Abstract
Objective
Autoimmune diseases predominantly affect women suggesting that female sex hormones may play a role in pathogenesis. We have previously shown that persistent mild-moderate elevations in serum prolactin levels induce a break in self-tolerance in mice with a BALB/c genetic background. In this study we evaluated the effects of hyperprolactinemia on mechanisms of B cell tolerance induction.
Methods
Effects of prolactin on splenic B cell subsets were studied in female Balb/c mice. BCR-mediated apoptosis and proliferation of transitional B cells were analyzed by flow-cytometry. Expression of apoptotic genes was examined by microarrays and real-time PCR. Kappa/lambda light chain-coexpressing B cells were assessed by flowcytometry and immunohistochemistry. Activation status of T3 B cells was evaluated by BCR-induced calcium influx studies.
Results
BCR-mediated apoptosis of the T1 B cell subset, a major checkpoint for negative selection of autoreactive specificities, was decreased in prolactin-treated mice. Microarray studies indicated that this event may be mediated by the prolactin-induced upregulation of the anti-apoptotic gene INF-γRII and downregulation of the pro-apoptotic gene Trp63. Prolactin treatment also altered the amount of receptor editing as indicated by the increased number of transitional B cells co-expressing kappa/lambda light chains. Additionally, hyperprolactinemia modified the level of B cell anergy by increasing the degree of BCR-induced calcium influx in the T3 B cells.
Conclusion
Persistently elevated serum prolactin levels interfere with B cell tolerance induction by impairing BCR-mediated clonal deletion, deregulating receptor editing, and decreasing the threshold for activation of anergic B cells, thereby promoting autoreactivity.
Keywords: Systemic Lupus Erythematosus, B cells, Tolerance/Suppression/Anergy, Autoantibodies
The strong predominance of females in autoimmune diseases suggests that female sex hormones may play a role in disease susceptibility. There is some clinical evidence in this regard for prolactin. Increased serum levels of this hormone have been reported in patients with systemic lupus erythematosis (SLE) (1), scleroderma (2) and multiple sclerosis (3), and have been correlated with disease activity in a subset of SLE (4) and scleroderma patients (5); high serum prolactin levels during breast feeding have also been linked to flares of rheumatoid arthritis (6). Further evidence for a role of prolactin in autoimmunity has been obtained from experimental studies in mice (7-10). For example, we have shown that treatment of ovariectomized non-lupus prone BALB/c mice bearing the appropriate transgenic marker with a dose of prolactin causing mild to moderate elevation of serum levels induced the development of a lupus-like disease (11). To understand the basis for prolactin-induced autoimmunity, we have evaluated the effects of the hormone on the induction of B cell tolerance. We demonstrate here that prolactin impairs the three crucial mechanisms for B cell tolerance induction: BCR-mediated deletion, receptor editing, and anergy.
Materials and Methods
Animals
Eight-10 week-old female BALB/c mice, purchased from Taconic Farms (Germantown, NY), were ovariectomized and subjected to the treatments described below. Mice were housed in the barrier animal facility at Albert Einstein College of Medicine, Bronx, NY in accordance with current guidelines.
Anti-mouse CD40L antibody
Anti-mouse CD40L antibody was prepared from culture-supernatants of the MR1 Armenian hamster B cell-hybridoma (ATCC cell line CRL-2580, Manassas, VA, USA). Antibody concentration was tested by ELISA (BD Pharmingen, San Jose, CA, USA) and prepared at 1mg/ml.
Treatments
Mice were injected subcutaneously with 0.1ml normal saline or 0.1mg ovine prolactin (Sigma-Aldrich, St. Louis, Missouri, USA) every day for 4 weeks. This prolactin treatments leads to twofold increase in serum prolactin concentration: serum prolactin levels of 68.3 ± 20.75 nanograms/ml (ng/ml) in prolactin-treated mice vs. 30.3 ± 19.7 ng/ml in placebo-treated mice (11). Anti-CD40L-antibody (250mg) was administered intraperitoneally 3 times/week for 4 weeks.
Flow-cytometry
Splenocytes isolated from sacrificed animals were subjected to RBC-depletion with ACK-lysis buffer. Single-cell suspensions were stained with PerCp-Cy5.5, PE-Cy7, PE, APC, FITC and biotin-conjugated antibodies to CD19 (BD Pharmingen, clone 1d3, BD Biosciences, San Jose, California), CD93 (eBioscience clone AA4.1, eBioscience, San Diego, California), CD23 (Caltag, Carlsbad, California), IgM (BD Pharmingen, clone R6-60.2), CD21 (BD Pharmingen, clone 7g6), CD22 (Chemicon International, Temecula, CA), CD40 (BD Pharmingen), BAFF-R (R&D Systems, McKinely Place N.E Minneapolis) kappa (κ) (BD Pharmingen, clone 187.1) and lambda (λ) (BD Pharmingen, clone r26-46) light chains at 4°C for 30 minutes. Cells were washed and stained with pacific blue-conjugated streptavidin (SA) (Invitrogen, Carlsbad, California), and then fixed with 2% paraformaldehype. After cell- permeabilization with 0.3% saponin, intracellular staining was performed for P-Syk (BD Pharmingen) and SHP-1 (Santa Cruz Biotechnology, Santa Cruz, CA).
CD19 and CD93-staining was used to differentiate the transitional (CD19+CD93+) from mature (CD19+CD93-) B cells. IgM and CD23-staining allowed for identification of the transitional T1 (CD19+CD93+IgM+CD23-), T2 (CD19+CD93+IgM+CD23+) and T3 (CD19+CD93+IgMlowCD23+) B cell subsets (12). CD21 and CD23-staining allowed for identification of the mature marginal zone (CD19+CD93-CD21++CD23-) and follicular (CD19+CD93-CD21-CD23++) B cells (13). Samples were sorted by MoFlow cell-sorter (Dako-Cytomation, Dako Colorado Inc., Fort Collins, Colorado, USA) (purity 95% or above) or acquired with the LSRII flow-cytometer (BD Biosciences). Sorted transitional B cells were subjected to RNA isolation for real-time PCR analysis of RAG expression (see below) whereas sorted λ+B cells were used for immunofluorescent-cytology studies or generation of hybridomas as described bellow. The data acquired by LSRII flow-cytometer was analyzed with FlowJo v.7.1 software (Treestar Inc, Ashlaand, OR, USA).
