Abstract
Proteinase-activated receptor2 (PAR2) is a GPCR that is activated by trypsin-like proteinases. PAR2 is detected in breast tumor specimens; however, it is not clear how PAR2 level in breast cancer cell/tissues compares to normal cell/tissues. Here, we show the elevation of PAR2 protein level in 76 out of 105 breast tumor specimens but only 5 out of 24 normal breast tissues. PAR2 level is also greater in breast cancer cell lines than that in normal breast cells and non-cancerous breast cell lines. To determine the role of PAR2 in breast carcinogenesis, we examined the effect of PAR2 agonists on cell proliferation and migration. Our studies show that PAR2 agonists (PAR2-activating peptide and trypsin) are neither potent growth enhancers nor chemoattractants to breast cancer cells. Instead, PAR2 agonists induce significant chemokinesis. PAR2-mediated chemokinesis is Gαi-dependent, and inhibiting Src kinase activity or silencing c-Src expression blocks PAR2-mediated chemokinesis. These results suggest that c-Src works downstream of Gαi to mediate this PAR2 agonist-induced event. To characterize c-Src effector, we reveal that PAR2 agonists activate JNKs in a Src-dependent manner and that JNK activity is essential for PAR2-mediated chemokinesis. Moreover, PAR2 agonist stimulation leads to paxillin Ser178 phosphorylation and paxillin(S178A) mutant inhibits PAR2-mediated chemokinesis. In conclusion, our studies demonstrate that PAR2 agonists facilitate breast cancer cell chemokinesis through the Gαi-c-Src-JNK-paxillin signaling pathway.
Keywords: PAR2, Src, JNK, paxillin, breast cancer, chemokinesis
INTRODUCTION
Proteinase-activated receptor 2 (PAR2) is the second member of the unique subfamily of four members within the superfamily of G protein-coupled receptor (GPCR) (Cottrell et al., 2003). Rather than simple ligand-receptor interaction that is responsible for receptor activation in most GPCRs, PAR2 is activated by the newly exposed amino-terminus that serves as a tethered ligand domain (Trejo, 2003). The tethered ligand domain is generated through proteolytical cleavage of the extracellular amino-terminus of PAR2 by PAR2 agonists including trypsin, tryptase, tissue factor-factor VIIa complex and factor Xa (Trejo, 2003). Synthetic receptor-activating peptide, whose sequence is identical to that of the tethered ligand domain, can mimic the action of natural PAR2 agonists (Cottrell et al., 2003; Trejo, 2003). PAR2 activation triggers robust cellular responses including cell proliferation and cytokine/protease expression (Cottrell et al., 2003; Ramachandran and Hollenberg, 2008). PAR2-mediated events are coupled to Gi and Gq-associated signaling pathways (Ramachandran and Hollenberg, 2008).
Accumulating evidences generated from recent in vitro and in vivo experimental studies strongly implicate an active role of PAR2 in tumor progression and development. For example, PAR2 is expressed in more pronounced level in prostate, gastric cancers and melanoma (Black et al., 2007; Caruso et al., 2006; Massi et al., 2005). PAR2 activation promotes cell proliferation in various cancer cell types including colon, gastric, cervical and pancreatic cancer cells (Caruso et al., 2006; Darmoul et al., 2004; Nishibori et al., 2005; Sanchez-Hernandez et al., 2008; Yada et al., 2005). PAR2 agonists induce Cox-2 expression in lung cancer cells (Wang et al., 2008), MMP 2/9 production in prostate cancer cells (Wilson et al., 2004) and VEGF secretion in breast cancer cells (Liu and Mueller, 2006). Genetic studies have linked PAR2 to VHL-associated renal cell carcinoma progression (Abdulrahman et al., 2007). Moreover, studies performed in in vivo experimental model show that PAR2 significantly contributes to hypoxia-induced angiogenesis (Uusitalo-Jarvinen et al., 2007) and melanoma metastasis (Shi et al., 2004). As monoclonal antibodies that functionally block PAR2 can effectively suppress tumor growth in xenograft models (Versteeg et al., 2008), PAR2 may represent an attractive therapeutic target for circumvention of breast malignancies.
A recent study reported the presence of PAR2 in several established breast cancer cell lines and infiltrative ductal breast tumors (Matej et al., 2007). PAR2 activation by tissue-factor-factor VIIa and Xa complex was shown to increase breast cancer cell migration (Hjortoe et al., 2004; Morris et al., 2006) and trypsin-like proteases secreted by breast cancer cells per se shown to stimulate cell migration through PAR2 in an autocrine manner (Ge et al., 2004). However, it is not clear how PAR2 levels in breast cancer cells and breast tumor specimens are compared to non-cancerous breast cells and normal breast tissues. Moreover, the intrinsic signaling mechanism associated with PAR2 agonist-induced breast cancer cell migration is also not well defined so far.
Paxillin is a focal adhesion molecule known to participate in cell adhesion and cell migration (Schaller, 2001). Throughout its protein sequence, paxillin contains a number of serine/threonine and tyrosine phosphorylation sites (Brown and Turner, 2004). Phosphorylation of these sites in response to extracellular stimuli can recruit signaling molecules to the focal adhesion and thus facilitate cell migration. For example, focal adhesion kinase (FAK) phosphorylates paxillin at Tyr31 and Tyr118 that generate binding sites for SH2-containing proteins such as CrkII (Schaller and Parsons, 1995). Also, JNK phosphorylates paxillin on Ser178 (Huang et al., 2003) that is required for FAK-paxillin interaction and FAK-mediated paxillin phosphorylation (Huang et al., 2008). JNK-mediated paxillin Ser178 phosphorylation has been found to be essential for cell migration of various cell types including rat bladder tumor cells (Huang et al., 2003), human hepatocellular carcinoma (Ching et al., 2007) and corneal epithelial cells (Kimura et al., 2008).
In this study, we analyzed PAR2 levels in both primary breast tumors and normal breast tissues. PAR2 levels are elevated in breast tumor specimens. Similarly, PAR2 expression is also high in almost all breast cancer cell lines we tested but is not detected in primary mammary epithelial cells and very low in immortalized, non-cancerous breast cell lines. In further studies, we show that PAR2 agonists (PAR2-activating peptide and trypsin) only promote significant cell migration when the agonists are in the direct contact with cells, indicating that they are chemokinesis stimulator rather than chemoattractants. In an effort to delineate the signaling mechanism associated with PAR2 agonist-induced breast cancer cell chemokinesis, we showed that the signaling pathway consisting of Gαi-c-Src-JNK is required for PAR2 agonist action. Moreover, we presented evidence that this pathway may impact PAR2 agonist-induced chemokinesis by facilitating paxillin Ser178 phosphorylation.
