Medicine. 1999;4(4):387–391. [Google Scholar]
- O’Hea EL, Grothe KB, Bodenlos JS, Boudreaux ED, White MA, Brantley PJ. Predicting Medical Regimen Adherence: The Interactions of Health Locus of Control Beliefs. Journal of Health Psychology. 2005;10(5):705–717. doi: 10.1177/1359105305055330. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. New England Journal of Medicine. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Ricker JH, Delamater AM, Hsu J. Correlates of regimen adherence in cystic fibrosis. Journal of Clinical Psychology in Medical Settings. 1998;5(2):159–172. [Google Scholar]
- Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychological Monographs. 1966;80(1):1–28. [PubMed] [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist. 2000;55(1):68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- Senecal C, Nouwen A, White D. Motivation and dietary self-care in adults with diabetes: are self-efficacy and autonomous self-regulation complementary or competing constructs? Health Psychology. 2000;19(5):452–457. doi: 10.1037//0278-6133.19.5.452. [DOI] [PubMed] [Google Scholar]
- Sensky T, Leger C, Gilmour S. Psychosocial and cognitive factors associated with adherence to dietary and fluid restriction regimens by people on chronic haemodialysis. Psychotherapy and Psychosomatics. 1996;65(1):36–42. doi: 10.1159/000289029. [DOI] [PubMed] [Google Scholar]
- Singh N, Squier C, Sivek C, Wagener M, Nguyen MH, Yu VL. Determinants of compliance with antiretroviral therapy in patients with human immunodeficiency virus: prospective assessment with implications for enhancing compliance. AIDS Care. 1996;8(3):261–269. doi: 10.1080/09540129650125696. [DOI] [PubMed] [Google Scholar]
- Stanton AL. Determinants of adherence to medical regimens by hypertensive patients. Journal of Behavioral Medicine. 1987;10(4):377–394. doi: 10.1007/BF00846477. [DOI] [PubMed] [Google Scholar]
- Ullman J. Structural Equation Modeling. In: Tabachnick B, Fidell L, editors. Using Multivariate Statistics. 4. Boston: Allyn and Bacon; 2001. pp. 653–771. [Google Scholar]
- Voils CI, Steffens DC, Flint EP, Bosworth HB. Social Support and Locus of Control as Predictors of Adherence to Antidepressant Medication in an Elderly Population. American Journal of Geriatric Psychiatry. 2005;13(2):157–165. doi: 10.1176/appi.ajgp.13.2.157. [DOI] [PubMed] [Google Scholar]
- Wallston KA. Hocus-Pocus, the focus isn’t strictly on locus: Rotter’s social learning theory modified for health. Cognitive Therapy and Research. 1992;16:183–189. [Google Scholar]
- Wallston KA. Control Beliefs. In: Smelser N, Baltes P, editors. International Encyclopedia of the Social and Behavioral Sciences. Oxford, England: Elsevier Science; 2001. [Google Scholar]
- Wallston KA, Stein MJ, Smith CA. Form C of the MHLC scales: a condition-specific measure of locus of control. Journal of Personality and Assessment. 1994;63(3):534–553. doi: 10.1207/s15327752jpa6303_10. [DOI] [PubMed] [Google Scholar]
- Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health Education Monographs. 1978;6(2):160–170. doi: 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. Journal of Personality and Social Psychology. 1996;70(1):115–126. doi: 10.1037//0022-3514.70.1.115. [DOI] [PubMed] [Google Scholar]
- Williams GC, McGregor HA, King D, Nelson CC, Glasgow RE. Variation in perceived competence, glycemic control, and patient satisfaction: relationship to autonomy support from physicians. Patient Education and Counseling. 2005;57(1):39–45. doi: 10.1016/j.pec.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Williams GC, McGregor HA, Zeldman A, Freedman ZR, Deci EL. Testing a self-determination theory process model for promoting glycemic control through diabetes self-management. Health Psychology. 2004;23(1):58–66. doi: 10.1037/0278-6133.23.1.58. [DOI] [PubMed] [Google Scholar]
- Williams GC, Minicucci DS, Kouides RW, Levesque CS, Chirkov VI, Ryan RM, et al. Self-determination, smoking, diet and health. Health Education Research. 2002;17(5):512–521. doi: 10.1093/her/17.5.512. [DOI] [PubMed] [Google Scholar]
- Williams GC, Rodin GC, Ryan RM, Grolnick WS, Deci EL. Autonomous regulation and long-term medication adherence in adult outpatients. Health Psychology. 1998;17(3):269–276. doi: 10.1037//0278-6133.17.3.269. [DOI] [PubMed] [Google Scholar]
- Wood E, Hogg RS, Yip B, Harrigan PR, O’Shaughnessy MV, Montaner JS. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 × 10(9) cells/L. Annals of Internal Medicine. 2003;139(10):810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]