Abstract
Background and Purpose
After stroke, individuals have decreased mobility of the hemiparetic leg, which demands less muscle oxygen consumption; thus, blood flow decreases. The purpose of this study was to determine the effect of single limb exercise (SLE) on femoral artery blood flow, diameter and peak flow velocity in the hemiparetic leg after stroke.
Methods
Twelve individuals (60.6 ± 14.5 years of age; 5 male) with chronic stroke (69.1 ± 82.2 months; 5 with right-side hemiparesis) participated in the study. The intervention consisted of a SLE knee extension/flexion protocol three times per week for 4 weeks. Using Doppler ultrasound, bilateral femoral artery blood flow, diameter and peak flow velocity was assessed at baseline, after 2 weeks and after 4 weeks of SLE.
Results
Using repeated measures ANOVA, femoral artery blood flow, arterial diameter, and blood flow velocity in the hemiparetic limb were significantly improved (p < 0.0001) after the SLE. No significant changes occurred in the non-trained limb for any outcome measures.
Conclusions
These data suggest that a 4-week SLE training program that increases muscular activity in the hemiparetic limb improves femoral artery blood flow, diameter, and peak velocity. SLE may be an important training strategy in stroke rehabilitation to minimize the vascular changes that occur post-stroke due to decreased activity of the hemiparetic limb.
Keywords: Vascular function, blood flow, stroke
Physiologic functional changes such as vascular resistance and arterial remodeling may be associated with aging 1, 2 and disease-induced changes3, 4 including stroke.5, 6 Specifically, people after stroke often present with decreased cardiorespiratory fitness 7, 8 and peripheral vascular adaptations (i.e. reduced blood flow, decreased arterial diameter and endothelial dysfunction) in the hemiparetic leg.5, 6 However, participation in regular physical activity such as aerobic exercise has altered these pathological vascular changes in people with obesity,9 Type 2 diabetes,3 spinal cord injury,10 and coronary artery disease.11
Most exercise interventions for people post-stroke focus on bilateral activity such as treadmill walking or cycling. However, during bilateral exercise, evidence has suggested a reduced work effort by the hemiparetic limb when compared to the other limb.6, 12, 13 This indicates a need to identify an exercise training strategy that would primarily focus on the hemiparetic limb to maximize work effort.
Participants in this study used single limb exercise (SLE) as an aerobic training intervention. The purpose of the present study was to characterize the effects of a 4-week SLE training intervention on cardiovascular function in the hemiparetic limb in people post-stroke. It was hypothesized that after the SLE training intervention significant improvements in femoral artery: 1) blood flow, 2) diameter, 3) peak blood flow velocity and 4) conductance would be observed after the training period when compared to baseline measures. Lastly, to determine if systemic vascular function would improve after SLE, we hypothesized that ankle-brachial index (ABI) would improve to the normal ranges (0.90 ≥ 1.40).14
Materials and Methods
Twelve participants with chronic stroke completed this within subject design study (Table 1). Inclusion criteria included: 1) a diagnosis of hemiparesis from a stroke at least 6 months ago confirmed by clinical assessment; 2) the ability to transfer from a sitting to standing position with minimal assist; 3) walking 10 meters independently with or without an orthotic or assistive device, 4) mild to moderate stroke deficits defined by a lower extremity Fugl-Meyer (LEFM) score from 20 to 33/34 15; 5) 35 degrees of active knee extension/flexion with movement against gravity and 6) medical clearance from primary care physician for exercise testing and prescription. Exclusion criteria consisted of the following: 1) type 1 or 2 diabetes; 2) current participation in single limb exercise or physical therapy; 3) peripheral vascular disease (ABI < 0.40) or known stenosis of the lower extremity vessels 4) taking alpha-adrenergics to improve peripheral vasodilation 5) a difference ≤ 2% between the hemiparetic and less affected limbs for arterial diameter and blood flow velocity and 6) any medical condition that would preclude participation in exercise testing and prescription. Institutionally approved informed consent was obtained in writing prior to enrollment in the research study.