RESULTS
PAR2 levels are elevated in breast cancer cell lines and breast tumor specimens
Although a recent study reported the detection of PAR2 expression in both established breast cancer cell lines and infiltrating ductal breast cancer surgical specimens (Matej et al., 2007), it is not clear how PAR2 levels in breast tumors are compared to non-tumorigenic breast cells and normal breast tissues. To answer this question, we examined PAR2 expression in human primary mammary epithelial cells (HMECs), immortalized non-cancerous breast cell lines (ME16C, 184A1 and MCF-10A) and established breast cancer cell lines (BT549, MCF-7, MDA-MB-231, MDA-MB-435S, MDA-MB-436, SK-BR3, T47D and ZR-75-1) by immunoblotting with anti-PAR2 mAb. PAR2 expression was not detectable in HMECs and low in non-cancerous breast cell lines (Fig.1A). In contrast, all breast cancer cell lines, with the exception of T47D line, exhibited much greater PAR2 expression (Fig.1A). To determine PAR2 levels in normal breast and breast tumors, we carried out PAR2 immunohistochemistry on tissue specimens from 105 breast tumors (90 invasive ductal carcinomas, 5 medullary carcinomas, 5 infiltrating lobular carcinomas and 5 mucinous adenocarcinomas) and 24 normal breast tissues (isolated from area adjacent to breast tumors) (Table 1). Considering specimens with over 25% of cells positive for PAR2 staining as PAR2 overexpression (staining grade ++ and +++), only 5 out of 24 normal breast tissues showed PAR2 overexpression (5/24, 20.8%) while PAR2 overexpression was seen in 68 out of 90 ductal carcinomas (75.6%, P<0.001 vs normal), 5 out of 5 medullary carcinomas (100%) and 3 out of 5 mucinous adenocarcinomas (60%) (Fig.1B, Fig.S1 and Table 1). Interestingly, none of the infiltrating lobular carcinomas displayed PAR2 overexpression (Fig.1B, Sig.S1 and Table 1). These results indicate that PAR2 levels are elevated in breast tumors in comparison with the normal breast tissue (tumor vs normal:72.4% vs 20.8%; P<0.001).
Fig.1. PAR2 levels are elevated in breast cancer cells and breast tumor tissues.
A. Overnight-cultured cells were lysed and cell lysates subjected to immunoblotting to detect PAR2 and β-actin with the respective antibodies. B. Paraffin-embedded tissue sections were examined by immunohistochemistry with anti-PAR2 mAb. PAR2 immunoreactivity is shown as brown-stained areas and areas that were not reactive are indicated by hemotoxylin counterstaining. a, normal breast tissue (staining grade 0); b, invasive ductal carcinoma (staining grade ++); c, invasive ductal carcinoma (staining grade +++); d, medullary carcinoma (staining grade +++); e, infiltrating lobular carcinoma (staining grade 0); and f, mucinous adenocarcinoma (staining grade ++).
Table 1.
PAR2 Levels in Normal Breast Tissue and Various Types of Breast Tumor Specimens
| Staining grade | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Overexpression rate % | Pa, | |
| Breast cancer (n=105) | 15 | 14 | 26 | 50 | 72.4 (76/105) | 0.000 |
| Invasive ductal carcinoma (n=90) | 10 | 12 | 24 | 44 | 75.6 (68/90) | 0.000 |
| Medullary carcinoma (n=5) | 0 | 0 | 0 | 5 | 100.0 (5/5) | |
| Infiltrating lobular carcinoma (n=5) | 4 | 1 | 0 | 0 | 0 (0/5) | |
| Mucinous adenocarcinoma (n=5) | 1 | 1 | 2 | 1 | 60.0 (3/5) | |
| Normal breast tissues (n=24) | 9 | 6 | 4 | 1 | 20.8 (5/24) | |
Total breast tumor samples and ductal carcinomas vs. normal breast tissues. No statistical analyses were done with other three type of breast cancer due to small sample sizes.
PAR2 agonists promote breast cancer cell chemokinesis
PAR2 activation leads to cell proliferation and migration in various cancer types (Abdulrahman et al., 2007; Caruso et al., 2006; Darmoul et al., 2004; Nishibori et al., 2005; Sanchez-Hernandez et al., 2008; Shi et al., 2004; Yada et al., 2005). As PAR2 expression is elevated in breast cancer cells, we reasoned that PAR2 activation might also promote breast cancer cell proliferation and migration. To test this possibility, we first examined the effect of PAR2 agonists, trypsin and PAR2-activating peptide SLIGRL (PAR2-AP), on cell growth of MDA-MB-231, MDA-MB-436, T47D and ZR-75-1 lines. Cells were cultured in the presence of trypsin (0.1, 0.3 and 1nM) or PAR2-AP (10, 50 and 100μM) for 3 days, and MTT assay was performed to measure cell numbers. No enhancement in cell growth was observed with T47D cells and statistically insignificant 10-20% increase detected in MDA-MB-231, MDA-MB-436 and ZR-75-1 at 1nM trypsin and 100μM PAR2-AP (data not shown). These results suggest that PAR2 agonists are not potent growth stimulators to breast cancer cells.
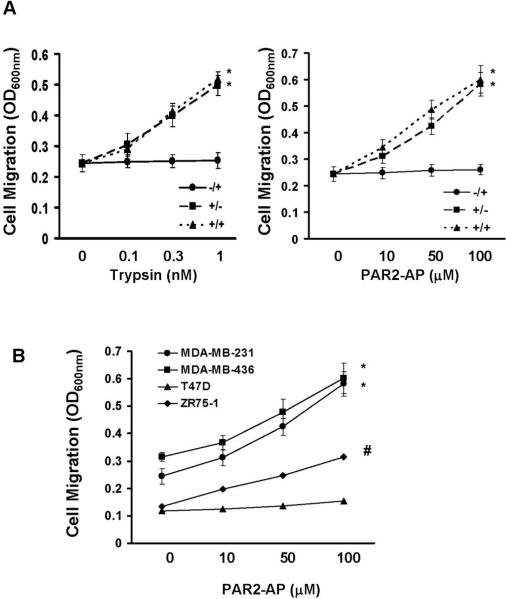
We next examined the effect of trypsin and PAR2-AP on MDA-MB-231 cell migration using Transwells. Various concentrations of trypsin (0.1, 0.3 and 1nM) or PAR2-AP (10, 50 and 100μM) were either added into underwells or upper wells or both, and cells were then allowed to migrate for 6 hrs. No significant stimulation in cell migration was observed when PAR2 agonists were present in underwells (Fig.2A). In contrast, more than 2-fold increase in cell migration was detected when 1nM trypsin or 100μM PAR2-AP was present in upper wells or in both under and upper wells (Fig.2A). In further experiments, we analyzed cell migration with MDA-MB-436, T47D and ZR-75-1 cells by adding PAR2-AP in both upper and underwells. PAR2-AP induced significant cell migration in MDA-MB-436 and ZR-75-1 cells while it did not significantly alter T47D cell migration (Fig.2B). The inability of PAR2-AP to induce T47D cell migration may be explained by the low PAR2 expression in this line (Fig.1A). These results suggest that PAR2 agonists can potently induce breast cancer cell migration in a chemokinesis, but not chemotaxis mechanism.
Fig.2. PAR2 agonists induce breast cancer cell migration in a chemokinesis mechanism.