Proliferation assay
Splenocytes from placebo and prolactin-treated mice were labeled with CFSE (carboxyfluorescein-diacetate-succidimyl ester, Molecular Probes, Eugene, OR). Cells were re-suspended in phenol-free RPMI-1640 medium supplemented with charcoal stripped 10% FCS. B lymphocyte-proliferation was induced by culturing the cells with anti-IgM (10μg/ml) for 3 days. Cells were stained for plasma-membrane markers to identify B cell subsets as depicted above. Samples were analyzed for proliferating B cells subtypes by flow-cytometry, as previously described (14).
Apoptosis assay
RBC-depleted splenocytes were incubated at 37°C in phenol-free RPMI-medium supplemented with 5% fetal calf serum in the presence and absence of 10μg/mL anti-IgM antibody (Jackson ImmunoResearch West Grove, PA, USA). After 10-14 hours, splenocytes were washed and stained for 30minutes at 4°C with fluorochrome-labeled antibodies to identify the T1 and T2 B cells as described above. Cells were washed again and stained with FITC-conjugated antibody to AnnexinV (Pharmingen), and, just prior to acquisition, samples were stained with impermeable dye Topro-3-labeled with APC (Invitrogen) in order to distinguish the necrotic from apoptotic (AnnexinV+ Topro3-) cells. Samples were acquired by an LSRII flow-cytometer (BD Biosciences) and data was analyzed with FlowJo v7.1 (Treestar).
B cell purification
Splenic B cells were isolated by negative selection using biotinylated anti-mouse CD43 antibody (BD PharMingen) and streptavidin conjugated Dynabeads according to the manufacturer's protocol (Dynal Invitrogen bead separations, Invtrogen Corporation). The purity of the isolated B cells was 95% or more as determined by flow-cytometry.
RNA isolation
Purified B cells were lysed using RLT buffer from RNeasy Mini-kit (Qiagen, Valencia, CA) and 1% betamercaptoethanol mix. Qiagen's protocol for the RNeasy Mini Kit with on-column DNA digestion was employed to isolate RNA from the lysates. The RNA samples were stored at -80°C until further use.
Microarray analysis
The gene expression in B cells was determined by Affymetrix genechips (genechip #45102) (Affymetrix, Inc., Santa Clara, CA). RNA was isolated from 5 placebo and 5 prolactin-treated mice by Trizol (Invitrogen). First-strand cDNA was synthesized from 30-50μg of RNA by using the first-strand cDNA-synthesis kit from Invitrogen as per the manufacturer's protocol. The reaction product was subjected to second-strand cDNA synthesis with the second-strand cDNA-synthesis kit (Invitrogen). Clean-up of double-stranded cDNA was done following the GeneChip sample-clean-up module #900371 (Affymetrix Inc). RNA transcript labeling was performed by ENZO BioArray™ Highyield™ RNA transcript-labeling kit (ENZO Lifesciences, Farmingdale, NY). Labeled RNA transcripts, cleaned according to the GeneChip sample clean-up module, were quantified by a spectrophotometer. Fragmentation of cRNA was done as per the Affimetrix protocol. Fragmented cRNA was sent to the Microarray facility at our institution for hybridization and scanning. The microarray data was analyzed by Array-assist software (Stratagene, La Jolla, CA).
Real-time PCR
The gene sequences were obtained from the Ensembl mouse genome database (http://www.ensembl.org/Mus_musculus/index.html). The primers were designed using Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Any primer pair generated with Primer3 was checked for gene specificity using the nucleotide-nucleotide BLAST database (http://130.14.29.110/BLAST/). The primer pair spanning the 5-6 intron for beta actin, which served as housekeeping gene, was the following: left primer 5′TGTACCCAGGCATTGCTGAC3′ and right primer 5′ACAGTGAGGCCAGGATGGAG3′. The RAG-1 primers were the following: left primer 5′CGGAACTCCTCTCCACCAAG3′ and right primer 5′ACCCGATTCATTTCCCTCAC 3′ the RAG-2 primers were the following: left primer 5′TGCATGGATTTGGAAGAACG 3′ and right primer 5′GGGGTTTCTTTTGGGAGTTTG3′. For the IFN-γRII, the following primers were used: left primer 5′CCTGCTTCACCCTGTTCCTC3′ and right primer 5′ CCGTCCTTGTCCAAGACCTC3′, whereas for Trp63 the left primer was 5′ CCACCATCTATCAGATTGAGCA3′ and right primer was 5′ GAGATGAGGAGGTGAGGAGAAG3′. cDNA was synthesized using the SuperScript™ First-Strand Synthesis System from Invitrogen.
Real-time PCR was done in a Light Cycler real-time PCR-machine (Bio Rad Laboratories, Hercules, CA) using Absolute QPCR SYBER Green Mix (ABgene, Rochester, USA). The conditions followed the standard ABgene protocol for the kit except for the annealing and extension step, where a temperature of 55°C for RAG1 and RAG2, 57°C for IFN-γRII, and 54°C for Trp63 were used for 30 seconds followed by 30 seconds at 72°C. A melting curve was generated at the end of the PCR and different samples containing the same primer pair showed matching amplicon melting temperatures.
Analysis of calcium mobilization
For measurements of free-intracellular-calcium concentration, splenocytes were loaded with Indo-1AM (Invitrogen), stained for CD19, CD93, CD23 and anti-IgM antibody (all from BD Pharmingen) and resuspended at 4×106 cells/ml in IMDM-medium (Hyclone, Logan, UT). Analysis was initiated with flow-cytometry and after establishing the baseline calcium concentration, cells were stimulated with 20μg/ml anti-IgM F(ab')2 (Southern Biotech). Calcium concentration was measured over time with LSR flow-cytometer and calcium influx was analyzed with FlowJo software (Tree Star).
B cell hybridomas
Hybridomas were generated from sorted λ+B cells obtained from mice treated with prolactin for 4 weeks and NSO-fusion partner at a 2:1 ratio, as described previously (15). Hybridoma-producing wells were screened for κ and λ-expression by ELISA using plates coated with anti-κ and anti-λ antibody (BD Biosciences). Positive clones were detected by alkaline phosphatase-conjugated anti-κ and anti-λ antibodies (Southern Biotech). Cell lines co-expressing κ and λ light chains were cloned on soft agarose, expended, and then tested by ELISA for reactivity to single and double-stranded (ds)DNA, as previously described (16).