Table 1.
Participant Demographics
| Characteristics n=12 | Mean ± SD |
|---|---|
| Sex: Male | 5 |
| Age (years) | 60.6 ± 14.5 |
| Race/ethnicity | |
| African American | 2 |
| Caucasian | 8 |
| Hispanic | 1 |
| Native American | 1 |
| Body Mass Index (DEXA scan) | 29.7 ± 4.0 |
| Medication | |
| Beta-blockers | 4 |
| Stroke Characteristics | |
| Time (months) post-stroke | 69.1 ± 82.2 |
| Right side weakness | 5 |
| Type of stroke: | |
| Ischemic | 9 |
| Hemorrhage | 3 |
| Stroke Severity | |
| LE Fugl-Meyer score | 26.7 ± 3.8 |
Dual Emission X-ray Absorptiometry (DEXA)
Lean tissue and fat mass for bilateral lower extremities was assessed at baseline and post-intervention using DEXA scans (GE Lunar, Madison, WI). This allowed for within and between-limb lean tissue comparison at the respective timepoints. The rationale for observing lean tissue changes was to ensure that improved blood flow was not the result of increased lean tissue.
Measures of Cardiovascular Function
At baseline, 2 and 4 weeks, Doppler ultrasound (MicroMaxx Doppler ultrasound, Sonosite, Inc; Bothell, WA) measurements were performed on bilateral femoral arteries for diameter and peak blood flow velocity. With the image frozen on the screen, femoral artery diameter was obtained at peak systole using the R-wave from an electrocardiogram (ECG). Peak blood flow velocity was recorded as the highest speed at which blood flowed through the vessel. All arterial measures for both limbs were taken in duplicate and values averaged for data analysis. The individual performing the second measurement was blinded to limb at the time of assessment. Blood flow was calculated using the equation: blood flow (BF) = ((π) * (femoral artery radius) 2 * (mean blood flow velocity (Vmean)) * (60)).16, 17 Vmean is the average blood flow velocity (Eq 1) of the Doppler waveform for one cardiac cycle.10 The average of the 6 cycles was used for data analysis. Food and drink intake was restricted 30 minutes prior to all ultrasound scans.18 All femoral artery measures were taken 24 hours after SLE to avoid the effects of an acute bout of exercise.16 Vascular conductance 19-21 was calculated using Eq 2.
| (1) |
| (2) |
To perform the ABI test, the participant rested for 10 minutes 22 in a supine position. Using a portable 5Mhz LifeDop hand-held Doppler probe (SummitDoppler, Golden CO) to obtain systolic blood pressure (BP), values were recorded and ABI was calculated. 22 Since the hemiparetic side was an area of interest, ABI was calculated for the affected side (ABIhemi).
Training Intervention
The single limb exercise (SLE) training intervention involved isokinetic (Biodex Medical Systems, Inc., Shirley, NY) extension/flexion using only the hemiparetic limb. Since no data were available in the literature to suggest a SLE protocol as an intervention in individuals post-stroke, we developed this training regimen based on pilot data. Therefore, we had the participants exercise at 150° *sec-1 with 40 repetitions per set. They were instructed to self-progress their exercise training with the goal of completing a total of 40 sets at the end of the intervention. A 30-second rest break in between each set allowed monitoring of heart rate and perceived exertion. Participants exercised 3 times per week for 4 weeks at an intensity of 60% - 70% of maximal heart rate. In an effort to limit compensatory movements to assist the hemiparetic leg, the participant was secured in place using straps for trunk, hip and leg stabilization.
Statistical Analysis
The arithmetic mean and standard error were used for descriptive statistics. At baseline, paired t-tests were used to determine significant differences in vascular function between the hemiparetic and less affected limbs. To elucidate the relationship between the blood flow and lean muscle tissue in the hemiparetic limb, Pearson product moment correlations were calculated using baseline values. Following the intervention, correlations were also performed to assess the relationship between percent change scores for blood flow and lean tissue.