A. Various concentrations of trypsin (0.1, 0.3 and 1 nM) or PAR2-AP (10, 50 and 100μM) were added in the medium in either upper wells or underwells or both. Overnight-starved MDA-MB-231 cells were detached with PBS containing 10 mM EDTA, then added to transwells and allowed to migrate for 4 hrs. Data are the mean ± SE. n = 3, *, P < 0.005 vs basal. -/+ represents agonist in underwell; +/- represents agonist in upper well; and +/+ represents agonist in both upper and underwells. B. Various concentrations of PAR2-AP (10, 50 and 100μM) were added in the medium in both upper and underwells. Overnight-starved MDA-MB-231, MDA-MB-436, T47D and ZR-75-1 cells were added to transwells and allowed to migrate for 4 hrs. Data are the mean ± SE. n = 3, *; P < 0.005 vs basal; #, P < 0.01 vs basal.
To confirm the role of PAR2 in trypsin and PAR2-AP-induced cell migration, we employed lentiviral vector to deliver two PAR2-specific shRNAs to knock down PAR2 expression in MDA-MB-231 cells (Fig.S1A). Both PAR2 shRNAs effectively inhibited PAR2-AP-induced chemokinesis (Fig.S1B) while control luciferase shRNA displayed no effect on this PAR2-AP-induced event (Fig.S1B). In a parallel experiment, we also examined the effect of various concentration of PAR2-AP on the migration of both control and PAR2 knockdown cells. A dose-dependent cell migration was observed with the control cells but not in PAR2 knockdown cells (data not shown). These results thus confirm the role of PAR2 in PAR2 agonist-induced breast cancer cell migration.
Gαi-associated signaling pathway mediates PAR2 agonist-induced chemokinesis
PAR2-associated events can be potentially mediated by Gi and Gq-coupled signaling pathways (Arora et al., 2007; Ramachandran and Hollenberg, 2008). G12/13-coupled signaling has also been reported to mediate cellular events associated with PAR1 (Ramachandran and Hollenberg, 2008), another member of PAR family. To take advantage of the availability of Gi inhibitor purtussis toxin and the knowledge that G12/13 and Gq can be specifically inhibited by the G protein signaling (RGS) regions of p115RhoGEF and GRK2 respectively (Kozasa and Ye, 2004), we either treated MDA-MB-231 and MDA-MB-436 cells with 1μg/ml pertussis toxin for 1 day to block Gi or expressed p115RhoGEF's RGS or GRK2's RGS for 2 days to block G12/13 or Gq respectively. Cells were added into transwells in the presence of 100μM PAR2-AP, and allowed to migrate for 4 hrs. Purtussis toxin completely abolished PAR2-AP-induced cell migration (Fig.3A); in contrast, the expression of p115RhoGEF-RGS or GRK2-RGS did not significantly alter cell migration (Fig.3A) though they were capable of blocking lysophosphotidic acid-induced SRE promoter and PLCβ activation respectively in our previous study (Bian et al., 2006). These results suggest that the Gi-coupled signaling pathway mediates PAR2 agonist-induced breast cancer cell chemokinesis.
Fig.3. Gαi-associated signaling pathway mediates PAR2-AP-induced chemokinesis.
A. MDA-MB-231 or MDA-MB-436 cells were either treated with 1μg/ml purtussis toxin (PTX) for 24 hrs or transfected with expression vector encoding p115RhoGEF-RGS or GRK2-RGS. Cells were detached with PBS containing 10mM EDTA, then added to transwells and allowed to migrate for 4 hrs in a chemokinesis condition (100μM PAR2-AP in both upper and under wells). Data are the mean ± SE. n = 3, *, P < 0.005 vs basal. B. MDA-MB-231 or MDA-MB-436 cells were transfected with expression vector encoding Gαi2 minigene or βARKct. Cells were detached, then added to transwells and allowed to migrate for 4 hrs in a chemokinesis condition. Data are the mean ± SE. n = 3, *, P < 0.005 vs basal.
As Gi-mediated events can be mediated through both Gαi and Gβγi subunits, we introduced Gα2i minigene (an 11-amino acid Gα2i carboxyl terminal) or the carboxyl-terminus of the β-adrenergic receptor kinase (β-ARKct) into MDA-MB-231 or MDA-MB-436 cells to respectively inhibit Gαi and Gβγi. The expression of Gαi2 minigene inhibited over 80% of PAR2-AP-induced cell migration (Fig.3B); in contrast, β-ARKct expression displayed little effect on cell migration (Fig.3B). To confirm the ability of Gαi2 to facilitate cell migration, we expressed constitutively active Gαi2 (Q205L) in MDA-MB-231 and T47D cells and observed that cells expressing Gαi2 Q205L displayed over 2-fold increase in cell migration over the empty vector-transfected cells (Fig.S2). These results suggest that Gαi-, but not Gβγi-coupled signaling pathway mediates PAR2 agonist-induced chemokinesis.
c-Src is involved in PAR2 agonist-induced chemokinesis
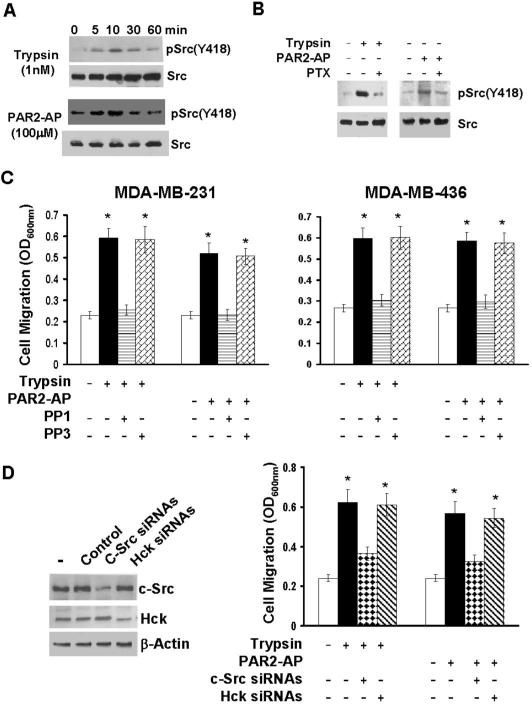
Recent studies have shown that Gαi directly regulates the activities of the Src family kinases c-Src and Hck (Ciccarelli et al., 2007; Ma et al., 2000). As Src family kinases are well identified as the key molecules in cell migration process (Parsons and Parsons, 2004), we hypothesized that PAR2 agonists induced breast cancer cell migration through Gαi regulation of Src family kinase activity. To test this hypothesis, we first determined whether PAR2 agonists could activate Src family kinases. MDA-MB-231 cells were treated with 1nM trypsin or 100μM PAR2-AP for various times, then lysed and cell lysates subjected to immunoblotting to detect active Src with anti-phosphor-Src(Tyr418) polyclonal antibody. Both trypsin and PAR2-AP induced Src Tyr418phosphorylation and peak phosphorylation observed between 5 and 10 min for both agonists (Fig.4A). However, 1-day pretreatment of purtussis toxin abolished PAR2 agonist-induced Src Tyr418 phosphorylation (Fig.4B). These results show that 1) PAR2 agonists can activate Src and 2) PAR2 agonist-induced Src activation is Gi-coupled.