PCR analysis
κ+λ+ double positive clones identified by ELISA of their supernatants were evaluated for Jκ rearrangement by PCR. DNA-amplification was carried out in two rounds of PCR using a PCR SPRINT Thermo-Cycler (Thermo Fisher Scientific Inc., Waltham, MA). The PCR conditions and primers used for the first and second round PCR for Vκ, Jκ1-5, universal Vλ and universal Jλ were performed as previously described (17, 18).
The studies described above have been reviewed and approved by the Animal Institute at Albert Einstein College of Medicine.
Statistical Analysis
Standard statistical tests (mean value, standard deviation, two-tailed Student's t test) were performed for data analysis. p-values less than 0.05 were considered significant.
Results
Effects of prolactin on antigen-mediated B cell deletion
Prolactin-mediated alterations of transitional B cell subsets
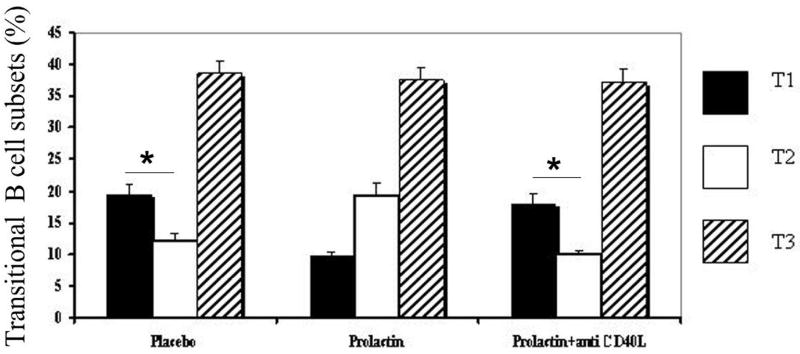
Mice treated with placebo, prolactin or prolactin+anti-CD40L antibody had similar absolute numbers of splenocytes (97.2×106±6×106, 100.4×106±1.26×106, 102×106±2.7×106 respectively) and B cells (38.8×106±3×106, 39.6×106±2.5×106, 40.2×106±1.5×106 respectively). All experimental groups displayed similar percentages of mature and transitional B cells. The absolute numbers of total transitional B cells did not differ among the mice treated with prolactin (6.7×106±0.7×106), placebo (6.6×106±0.5×106), and prolactin+anti-CD40L antibody (6.7×106±0.4+106). However, placebo-treated mice have more T1 than T2 B cells; the lower number of T2 cells reflects the negative selection of autoreactive specificities that occurs at the T1/T2 junction (19-21). Prolactin-treated mice show an increased percentage of T2 B cells and a decreased percentage of T1 B cells, resulting in a T1/T2 ratio of less than 1. Prolactin-mediated alteration of the transitional B cell subsets was reversed by treatment with anti-CD40L-antibody; the mice that received simultaneous treatment with prolactin and anti-CD40L-antibody displayed T1/T2 ratios similar to that of placebo-treated mice indicating that CD40-CD40L interactions are necessary for prolactin-induced alterations of the transitional B cell subsets (Figure 1). The absolute numbers of T1 B cells were higher in placebo (1.2×106±0.9×106) and prolactin+anti-CD40L antibody-treated mice (1.1×106±0.4×106) than in mice treated with prolactin (0.6×106±0.1+106) (p=0.0002 and 0.0001, respectively). The absolute number of T2 B cells was larger in prolactin-treated mice (1.3×106±0.2×106) than in placebo (0.8×106±0.06×106) and prolactin+anti-CD40L antibody-treated mice (0.6×106± 0.04×106), (p=0.0005 and 0.0003, respectively). Since T2 is a cycling subset, we wanted to evaluate whether the increased number of T2 B cells in prolactin-treated mice is induced by prolactin-mediated proliferation of that subset. As determined by CFSE assay, T2 subsets from placebo and prolactin-treated mice showed similar percentages of proliferating cells upon stimulation with anti-IgM antibody (29.81±10.74 and 37.37±7.31, respectively) (p=0.1). These data indicated that prolactin-induced expansion of T2 B cells is not caused by prolactin-mediated increase in T2 cycling.
Figure 1. Flowcytometric analysis of the transitional B cell subsets.
In mice treated with placebo (n=5) or prolactin+anti-CD40L antibody (n=5), the percentage of T1 B cells (CD19+CD93+IgM+CD23-) was significantly higher than the T2 B cells (CD19+CD93+IgM+CD23+) (p=0.0003 and p=0.0004, respectively), but that was not the case with prolactin-treated mice (n=5) in which T1/T2 B cell ratio was <1. No significant difference was found in the size of the T3 (CD19+CD93+IgMlowCD23+) subset among the mice treated with placebo, prolactin or prolactin+anti-CD40L antibody.
In contrast to the transitional T2 subset that gives rise to mature B cells, the T3 subset consists of anergic B cells (see below) and does not directly contribute to the mature subsets (12, 22). The size of T3 B cell compartment was similar in all experimental groups (Figure 1).
There were no significant differences in the percentages and absolute numbers of marginal zone and follicular B cells among the three experimental groups (data not shown).