Fig.4. c-Src is involved in PAR2 agonist-induced chemokinesis.
A. Overnight-starved MDA-MB-231 cells were stimulated with either 1 nM trypsin or 100μM PAR2-AP for various times (5, 10, 30 and 60 min), then lysed and cell lysates subjected to immunoblotting to detect phosphor-Src(Y418) and c-Src with the respective antibodies. B. MDA-MB-231 cells were treated with 1μg/ml purtussis toxin (PTX) for 24 hrs and then stimulated with 1 nM trypsin or 100μM PAR2-AP for 10 min. Cells were lysed and cell lysates subjected to immunoblotting to detect phosphor-Src(Y418) and c-Src. C. MDA-MB-231 or MDA-MB-436 cells were treated with 10μM PP1 or PP3 for 2 hrs. Cells were detached with PBS containing 10mM EDTA, then added to transwells and allowed to migrate for 4 hrs in a chemokinesis condition (1nM trypsin or 100μM PAR2-AP in both upper and underwells). Data are the mean ± SE. n = 3, *, P < 0.005 vs basal. D. MDA-MB-231 cells were treated with 100 nM control, c-Src or Hck siRNA pool for 3 days. Data are the mean ± SE. n = 3, *, P < 0.005 vs basal. Cells were detached with PBS containing 10mM EDTA, then washed with serum-free medium and resuspended in serum-free medium. Half of the cell suspension was spun and the pellets lysed for immunoblotting to detect c-Src, Hck or β-actin with the respective antibodies. The other half of the cell suspension was analyzed for both trypsin and PAR2-AP-induced chemokinesis.
To determine the importance of Src activity in PAR2 agonist-induced chemokinesis, we treated MDA-MB-231 and MDA-MB-436 cells with PP1 (a Src kinase chemical inhibitor) or PP3, a non-functional structural analog of PP1 for 2 hrs and then assayed PAR2 agonist-induced cell migration. PP1 but not PP3 diminished trypsin or PAR2-AP-induced chemokinesis (Fig.4C). To further confirm the role of Src in PAR2-mediated cell migration, we overexpressed wild-type Csk (kinase that inactivates Src) or kinase-dead Csk in MDA-MB-231 and MDA-MB-436 cells with the aid of adenovirus. Forced expression of wild-type Csk, but not kinase-dead Csk, inhibited trypsin or PAR2-AP-induced cell migration (data not shown). These results clearly show that the activity of Src family kinase is essential for PAR2 agonist-induced chemokinesis.
We next attempted to define the importance of c-Src and Hck in PAR2 agonist-induced chemokinesis by individually silencing c-Src or Hck with specific siRNAs. Though c-Src and Hck siRNAs (but not control siRNA) specifically reduced their respective target expression, only c-Src siRNAs significantly inhibited trypsin or PAR2-AP-induced MDA-MB-231 chemokinesis (Fig.4D). These results place c-Src as the Gαi downstream effector to mediate PAR2 agonist-induced chemokinesis.
JNKs work downstream of c-Src to mediate PAR2 agonist-induced chemokinesis
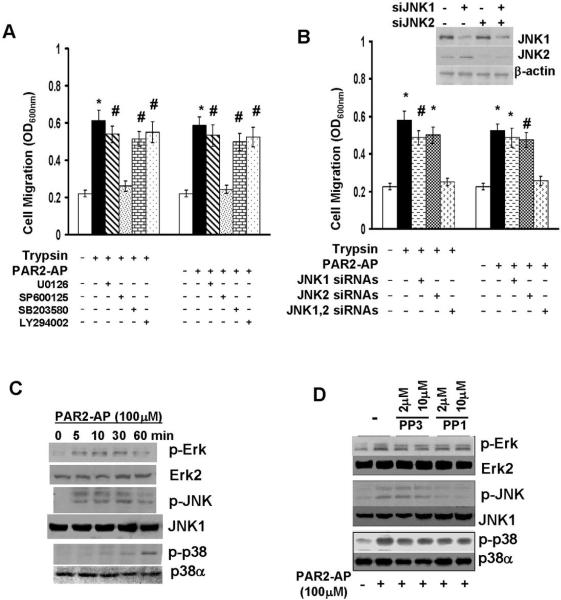
Recent studies show that c-Src may facilitate cell migration through various signaling molecules including members of MAPK families and PI3K (Parsons and Parsons, 2004). To define the signaling pathway-associated with c-Src action, we treated MDA-MB-231 cells with 2μM U0126 (MEK1/2 inhibitor), 10μM SP600125 (JNK inhibitor), 10μM SB203580 (p38 MAPK inhibitor) or 50μM LY294002 (PI3K inhibitor) for 2 hrs, and then analyzed trypsin or PAR2-AP-induced chemokinesis. No significant inhibitory effect in PAR2 agonist-induced cell migration was observed with cells treated with U0126, SB203580 or LY294002 (Fig.5A). However, prior SP600125 treatment resulted in almost complete inhibition in trypsin or PAR2-AP-induced chemokinesis (Fig.5A). To confirm the role of JNKs in PAR2 agonist-induced cell migration, MDA-MB-231 cells were transfected with JNK1 or JNK2 siRNAs either individually or together for 3 days. Although JNK1 or JNK2 siRNA was capable of effectively silencing their respective targets (Fig.5B), single JNK1 and JNK2 siRNA treatment led to slight to moderate reduction in PAR2-AP-induced chemokinesis (Fig.5B). However, treating cells with JNK1 and JNK2 siRNAs together resulted in over 90% reduction in cell migration (Fig.5B). Similar results were also obtained with MDA-MB-436 cells (data not shown). These results suggest that 1) JNK activity is required for PAR2 agonist-induced chemokinesis; and 2) both JNK1 and JNK2 contribute to this PAR2 agonist-induced event.
Fig.5. JNKs are involved in PAR2 agonist-induced chemokinesis.
A. MDA-MB-231 cells were treated with 2μM U0126, 10μM SP600125, 10μM SB203580 or 50μM LY294002 for 2 hrs. Cells were detached with PBS containing 10mM EDTA, then added to transwells and allowed to migrate for 4 hrs in a chemokinesis condition (1nM trypsin or 100μM PAR2-AP in both upper and underwells). Data are the mean ± SE. n = 3, *, P < 0.005 vs basal; #, P < 0.01 vs basal. B. MDA-MB-231 cells were treated with 100 nM JNK1, JNK2 siRNA pool or together for 3 days and then detached with PBS containing 10μM EDTA. Half of the cells were lysed and cell lysates subjected to immunoblotting to detect JNK1, JNK2 and β-actin with the respective antibodies. Other half of the cells were analyzed for both trypsin and PAR2-AP-induced chemokinesis. Data are the mean ± SE. n = 3, *, P < 0.005 vs basal; #, P < 0.01 vs basal. C. Overnight-starved MDA-MB-231 cells were stimulated with 100μM PAR2-AP for various times (5, 10, 30 and 60 min), then lysed and cell lysates subjected to immunoblotting to detect phosphor-Erk, Erk2, phosphor-JNK, JNK1 phosphor-p38 and p38α with the respective antibodies. D. MDA-MB-231 cells were treated with PP1 or PP3 (2 and 10μM) for 2 hrs and then stimulated with 100μM PAR2-AP for 20 or 60 min. Cell lysates harvested from 20-min stimulation group were subjected to immunoblotting to detect phosphor-Erk, Erk2, phosphor-JNK and JNK1. Cell lysates harvested from 60-min stimulation group were analyzed for level of phosphor-p38 and p38α. In C and D, The levels of Erk1 and JNK2 were also measured with the respective polyclonal antibodies. We detected no difference in their protein levels upon the treatment.
To investigate whether c-Src and JNKs were functionally linked in the process of PAR2 agonist-induced chemokinesis, we first examined the effect of PAR2-AP on the activation of MAPKs including Erk, JNK and p38 MAPK. MDA-MB-231 cells were stimulated with 100μM PAR2-AP for varying times, then lysed and cell lysates subjected to immunoblotting to detect Erk, JNK and p38 phosphorylation with the respective phosphor-specific antibodies. PAR2-AP activated Erk and JNK in a similar pattern with the detectable induction of phosphorylation at 5 min and peak phosphorylation between 10 and 30 min (Fig.5C). However, the induction of p38 MAPK phosphorylation occurred at much later times (> 30min) (Fig.5C). To determine the importance of c-Src activity in PAR2-AP-induced MAPK activation, we treated MDA-MB-231 cells with PP1 or PP3 for 2 hrs followed by the stimulation of PAR2-AP for 10 (for analyzing Erk and JNK) or 60 min (for analyzing p38). Both PP1 and PP3 did not alter the degree of PAR2-AP-induced Erk or p38 MAPK phosphorylation (Fig.5D). In contrast, PP1 but not PP3 diminished PAR2-AP-induced JNK phosphorylation (Fig.5D). These results suggest that 1) the distinct signaling mechanisms are responsible for PAR2-AP-induced Erk, JNK and p38 MAPK activation; 2) Src activity is specifically required for JNK activation; and 3) JNKs may work downstream of c-Src to facilitate breast cancer cell chemokinesis.
Recent studies show that c-Src is capable of activating small GTPases Ras (Xie and Herschman, 1995), Rac (Yamauchi et al., 2002), Cdc42 (Miyamoto et al., 2003; Yamauchi et al., 2002) and RhoA (Nagao et al., 1999), and all of them can activate JNKs by directly interacting with JNK upstream kinase MEKK1 (Fanger et al., 1997; Gallagher et al., 2004; Russell et al., 1995). To determine the potential involvement of those small GTPases in PAR2 agonist-induced JNK activation, we expressed Myc-tagged dominant negative forms of H-Ras (N17), Rac1 (N17), Cdc42 (N17) or RhoA (N19) in MDA-MB-231 cells. Cells were stimulated with 100μM PAR2-AP for 10 min and cell lysates analyzed for JNK phosphorylation. Immunoblotting with Myc tag mAb showed that all dominant negative molecules expressed at similar levels (Fig.S3), but only dominant negative Rac1 significantly blocked PAR2-AP-induced JNK phosphorylation (Fig.S3). These results suggest that a signaling pathway consisting of Gαi-c-Src-Rac1-JNK1/2 mediates PAR2 agonist-induced chemokinesis.
JNK-mediated Paxillin serine phosphorylation is essential for PAR2 agonist-induced chemokinesis
Recent studies demonstrate that direct phosphorylation of paxillin at Ser178 by JNK play an important role in the regulation of cell migration (Ching et al., 2007; Huang et al., 2003). We hypothesized that JNK participated in PAR2 agonist-induced cell migration by promoting paxillin Ser178 phosphorylation. To test this hypothesis, MDA-MB-231 cells were treated with 1nM trypsin or 100μM PAR2-AP for varying times, then lysed and cell lysates analyzed for paxillin Ser178 phosphorylation. Both trypsin and PAR2-AP induced paxillin phosphorylation at 10 min and maximum phosphorylation detected between 20 and 30 min (Fig.6A). However, prior treatment of cells with PP1 or SP600125 inhibited PAR2 agonist-induced paxillin Ser178 phosphorylation (Fig.6B).
Fig.6. Paxillin (PXN) Ser178 phosphorylation is linked to PAR2 agonist-induced chemokinesis.
A. Overnight-starved MDA-MB-231 cells were stimulated with 1nM trypsin or 100μM PAR2-AP for various times (10, 20, 30 and 60 min), then lysed and cell lysates subjected to immunoblotting to detect phosphor-paxillin(S178) and paxillin with the respective antibodies. B. MDA-MB-231 cells were treated with PP1 (2 and 10μM) or SP600125 (5 and 20μM) for 2 hrs and then stimulated with 100μM PAR2-AP for 30 min. Cells were lysed and cell lysates subjected to immunoblotting to detect phosphor-paxillin(S178) and paxillin. C. MDA-MB-231 or MDA-MB-436 cells were transfected with empty vector (control) or vector encoding wild-type paxillin [PXN(wt)] or paxillin containing S178A mutation [PXN(S178A)]. Cells were detached, then added to transwells and allowed to migrate for 4 hrs in a chemokinesis condition (1nM trypsin or 100 μM PAR2-AP in both upper and underwells). Data are the mean ± SE. n = 3, *, P < 0.005 vs basal.
To determine the importance of paxillin Ser178 phosphorylation in PAR2 agonist-induced cell migration, we overexpressed wild-type paxillin or paxillin(S178A) mutant in MDA-MB-231 and MDA-MB-436 cells. Forced expression of wild-type paxillin or paxillin(S178A) overexpression did not display apparent effect on basal cell migration (Fig.6C), suggesting that the levels of paxillin alone are not sufficient to regulate cell migration. In contrast, paxillin(S178A), but not wild-type paxillin, inhibited over 70% of PAR2-AP or trypsin-induced cell migration (Fig.6C). These results suggest a signaling pathway in which PAR2 agonist activates JNKs via Gαi-c-Src, which in turn phosphorylates paxillin Ser178, thus promoting chemokinesis.
DISCUSSION
PAR2 levels are elevated in prostate, gastric cancers and melanoma (Black et al., 2007; Caruso et al., 2006; Massi et al., 2005). Recent studies also show the presence of PAR2 expression in ductal breast tumor specimens and surrounding stromas (D'Andrea et al., 2001; Matej et al., 2007). However, it has not been examined whether PAR2 expression is elevated in breast tumors. In this study, we found that PAR2 was present in 72.4% of breast tumor specimens but in only 20.8% of normal breast tissues (Fig.1B, Fig.S1 and Table 1). Similarly, PAR2 levels are significantly higher in breast cancer cell lines than normal breast and non-cancerous breast cell lines. The observation made with the established cell lines and limited size of primary tissue samples show that PAR2 levels are elevated in breast cancer cells.