Effects of prolactin on BCR-mediated apoptosis
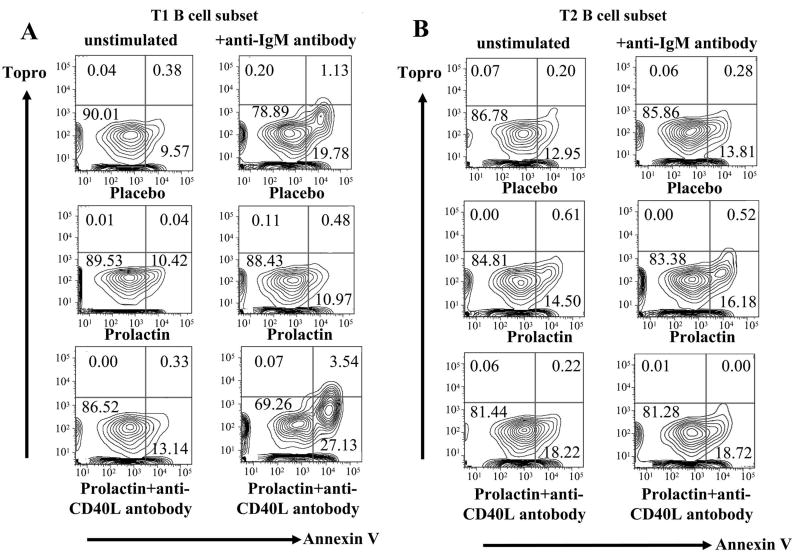
To study the impact of prolactin on the negative selection of B cells, we evaluated the effect of the hormone on BCR-mediated apoptosis of transitional B cells. Upon stimulation with anti-IgM antibody as a surrogate antigen, T1 B cells from placebo-treated mice showed a higher degree of apoptosis than T1 B cells from prolactin-treated mice (p=0.008). T1 B cells from mice treated with prolactin+anti-CD40L antibody displayed a degree of apoptosis similar to that of T1 B cells from placebo-treated mice (p=0.003) (Figure 2A). No significant difference in anti-IgM antibody-induced apoptosis of T2 B cells was found among the placebo, prolactin, and prolactin+anti-CD40L-treated mice (Figure 2B). These findings indicated that persistent elevation of serum prolactin level inhibits BCR-mediated apoptosis of T1 B cells, and that this effect of the hormone is dependent upon the CD40-CD40L pathway.
Figure 2. BCR-mediated apoptosis in transitional B cell subsets.
The percentage of apoptotic cells (topro- annexin V+) was determined for T1 and T2 B cell subsets in placebo, prolactin, and prolactin+anti-CD40L antibody-treated mice (n=5 in each group). A) As shown in these representative experiments, after anti-IgM stimulation T1 B cells from mice treated with placebo or prolactin+anti-CD40L antibody showed higher increases in the percentage of apoptotic cells than T1 B cells from prolactin-treated mice (p=0.008 and p=0.003, respectively). B) The degree of anti-IgM-induced apoptosis in T2 B cell subsets of placebo, prolactin and prolactin+anti-CD40L-treated mice was not significantly different.
Prolactin modulates the expression of apoptosis-related genes
To understand the molecular basis for the effects of prolactin, microarray analysis of purified B cells from placebo and prolactin-treated mice was performed using Affymetrix genechips. 160 apoptosis-related genes were affected by prolactin; Mcl1, Birc1, NF-κB2, IFN-γRII, Trp63, Trp73, E2f1, and chk2 were chosen for further evaluation by real-time PCR. mRNA expression of IFN-γRII, a potent anti-apoptotic molecule, was up-regulated 2.73-fold by prolactin, whereas Trp63, a member of p53 family of pro-apoptotic molecules, was down-regulated 3.7-fold. Although the changes by real-time PCR were always in the same direction as the changes identified by the microarrays, the expression differences by real-time PCR did not reach statistical significance for the remaining apoptosis-related genes mentioned above.
Prolactin upregulates CD40 expression
Hyperactivity of CD40-CD40L interactions may contribute to autoantibody production (23). Treatment with prolactin upregulates the expression of CD40 on transitional T1 B cells (p=0.05) (Table 1). This upregulation of CD40 may be a contributory factor to the increased survival of T1 B cells in prolactin-treated mice since CD40-engagement can rescue transitional B cells from antigen-mediated apoptosis (24). CD40-engagement is also linked to overexpression of the antiapoptotic molecule Bcl-2 (25) which we have previously shown to be upregulated by prolactin (11).
Table 1. Expression of CD40 and BAFF-R *.
| MFI | Placebo | Prolactin | P value |
|---|---|---|---|
| CD40 expression on T1 B cells | 527±98 | 740±78 | p=0.05 |
| BAFF-R expression on T2 B cells | 419.33±41.96 | 555.96±17.73 | p=0.006 |
Values are the mean ± SEM results from 4 mice per group for the CD40 experiment and 5 mice per group for the BAFF-R expression experiment.
Prolactin upregulates the expression of BAFF-R
BAFF is a B cell-activating factor belonging to the TNF family. BAFF-BAFF-R interaction plays a critical role in B cell development at the transition between T1 and T2 B cells, and functions as a survival factor for T2 B cells (26). T2 B cells from prolactin-treated mice express more BAFF-R than T2 B cells from placebo-treated mice (p=0.006) (Table 1).
Effects of prolactin on receptor editing
Prolactin alters the number of κ+λ+ dual positive B cells
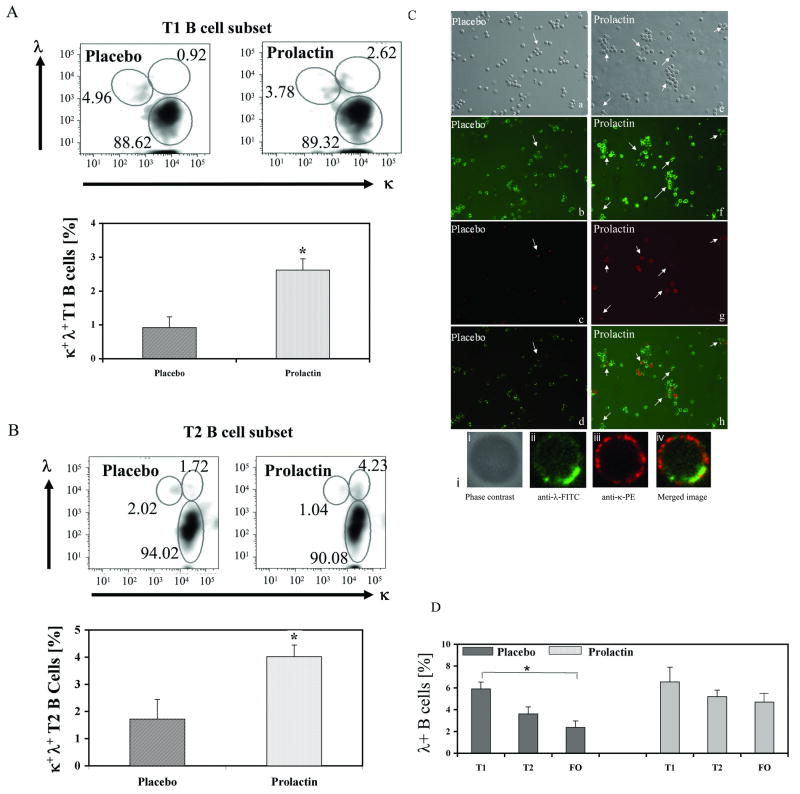
Co-expression of dual light chains on a B cell reflects an ongoing process of receptor editing (27, 28). By flowcytometric analysis we found that prolactin-treated mice displayed higher numbers of κ+λ+B cells with T1 and T2 phenotype than placebo-treated mice (p=0.003 and 0.004, respectively) (Fig 3A and B). In addition, immuncytochemical enumeration of κ+λ+ B cells showed that compared to placebo-treated mice, the mice treated with prolactin had elevated numbers of B cells co-expressing κ and λ light chains (Fig 3C), indicating that prolactin increases the number of B cells that escape allelic exclusion. The elevated number of dual light chain-expressing transitional B cells in prolactin-treated mice was also accompanied by 2.17 and 2.67-fold higher expression of RAG-1 and RAG-2 mRNA, as determined by real-time PCR.
Figure 3. Prolactin increases the number of B cells co-expressing κ and λ light chains.
Flow-cytometric analysis showed that prolactin-treated mice (n=5) have a higher number of κ and λ dual-expressing T1 (A) and T2 B cells (B) than placebo-treated mice (n=5) (p=0.003 and p=0.004, respectively). C) Detection of κ+λ+ B cells by immunocytochemistry. The images a (phase-contrast), b (λ+ cells (green)), c (κ+ cells (red)) and d (the merged images) are from mice treated with placebo, and e, f, g and h show the phase-contrast, λ+ cells (green), κ+ cells (red) and merged images, respectively, from prolactin-treated mice. The arrows point out κ+λ+ cells. i) Confocal microscopy demonstrates that κ (iii) and λ (ii) light chains are co-expressed on the surface of the same B cell of a prolactin-treated mouse (iv). D). Effect of prolactin on λ+ B cells in the splenic B cell subsets. Placebo-treated mice showed a significant drop in the percentage of λ+ B cells during their maturation from T1 (5.9±0.63) to follicular (FO) (2.37± 0.58) B cells (p=0.0001). In prolactin-treated mice, the drop in the percentage of λ+ B cells from T1 to FO B cells was not statistically significant..
During the maturation process from transitional to mature stage, prolactin-treated mice exhibited significantly lesser drop in λ-expressing B cells than placebo-treated mice (Figure 3D). Although we observed a drop in the percentage of λ-expressing B cells during maturation from T1 to follicular stage in both placebo and prolactin-treated mice, the decline was statistically significant only for the placebo-treated mice (p=0.0001 vs p=0.08).
B cell hybridomas
Three fusions of B cells from prolactin-treated mice produced 29 κ+λ+ hybridomas which were then subjected to soft-agarose cloning. The clones were expanded and tested for DNA-reactivity by ELISA. Nine clones were DNA-reactive. When the supernatant protein concentration was normalized to 5μg/ml, two clones showed reactivity to ssDNA and one clone showed reactivity to dsDNA. In addition, 14 κ+λ+ clones were chosen for PCR analysis of the κ light chain usage. Nine clones were VκJκ2, 2 were VκJκ4 and 3 were VκJκ5.
Effect of prolactin on anergy induction
Prolactin decreases the activation threshold of anergic T3 B cells
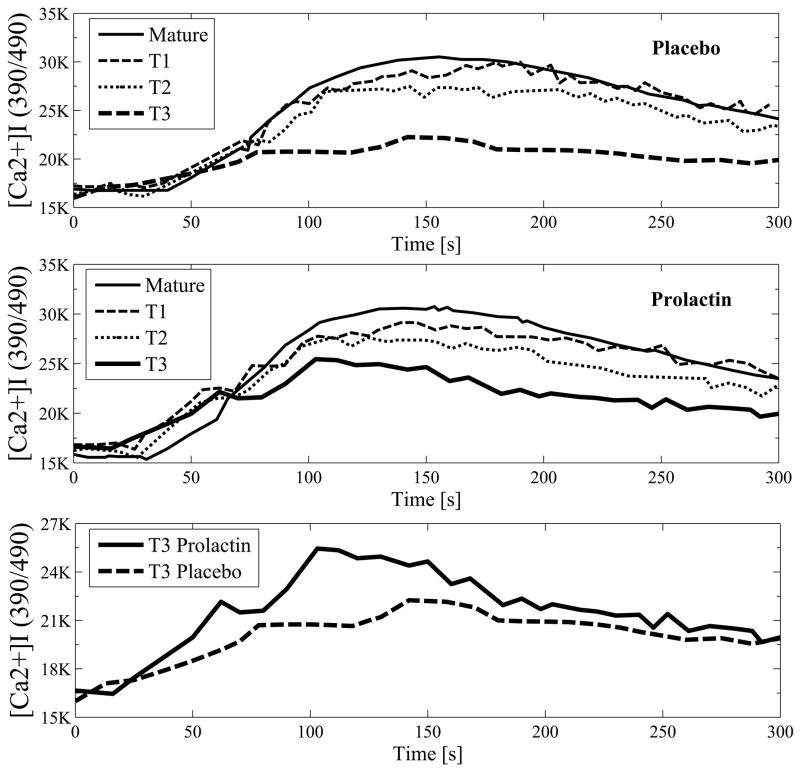
T3 transitional B cell subset (CD19+CD93+IgMlowCD23+) consists of anergic cells (12, 22). To evaluate the effect of prolactin on anergy induction, we studied the impact of the hormone on the size of T3 B cell subset and found that treatment with prolactin did not affect the number of T3 B cells (Fig 1). However, treatment with prolactin decreased the threshold for BCR-mediated activation of T3 B cells. Stimulation with anti-IgM antibody induced a higher degree of calcium influx in T3 B cells from prolactin-treated mice than in T3 B cells from placebo-treated mice (p=0.008) (Figure 4). To study the mechanisms by which prolactin affects BCR-mediated calcium influx in T3 B cells, we examined CD22/SHP-1 expression and Syk-phosphorylation upon BCR-engagement. T3 B cells from prolactin-treated mice expressed more phosphorylated syk that the T3 cells from placebo-treated mice (p= 0.01). No differences in the expression of BCR-inhibitory molecules CD22/SHP-1 was found between the T3 cells from placebo and prolactin-treated mice.