Recent studies with colorectal cancer show that PAR2-trypsin system facilitates colorectal carcinogenesis by promoting cell proliferation and migration/invasion (Soreide et al., 2006). In this study, we found that PAR2 agonists (trypsin and PAR2-AP) are neither potent growth stimulators nor chemoattractants to breast cancer cells. Instead, PAR2 agonists have the pronounced effect on breast cancer cell chemokinesis (Fig.2). These findings suggest that PAR2 system most likely facilitates breast carcinogenesis by assisting cells departing from the primary sites rather than serving as a chemotactic signal for cancer cells traveling to the distant sites.
Two early studies showed that PAR2 mediated tissue factor (TF)-factor VIIa and Xa-induced breast cancer cell migration, and that both were able to stimulate cell migration as chemoattractants (Hjortoe et al., 2004; Morris et al., 2006). These reports are different from our observation that trypsin or PAR2-AP can only potently induce breast cancer cell migration as a chemokinesis stimulant rather than a chemoattractant (Fig.2). Factor VIIa and Xa are known to activate both PAR1 and PAR2 (Ruf and Mueller, 2006), and it thus is possible that PAR2 agonist can induce chemotaxis when PAR1 is also activated. This possibility is supported by a recent study that PAR2-AP induced significant chemotaxis in melanoma and prostate cancer cells only when PAR1-activating peptide was present (Shi et al., 2004). Interestingly, an early study showed that TF alone could induce smooth muscle cell chemotaxis (Sato et al., 1996). It is likely that the disparity in PAR2's ability to induce chemotaxis is simply due to different cell lineage. A recent study shows that tissue factor can interact with β1 integrin and disruption of TF-β1 integrin interaction with specific monoclonal antibody inhibits TF-PAR2 signaling (Versteeg et al., 2008). We reason that TF's ability to induce chemotaxis can also attribute to its interaction with β1 integrin. The absence of interaction between trypsin or PAR2-AP and β1 integrin may explain their inability to induce chemotaxis in breast cancer cells.
A recent study reports that factor VIIa-induced upregulation of IL8 expression facilitates PAR2-mediated breast cancer cell migration (Hjortoe et al., 2004). Another study shows that constitutive PAR2-mediate breast cancer cell migration requires both β-arrestin-1 and 2 (Ge et al., 2004). However, these studies have not revealed the signaling cascade associated with PAR2-agonits-induced cell migration. By specifically blocking the functionality or expression of the pertinent signaling molecules, we show that the signaling pathway consisting of Gαi-c-Src-JNK mediates PAR2 agonist-induced breast cancer cell chemokinasis (Fig.3, 4, 5, S3 and S4). An early study shows that JNKs facilitates cell migration by directly phosphorylating paxillin Ser178 (Huang et al., 2003). Ser178 phosphorylation of paxillin is suggested to be important for adhesion turnover that is required for cell migration (Huang et al., 2004). We found that both trypsin and PAR2-AP induced paxillin Ser178 phosphorylation and intercepting JNK signaling prevented PAR2 agonist-induced paxillin Ser178 phosphorylation (Fig.6). AS non-JNK-phosphorylable paxillin(S178A) blocked PAR2 agonist-induced cell migration (Fig.6), we rationalize that PAR2 agonist activates JNKs, which promote breast cancer cell migration through the Ser178 phosphorylation of paxillin.
In conclusion, we have shown that PAR2 level is elevated in both established breast cancer cell lines and primary breast tumor specimens. We have also identified the Gαi-c-Src-JNK-paxillin signaling pathway that is important for PAR2 agonist-induced breast cancer cell chemokinesis. As cell migration is one of the most critical components of cancer progression, we speculate that PAR2 may represent an attractive target for breast malignancies.
MATERIALS AND METHODS
Breast cancer cell lines and breast tumor tissue arrays
All breast cells and breast cancer cell lines were obtained from ATCC (Manassas, VA) and cultured in the condition recommended by ATCC. Human primary mammary epithelial cells (HMECs) were purchased from Lonza, Inc (Allendale, NJ). Breast tumor tissue arrays (Od-CT-RpBre01-02 and T07-001) were obtained from Shanghai Outdo Biotech Co. (Shanghai, China). The array contains 105 cases of primary carcinomas including 90 invasive ductal carcinomas, 5 medullary carcinomas, 5 infiltrating lobular carcinomas and 5 mucinous adenocarcinomas. In parallel, 24 normal breast tissues from the regions around cancers were also included in the array. All experiments involving human tissues were conducted in accordance with protocols approved by the Shanghai University of Traditional Chinese Medicine Research Ethics Board.
Immunohistochemistry
Immunostaining was performed using DAKO SLAB Kit (Dako Co. Carpinteria, CA) as previously described (Su et al., 2001a; Su et al., 2001b). Briefly, the sections were deparaffinized, blocked and then incubated with anti-PAR2 mAb (SAM11, Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:200 followed by incubation with the biotinylated secondary antibody. The sections were subsequently incubated with peroxidase-labeled streptavidin and visualized by DAB. The positive PAR2 staining was confirmed by competition with PAR2 peptide (Santa Cruz biotechnology) in the parallel experiment. The intensity of PAR2 immunostaining was graded based on the percentage of cells displaying PAR2 staining and average 1,000 cells counted for each sample. “-” represents negative PAR2 staining; “+” represents weak PAR2 staining (<25% positive); “++” and “+++” were considered as PAR2 overexpression (25~50% and >50% respectively).
Transwell migration assay
The migration was performed using Costar Transwell. Briefly, the undersurface of transwell was coated with 10μg/ml collagen I, 105 cells then added to the upper wells and allowed to migrate for 4 hrs. Cotton swabs were used to remove cells in the upper surface of the transwells, and migratory cells attached on the undersurface were stained with crystal violet solution. Wells were gently rinsed with water and dried in the air. Crystal violet-stained attached cells were solubilized with 100 μl of 10% acetic acid and quantitated on a microplate reader at 600 nm. As indicated, various concentrations of trypsin and PAR2-AP were added into medium in either upper wells or underwells or both to stimulate cell migration. To identify the signaling molecules potentially involved in PAR2 agonist-induced cell migration, cells were either treated for 24 hrs (purtussis toxin) or 2 hrs (all other inhibitors) prior to migration assay. Alternatively, cells were transfected with mammalian expression vectors encoding constitutively active or dominant negative forms of signaling molecules or siRNAs with AMAXA electroporation apparatus using manufacturer's protocols for each individual cell line. Migration was assayed 2 (plasmid transfection) or 3 days (for siRNA transfection) after transfection. We constantly achieve over 80% of transfection efficiencies in these cell lines as determined by GFP reporter plasmid transfection.
Cell growth
Cell growth was analyzed by MTT assay. Briefly, 3×104 cells were plated into each well on 24-well plates for overnight, and then switched to 0.5% FCS-containing medium with 0.1 to 1 nM trypsin or 10 to 100 μM PAR2-AP. After 3 days, 200μl of 40mg/ml MTT was added into each well and incubated at 37°C for 2 hrs. The formed crystals were dissolved with DMSO and cell number quantitated by a microplate reader at 560nm.