Figure 4. Prolactin decreases the threshold for activation of T3 B cells.
After stimulation with anti-IgM F(ab')2,, the calcium concentration was measured over time in B cells from placebo and prolactin-treated mice (n=5 for both groups). BCR-engagement induced higher calcium influx, determined as MFI from all time points, in T3 B cells from prolactin-treated mice than in T3 B cells from placebo-treated mice (p=0.008).
Discussion
Prolactin is a peptide hormone with lactogenic and immunomodulatory functions. Several clinical observations have suggested that prolactin stimulates a number of autoimmune diseases such as lupus (4), scleroderma (5), and multiple sclerosis (3). In addition, numerous murine studies have provided insight into modulation of autoimmunity by prolactin. Data from lupus-prone mice have indicated that prolactin affects the onset of disease and disease activity (29). We have shown that mild-moderately increased serum prolactin levels induce an autoimmune state in BALB/c mice expressing a transgene for the heavy chain of a pathogenic anti-DNA antibody (11). In these mice, hyperprolactinemia increases the number of autoreactive B cells with the follicular phenotype and leads to their activation with subsequent anti-DNA antibody production, and IgG deposition in the kidneys. These findings indicate that modestly increased serum prolactin levels break B cell tolerance, but they do not indicate how this is accomplished. In this report, we demonstrate that the same level of hyperprolactinemia interferes with the three known mechanisms for B cell tolerance induction: BCR-mediated deletion, receptor editing and anergy
Autoreactive B cells are constantly generated in the bone marrow and periphery (30). High affinity autoreactive immature B cells are negatively selected and purged from the normal B cell repertoire by clonal deletion (31). Clonal deletion, the main mechanism of central tolerance, also occurs in the periphery during the transitional stage of B cell development in the spleen. A significant number of immature B cells that have survived the negative selection in the bone marrow undergo deletion at the T1/T2 junction (32, 33), so that the T1 B cell subset is normally larger than the T2 subset. The T1/T2 interphase thus is a major checkpoint for negative selection of autorecative specificities (19-21). B cell selection at this point is mediated by BCR-signaling, with strong signals inducing deletion of autoreactive B cells through apoptosis (34).
Treatment with prolactin alters the B cell-maturation pattern in the spleen and leads to an inverted T1/T2 B cell ratio (11). Prolactin decreases the degree of BCR-mediated apoptosis of T1 B cells. Prolactin thereby overrides the negative selection that occurs at this stage of B cell development, leading to the survival of autoreactive specificities normally destined for deletion and allowing their maturation into T2 B cells. This effect of prolactin is dependent on the CD40-CD40L costimulatory pathway. Prolactin upregulates the expression of CD40 on T1 B cells, and CD40 engagement is known to increase the expression of anti-apoptotic molecule Bcl-2 (25), which we have shown to be overexpressed by prolactin treatment (11). Prolactin also upregulates the transcription of anti-apoptotic factors INFγRII and Trp3 in B cells, but it is not known whether this contributes to the impact of the hormone on BCR-mediated apoptosis.
T2 B cells from prolactin-treated mice show a trend toward increased resistance to BCR-induced apoptosis, which may be linked to the prolactin-induced overexpression of BAFF-R. Both BAFF-BAFF-R and the CD40-CD40L-costimulatory pathway provide stimulatory and survival signals to B cells (23, 26) so that the prolactin-induced small increases in the expression of BAFF-R and CD40 might be responsible for the accelerated and skewed maturation of transitional B cells into mature B cells. This may explain our previous finding of the increased numbers of autoreactive follicular B cells in prolactin-treated BALB/c mice (11).
B cells can escape clonal deletion by co-expression of more than one light chains on their surface. Dual light chain expressing B cells have been observed in a variety of lymphoid neoplasmas and in transgenic mouse models, but also in normal humans and mice (35, 36). Receptor editing, an attempt to rescue autoreactive B cells from deletion by replacing self-reactive Ig light or heavy chains with non-autoreactive ones (28), plays a role in the generation of dual isotype-expressing B cells (27). This is of special importance in autoimmunity since the coexpression of two BCRs may allow autoreactive B cells to escape clonal deletion (36-38). Studies in PC and anti-DNA models have demonstrated that altered receptor editing may result in autoreactivity (39, 40). We found that prolactin-treated mice have higher numbers of κ+λ+ transitional B cells than placebo-treated mice. Analysis of the light chain expression of the hybridomas generated from κ+λ+ B cells from prolactin-treated mice showed that these clones predominantly use Jk2, Jk4, and Jk5 genes. The fact that no Jk1 clones were detected along with the increased number of Jk4 and Jk5 clones indicates that prolactin accelerates the process of receptor editing. This is supported by the finding of increased expression of RAG-1 and RAG-2 in transitional B cells of prolactin-treated mice. Furthermore, prolactin-induced dysregulation of receptor editing leads to the generation of autoreactivity, as shown here by the finding of DNA-reactive clones among the hybridomas generated from dual receptor-expressing B cells.
Autoreactive B cells with lower affinity are not subjected to deletion or receptor editing, but may be rendered anergic, retaining their antigen binding capacity but not responding to their specific antigen under optimal conditions of stimulation (41). The continuous presence of autoantigens is required for the anergic cells to remain in the anergic state (42); removal of the autoantigen changes the anergic B cells into naïve B cells that can be activated and induced to secrete autoantibodies (43). Thus, anergic B cells pose a potential threat because they can become autoreactive. Given the fact that almost 30-50% of newly produced B cells are destined to become anergic (12, 43), both the frequency of the anergic B cells as well as the level of anergy are very important variables in the induction and maintenance of B cell tolerance.
It has been shown that a substantial number of anergic B cells exist even under physiologic conditions and comprise the majority of the transitional T3 B cell subset (12). Unlike T2 B cells, T3 B cells do not give rise to mature B cells (12, 22). T3 B cells do not mobilize Ca++, proliferate, upregulate activation markers or mount an immune response upon BCR engagement (12), and they preferentially use Ig JH3 segment that has been linked to autoreactivity (22). Prolactin did not affect the size of the T3 B cell subset, but did decrease the threshold for T3 activation. B cell overactivity is a well-known feature of SLE, and it has been shown that lupus B cells exhibit aberrant early signal transduction events including increased anti-IgM-mediated free intracytoplasmic Ca++ responses (44, 45).
These findings demonstrate for the first time that a hormone affects all three known mechanisms of B cell tolerance induction: BCR-mediated deletion, receptor editing, and anergy. It remains unclear whether these effects of prolactin are unique. Murine studies have shown that persistently increased levels of estrogen can break B cell tolerance and induce a lupus-like syndrome in mice with a BALB/c genetic background (46) by impairing the negative selection of autoreactive B cells (47). However, the effects of estrogen on other mechanisms for B cell tolerance induction have not yet been explored.
Like prolactin, estrogen exerts immunostimulatory effects and induces autoantibody production (46). Additive or synergistic effects of the two hormones on the immune system likely contribute to gender-distinct autoimmune responses, but it may be difficult to differentiate individual hormone-specific immunomodulatory effects because estrogen and prolactin affect each other's serum concentration - estrogen stimulates prolactin secretion and increased prolactin levels suppress secretion of estrogen (48). Some previous studies in mice have suggested that estrogen's effects on autoimmunity are mediated, at least in part, by prolactin (16, 49). However, the observation that estrogen induces autoreactive B cells of the marginal zone phenotype (50) whereas prolactin induces autoreactive B cells of the follicular phenotype (11), indicates that the effects of estrogen and prolactin on autoimmunity are hormone specific. Since the studies reported here utilized ovariectomized mice, it is clear that the effects of prolactin on B cell tolerance we describe are independent of those of estrogen; to our knowledge there have been no similar studies of the effects of estrogen on the mechanisms of B cell tolerance induction. In view of the strong gender bias in autoimmune disease, targeted manipulation of specific pathways of immune function affected by prolactin and/or estrogen may provide new and more effective forms of treatment.
Acknowledgments
Grant Support: This work was supported by Grant AI 057924 from the National Institutes of Health (to E.P.).
The authors would like to thank George Tsokos and Moncef Zouali for critical reading of the manuscript, Martin Pepeljugoski for technical support during the preparation of the manuscript for publication, Yi Bio for procedural help with the microarrays, Susan Buhl for her help with the generation of the hybridomas, and Milagros Mejia for the maintenance of the mouse colony and administration of the treatments.
Abbreviations used in this paper
- SLE
systemic lupus erythematosus
- trp63
transformation related protein 63
- BAFF
B cell-activating factor of the TNF family
Footnotes
Disclosures: The authors have declared that no conflicts of interest exist.
References
- 1.Walker S, McMurray R, Houri J, Allen S, Keisler D, Sharp G, et al. Effects of prolactin in stimulating disease activity in systemic lupus erythematosus. Ann N Y Acad Sci. 1998;840:762–72. doi: 10.1111/j.1749-6632.1998.tb09615.x. [DOI] [PubMed] [Google Scholar]
- 2.Kucharz E, Jarczyk R, Jonderko G, Rubisz-Brezeziska J, L BW. High serum level of prolactin in patients with systemic sclerosis. Clin Rheumatol. 1996;15:314. doi: 10.1007/BF02229718. [DOI] [PubMed] [Google Scholar]
- 3.Azar S, Yamout B. Prolactin secretion is increased in patients with multiple sclerosis. Endocr Res. 1999 May;25(2):207–14. doi: 10.1080/07435809909066142. [DOI] [PubMed] [Google Scholar]
- 4.Jara L, Gomez-Sanchez C, Silveira LH, Martinez-Osuna P, Vasey FB, Espinoza LR. Hyperprolactinemia in systemic lupus erythematosus: association with disease activity. Am J Med Sci. 1992;303:222–6. doi: 10.1097/00000441-199204000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Mirone L, Barini A, Barini A. Androgen and prolactin (Prl) levels in systemic sclerosis (SSc): relationship to disease severity. Ann N Y Acad Sci. 2006;1069:257–62. doi: 10.1196/annals.1351.023. [DOI] [PubMed] [Google Scholar]
- 6.Olsen N, Kovacs W. Hormones, pregnancy, and rheumatoid arthritis. Gend Specif Med. 2002:28–37. [PubMed] [Google Scholar]
- 7.Mattsson R, Mattsson A, Hansson I, Holmdahl R, Rook G, Whyte A. Increased levels of prolactin during, but not after, the immunisation with rat collagen II enhances the course of arthritis in DBA/1 mice. Autoimmunity. 1992;11:163–70. doi: 10.3109/08916939209035151. [DOI] [PubMed] [Google Scholar]
- 8.Berczi I, Nagy E. A possible role of prolactin in adjuvant arthritis. Arthritis Rheum. 1982;25:591–4. doi: 10.1002/art.1780250517. [DOI] [PubMed] [Google Scholar]
- 9.Dijkstra C, van der Voort E, De Groot C, Huitinga I, Uitdehaag B, Polman C, et al. Therapeutic effect of the D2-dopamine agonist bromocriptine on acute and relapsing experimental allergic encephalomyelitis. Psychoneuroendocrinology. 1994;19:135–42. doi: 10.1016/0306-4530(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 10.Mc Murray R. Prolactin in murine systemic lupus erythematosus. Lupus. 2001;10:742–7. doi: 10.1191/096120301717164985. [DOI] [PubMed] [Google Scholar]
- 11.Peeva E, Michael D, Cleary J, Rice J, Chen X, Diamond B. Prolactin modulates the naive B cell repertoire. J Clin Invest. 2003;111:275–83. doi: 10.1172/JCI16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, et al. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–62. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Peeva E, Venkatesh J, Diamond B. Tamoxifen blocks estrogen-induced B cell maturation but not survival. J Immunol. 2005;175:1415–23. doi: 10.4049/jimmunol.175.3.1415. [DOI] [PubMed] [Google Scholar]
- 14.Lyons A, Parish C. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 15.de StGroth S, Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35:1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]
- 16.Peeva E, Grimaldi C, Spatz L, Diamond B. Bromocriptine restores tolerance in estrogen-treated mice. J Clin Invest. 2000;106:1373–9. doi: 10.1172/JCI10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehlich A, Martin V, Muller W, Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr Biol. 1994;4:573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 18.Yamagami T, ten Boekel E, Schaniel C, Andersson J, Rolink A, Melchers F. Four of five RAG-expressing JCκ-/- small pre-BII cells have no L chain gene rearrangements: detection by high-efficiency single cell PCR. Immunity. 1999;11:309–316. doi: 10.1016/s1074-7613(00)80106-5. [DOI] [PubMed] [Google Scholar]