Immunoblotting
Overnight-seeded cells were starved for 24 hrs and then stimulated with 1 nM trypsin or 100 μM PAR2-AP for varying times. Cells were washed with ice-cold PBS and then lysed in RIPA buffer (PBS containing 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM Na3 VO4, and 100 μg/ml phenylmethylsulfonyl fluoride). Cell lysates (50 μg of protein) were boiled in non-reducing SDS sample buffer, electrophoresed on a 10% polyacrylamide SDS gel, and transferred onto nitrocellulose membranes (Millipore, Billerica, MA). PAR2 was detected by anti-PAR2 mAb (SAM11). Information about the other antibodies used in immunoblotting is included in Supplemental Materials.
Statistical Analysis
Statistical analysis for immunohistochemistry of PAR2 expression was performed using the SPSS 14.0 software (SPSS Inc. Chicago, IL). The χ2 and Fisher's exact tests were used to examine the correlation between PAR2 expression and other clinicopathological characteristics. Statistical analysis of cell migration was done by student t test using Microsoft Excel software.
Supplementary Material
ACKNOWLEDGEMENTS
This work is supported by MOST of China (2006BAI08B02-06) (SS), Shanghai Municipal Science, Technology Commission (06DZ19728) (SS), E-institutes of Shanghai Municipal Education Commission (E 03008) (SS), NIH grant CA093926 and HL083335 (SH).
REFERENCES
- Abdulrahman M, Maina EN, Morris MR, Zatyka M, Raval RR, Banks RE, Wiesener MS, Richards FM, Johnson CM, Latif F, Maher ER. Identification of novel VHL targets that are associated with the development of renal cell carcinoma. Oncogene. 2007;26:1661–1672. doi: 10.1038/sj.onc.1209932. [DOI] [PubMed] [Google Scholar]
- Arora P, Ricks TK, Trejo J. Protease-activated receptor signalling, endocytic sorting and dysregulation in cancer. J Cell Sci. 2007;120:921–928. doi: 10.1242/jcs.03409. [DOI] [PubMed] [Google Scholar]
- Bian D, Mahanivong C, Yu J, Frisch SM, Pan ZK, Ye RD, Huang S. The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration. Oncogene. 2006;25:2234–2244. doi: 10.1038/sj.onc.1209261. [DOI] [PubMed] [Google Scholar]
- Black PC, Mize GJ, Karlin P, Greenberg DL, Hawley SJ, True LD, Vessella RL, Takayama TK. Overexpression of protease-activated receptors-1,-2, and-4 (PAR-1, -2, and -4) in prostate cancer. Prostate. 2007;67:743–756. doi: 10.1002/pros.20503. [DOI] [PubMed] [Google Scholar]
- Brown MC, Turner CE. Paxillin: adapting to change. Physiological reviews. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- Caruso R, Pallone F, Fina D, Gioia V, Peluso I, Caprioli F, Stolfi C, Perfetti A, Spagnoli LG, Palmieri G, Macdonald TT, Monteleone G. Protease-activated receptor-2 activation in gastric cancer cells promotes epidermal growth factor receptor trans-activation and proliferation. Am J Pathol. 2006;169:268–278. doi: 10.2353/ajpath.2006.050841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching YP, Leong VY, Lee MF, Xu HT, Jin DY, Ng IO. P21-activated protein kinase is overexpressed in hepatocellular carcinoma and enhances cancer metastasis involving c-Jun NH2-terminal kinase activation and paxillin phosphorylation. Cancer Res. 2007;67:3601–3608. doi: 10.1158/0008-5472.CAN-06-3994. [DOI] [PubMed] [Google Scholar]
- Ciccarelli M, Cipolletta E, Santulli G, Campanile A, Pumiglia K, Cervero P, Pastore L, Astone D, Trimarco B, Iaccarino G. Endothelial beta2 adrenergic signaling to AKT: role of Gi and SRC. Cell Signal. 2007;19:1949–1955. doi: 10.1016/j.cellsig.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Cottrell GS, Amadesi S, Schmidlin F, Bunnett N. Protease-activated receptor 2: activation, signalling and function. Biochem Soc Trans. 2003;31:1191–1197. doi: 10.1042/bst0311191. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Derian CK, Santulli RJ, Andrade-Gordon P. Differential expression of protease-activated receptors-1 and -2 in stromal fibroblasts of normal, benign, and malignant human tissues. Am J Pathol. 2001;158:2031–2041. doi: 10.1016/S0002-9440(10)64675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmoul D, Gratio V, Devaud H, Laburthe M. Protease-activated receptor 2 in colon cancer: trypsin-induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J Biol Chem. 2004;279:20927–20934. doi: 10.1074/jbc.M401430200. [DOI] [PubMed] [Google Scholar]
- Fanger GR, Johnson NL, Johnson GL. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 1997;16:4961–4972. doi: 10.1093/emboj/16.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher ED, Gutowski S, Sternweis PC, Cobb MH. RhoA binds to the amino terminus of MEKK1 and regulates its kinase activity. J Biol Chem. 2004;279:1872–1877. doi: 10.1074/jbc.M309525200. [DOI] [PubMed] [Google Scholar]
- Ge L, Shenoy SK, Lefkowitz RJ, DeFea K. Constitutive protease-activated receptor-2-mediated migration of MDA MB-231 breast cancer cells requires both beta-arrestin-1 and -2. J Biol Chem. 2004;279:55419–55424. doi: 10.1074/jbc.M410312200. [DOI] [PubMed] [Google Scholar]
- Hjortoe GM, Petersen LC, Albrektsen T, Sorensen BB, Norby PL, Mandal SK, Pendurthi UR, Rao LV. Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood. 2004;103:3029–3037. doi: 10.1182/blood-2003-10-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. A role for JNK-paxillin signaling in cell migration. Cell Cycle. 2004;3:4–6. [PubMed] [Google Scholar]
- Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- Huang Z, Yan DP, Ge BX. JNK regulates cell migration through promotion of tyrosine phosphorylation of paxillin. Cell Signal. 2008;20:2002–12. doi: 10.1016/j.cellsig.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Kimura K, Teranishi S, Yamauchi J, Nishida T. Role of JNK-dependent serine phosphorylation of paxillin in migration of corneal epithelial cells during wound closure. Invest Ophthalmol Vis Sci. 2008;49:125–132. doi: 10.1167/iovs.07-0725. [DOI] [PubMed] [Google Scholar]
- Kozasa T, Ye RD. Selective inhibition of G protein-mediated pathways using RGS domains. Methods Mol Biol. 2004;2004;237:153–67. doi: 10.1385/1-59259-430-1:153. [DOI] [PubMed] [Google Scholar]
- Liu Y, Mueller BM. Protease-activated receptor-2 regulates vascular endothelial growth factor expression in MDA-MB-231 cells via MAPK pathways. Biochem Biophys Res Commun. 2006;344:1263–1270. doi: 10.1016/j.bbrc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Massi D, Naldini A, Ardinghi C, Carraro F, Franchi A, Paglierani M, Tarantini F, Ketabchi S, Cirino G, Hollenberg MD, Geppetti P, Santucci M. Expression of protease-activated receptors 1 and 2 in melanocytic nevi and malignant melanoma. Hum Pathol. 2005;36:676–685. doi: 10.1016/j.humpath.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Matej R, Mandakova P, Netikova I, Pouckova P, Olejar T. Proteinase-activated receptor-2 expression in breast cancer and the role of trypsin on growth and metabolism of breast cancer cell line MDA MB-231. Physiol Res. 2007;56:475–484. doi: 10.33549/physiolres.930959. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Itoh H. Src kinase regulates the activation of a novel FGD-1-related Cdc42 guanine nucleotide exchange factor in the signaling pathway from the endothelin A receptor to JNK. J Biol Chem. 2003;278:29890–29900. doi: 10.1074/jbc.M301559200. [DOI] [PubMed] [Google Scholar]
- Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res. 2006;66:307–314. doi: 10.1158/0008-5472.CAN-05-1735. [DOI] [PubMed] [Google Scholar]
- Nagao M, Kaziro Y, Itoh H. The Src family tyrosine kinase is involved in Rho-dependent activation of c-Jun N-terminal kinase by Galpha12. Oncogene. 1999;18:4425–4434. doi: 10.1038/sj.onc.1202832. [DOI] [PubMed] [Google Scholar]
- Nishibori M, Mori S, Takahashi HK. Physiology and pathophysiology of proteinase-activated receptors (PARs): PAR-2-mediated proliferation of colon cancer cell. J Pharmacol Sci. 2005;97:25–30. doi: 10.1254/jphs.fmj04005x5. [DOI] [PubMed] [Google Scholar]
- Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Hollenberg MD. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008;153(Suppl 1):S263–282. doi: 10.1038/sj.bjp.0707507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf W, Mueller BM. Thrombin generation and the pathogenesis of cancer. Sem Thromb Hemost. 2006;32(Suppl 1):61–68. doi: 10.1055/s-2006-939555. [DOI] [PubMed] [Google Scholar]
- Russell M, Lange-Carter CA, Johnson GL. Direct interaction between Ras and the kinase domain of mitogen-activated protein kinase kinase kinase (MEKK1) J Biol Chem. 1995;270:11757–11760. doi: 10.1074/jbc.270.20.11757. [DOI] [PubMed] [Google Scholar]
- Sanchez-Hernandez PE, Ramirez-Duenas MG, Albarran-Somoza B, Garcia-Iglesias T, del Toro-Arreola A, Franco-Topete R, Daneri-Navarro A. Protease-activated receptor-2 (PAR-2) in cervical cancer proliferation. Gynecol Oncol. 2008;108:19–26. doi: 10.1016/j.ygyno.2007.08.083. [DOI] [PubMed] [Google Scholar]
- Sato Y, Asada Y, Marutsuka K, Hatakeyama K, Sumiyoshi A. Tissue factor induces migration of cultured aortic smooth muscle cells. Thromb Haemost. 1996;75:389–392. [PubMed] [Google Scholar]
- Schaller MD. Paxillin: a focal adhesion-associated adaptor protein. Oncogene. 2001;20(44):6459–6472. doi: 10.1038/sj.onc.1204786. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Gangadharan B, Brass LF, Ruf W, Mueller BM. Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol Cancer Res. 2004;2:395–402. [PubMed] [Google Scholar]
- Soreide K, Janssen EA, Korner H, Baak JP. Trypsin in colorectal cancer: molecular biological mechanisms of proliferation, invasion, and metastasis. J Pathol. 2006;209:147–156. doi: 10.1002/path.1999. [DOI] [PubMed] [Google Scholar]
- Su SB, Motoo Y, Iovanna JL, Berthezene P, Xie MJ, Mouri H, Ohtsubo K, Matsubara F, Sawabu N. Overexpression of p8 is inversely correlated with apoptosis in pancreatic cancer. Clin Cancer Res. 2001a;7:1320–1324. [PubMed] [Google Scholar]
- Su SB, Motoo Y, Iovanna JL, Xie MJ, Mouri H, Ohtsubo K, Yamaguchi Y, Watanabe H, Okai T, Matsubara F, Sawabu N. Expression of p8 in human pancreatic cancer. Clin Cancer Res. 2001b;7:309–313. [PubMed] [Google Scholar]
- Trejo J. Protease-activated receptors: new concepts in regulation of G protein-coupled receptor signaling and trafficking. J Pharmacol Exp Ther. 2003;307:437–442. doi: 10.1124/jpet.103.052100. [DOI] [PubMed] [Google Scholar]
- Uusitalo-Jarvinen H, Kurokawa T, Mueller BM, Andrade-Gordon P, Friedlander M, Ruf W. Role of protease activated receptor 1 and 2 signaling in hypoxia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:1456–1462. doi: 10.1161/ATVBAHA.107.142539. [DOI] [PubMed] [Google Scholar]
- Versteeg HH, Schaffner F, Kerver M, Petersen HH, Ahamed J, Felding-Habermann B, Takada Y, Mueller BM, Ruf W. Inhibition of tissue factor signaling suppresses tumor growth. Blood. 2008;111:190–199. doi: 10.1182/blood-2007-07-101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wen S, Bunnett NW, Leduc R, Hollenberg MD, MacNaughton WK. Proteinase-activated receptor-2 induces cyclooxygenase-2 expression through beta-catenin and cyclic AMP-response element-binding protein. J Biol Chem. 2008;283:809–815. doi: 10.1074/jbc.M703021200. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gallagher S, Warpeha K, Hawthorne SJ. Amplification of MMP-2 and MMP-9 production by prostate cancer cell lines via activation of protease-activated receptors. Prostate. 2004;60:168–174. doi: 10.1002/pros.20047. [DOI] [PubMed] [Google Scholar]
- Xie W, Herschman HR. v-src induces prostaglandin synthase 2 gene expression by activation of the c-Jun N-terminal kinase and the c-Jun transcription factor. J Biol Chem. 1995;270:27622–27628. doi: 10.1074/jbc.270.46.27622. [DOI] [PubMed] [Google Scholar]
- Yada K, Shibata K, Matsumoto T, Ohta M, Yokoyama S, Kitano S. Protease-activated receptor-2 regulates cell proliferation and enhances cyclooxygenase-2 mRNA expression in human pancreatic cancer cells. J Surg Oncol. 2005;89:79–85. doi: 10.1002/jso.20197. [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Miyamoto Y, Kokubu H, Nishii H, Okamoto M, Sugawara Y, Hirasawa A, Tsujimoto G, Itoh H. Endothelin suppresses cell migration via the JNK signaling pathway in a manner dependent upon Src kinase, Rac1, and Cdc42. FEBS lett. 2002;527:284–288. doi: 10.1016/s0014-5793(02)03231-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.