- 19.Loder F, Mutschler B, Ray R, Paige C, Sideras P, Torres R, et al. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su T, Rawlings D. Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J Immunol. 2002;168:2101–10. doi: 10.4049/jimmunol.168.5.2101. [DOI] [PubMed] [Google Scholar]
- 21.Petro J, Gerstein R, Lowe J, Carter R, Shinners N, Khan W. Transitional type 1 and 2 B lymphocyte subsets are differentially responsive to antigen receptor signaling. J Biol Chem. 2002;277:48009–19. doi: 10.1074/jbc.M200305200. [DOI] [PubMed] [Google Scholar]
- 22.Teague B, Pan Y, Mudd P, Nakken B, Zhang Q, Szodoray P, et al. Cutting edge: Transitional T3 B cells do not give rise to mature B cells, have undergone selection, and are reduced in murine lupus. J Immunol. 2007;178:7511–5. doi: 10.4049/jimmunol.178.12.7511. [DOI] [PubMed] [Google Scholar]
- 23.Koshy M, Berger D, Crow M. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J Clin Invest. 1996;98:826–37. doi: 10.1172/JCI118855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sater R, Sandel P, Monroe J. B cell receptor-induced apoptosis in primary transitional murine B cells: signaling requirements and modulation by T cell help. Int Immunol. 1998;10:1673–82. doi: 10.1093/intimm/10.11.1673. [DOI] [PubMed] [Google Scholar]
- 25.Buckley A. Prolactin, lymphocyte growth and survival factor. Lupus. 2001;10:684–690. doi: 10.1191/096120301717164912. [DOI] [PubMed] [Google Scholar]
- 26.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, et al. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–66. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezanka L, Kenny J, Longo D. Dual isotype expressing B cells [kappa(+)/lambda(+)] arise during the ontogeny of B cells in the bone marrow of normal nontransgenic mice. Cell Immunol. 2005;238:38–48. doi: 10.1016/j.cellimm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Nemazee D. Receptor editing in B cells. Adv Immunol. 2000;74:89–126. doi: 10.1016/s0065-2776(08)60909-8. [DOI] [PubMed] [Google Scholar]
- 29.Peeva E, Venkatesh J, Michael D, Diamond B. Prolactin as a modulator of B cell function; implications for SLE. Biomed Pharmacother. 2004;58:310–19. doi: 10.1016/j.biopha.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Wardemann H, Yurasov S, Schaefer A, Young J, Meffre E, Nussenzweig M. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 31.Nemazee DA, Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 32.Chung JB, Sater RA, Fields ML, Erikson J, Monroe JG. CD23 defines two distinct subsets of immature B cells which differ in their responses to T cell help signals. Int Immunol. 2002;14:157–66. doi: 10.1093/intimm/14.2.157. [DOI] [PubMed] [Google Scholar]
- 33.Carsetti R, Kohler G, Lamers M. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2:945–56. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 35.Rice JS, Newman J, Wang C, Michael DJ, Diamond B. Receptor editing in peripheral B cell tolerance. Proc Natl Acad Sci U S A. 2005;102:1608–13. doi: 10.1073/pnas.0409217102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radic MZ, Erikson J, Litwin S, Weigert M. B lymphocytes may escape tolerance by revising their antigen receptors. J Exp Med. 1993;177:1165–73. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiegs SL, Russell DM, Neamazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–20. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Li H, Ni D, Weigert M. Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern. J Exp Med. 2002;196:1543–52. doi: 10.1084/jem.20021560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenny JJ, Rezanka LJ, Lustig A, Fischer RT, Yoder J, Marshall S, et al. Autoreactive B cells escape clonal deletion by expressing multiple antigen receptors. J Immunol. 2000;164:4111–9. doi: 10.4049/jimmunol.164.8.4111. [DOI] [PubMed] [Google Scholar]
- 41.Nossal GJ, Pike BL. Clonal anergy: persistence in tolerant mice of antigen-binding B lymphocytes incapable of responding to antigen or mitogen. Proc Natl Acad Sci U S A. 1980;77:1602–6. doi: 10.1073/pnas.77.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gauld SB, Benschop RJ, Merrell KT, Cambier J. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6:1160–7. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 43.Melchers F. Anergic B cells caught in the act. Immunity. 2006;25:864–7. doi: 10.1016/j.immuni.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Liossis S, Kovacs B, Dennis G, Kammer G, Tsokos G. B cells from patients with systemic lupus erythematosus display abnormal antigen receptor-mediated early signal transduction events. J Clin Invest. 1996;98:2549–57. doi: 10.1172/JCI119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liossis SN, Sfikakis PP, Tsokos GC. Immune cell signaling aberrations in human lupus. Immunol Res. 1998;18:27–39. doi: 10.1007/BF02786511. [DOI] [PubMed] [Google Scholar]
- 46.Bynoe M, Grimaldi C, Diamond B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proc Natl Acad Sci U S A. 2000;97:2703–8. doi: 10.1073/pnas.040577497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grimaldi CM, Jeganathan V, Diamond B. Hormonal regulation of B cell development: 17 beta-estradiol impairs negative selection of high-affinity DNA-reactive B cells at more than one developmental checkpoint. J Immunol. 2006;176:2703–10. doi: 10.4049/jimmunol.176.5.2703. [DOI] [PubMed] [Google Scholar]
- 48.Wilson J, Foster D. Textbook of endocrinology. 8th. Philadelphia, PA: WB Saunders; 1992. [Google Scholar]
- 49.Elbourne K, Keisler D, McMurray RW. Differential effects of estrogen and prolactin on autoimmune disease in the NZB/NZW F1 mouse model of systemic lupus erythematosus. Lupus. 1998;7:420–7. doi: 10.1191/096120398678920352. [DOI] [PubMed] [Google Scholar]
- 50.Grimaldi C, Michael D, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001;167:1886–90. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]