Three different 2 side (hemiparetic; less affected) × 3 time (baseline, T1, post) within subject, repeated measures ANOVA with time as the repeating factor, investigated the effects of SLE on the dependent measures 1) blood flow, 2) arterial diameter and 3) peak blood flow velocity. A significant side *time interaction suggested a statistically significant SLE effect and warranted further post-hoc analysis. For post-hoc analysis, the percent change was calculated from baseline to post for arterial diameter and blood flow velocity. Testing each side, a second set of repeated measures ANOVA determined if the percent change was significantly different from zero. Further, vascular conductance was assessed using paired t-tests to determine significant differences between baseline and post-intervention values.
Because the pattern of variability of ABI and ABIhemi across time, which traditional repeated measures ANOVA does not account for, a linear mixed model23 was used to test statistical significance across time. All statistical analyses were conducted with alpha = 0.05.
Results
Baseline Measures
Baseline values between the hemiparetic and less affected limb were significantly different for resting femoral artery blood flow, diameter and peak blood flow velocity (p < 0.0001). Furthermore, femoral artery blood flow to the hemiparetic limb was 29.57% less compared to the other side. Baseline descriptive data are reported in Table 2.
Table 2.
Effect of SLE on Hemodynamic Outcomes and Body Composition * (n = 12)
| PRE | T1 | POST | |
|---|---|---|---|
| Blood Flow(m*min-1) | |||
| HP | 519.18 ± 47.76† | 627.70 ± 45.95† | 735.72 ± 50.90 |
| NHP | 736.43 ± 59.17 | 719.07 ± 36.38 | 769.29 ± 54.63 |
| Diameter(cm) | |||
| HP | 0.85 ± 0.03† | 0.91 ± 0.04† | 0.93 ± 0.04 |
| NHP | 0.93 ± 0.03 | 0.95 ± 0.03 | 0.95 ± 0.03 |
| Peak Velocity(cm*sec-1) | |||
| HP | 70.14 ± 4.90† | 76.51 ± 4.81 | 81.74 ± 4.38 |
| NHP | 82.15 ± 4.50 | 83.82 ± 4.09 | 84.45 ± 4.79 |
| LE Lean Tissue(g) | |||
| HP | 7380.42 ± 544.85 | N/A | 7317.5 ± 498.21 |
| NHP | 7584.00 ± 567.76 | N/A | 7527.42 ± 519.98 |
| MAP(mmHg) | 69.59 ± 1.97 | N/A | 67.12 ± 2.09 |
| ABI | 0.96 ± 0.05 | N/A | 0.94 ± 0.02 |
| ABIhemi | 0.97 ± 0.04 | N/A | 1.00 ± 0.02 |
Values are expressed as Mean ± SE
denotes significance using paired t-tests between HP and NHP at each time point.
LE = lower extremity; N/A= not assessed
At baseline, DEXA scans were performed to assess lean tissue composition. Lean tissue between the hemiparetic and less affected limb was significantly different at baseline (p = 0.05). A weak, non-significant relationship between lean lower extremity tissue and femoral artery blood flow (r = 0.14, p = 0.665) was found.
Femoral Artery Adaptation to SLE
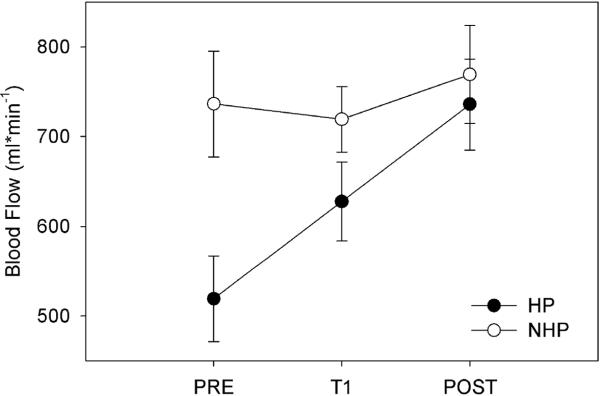
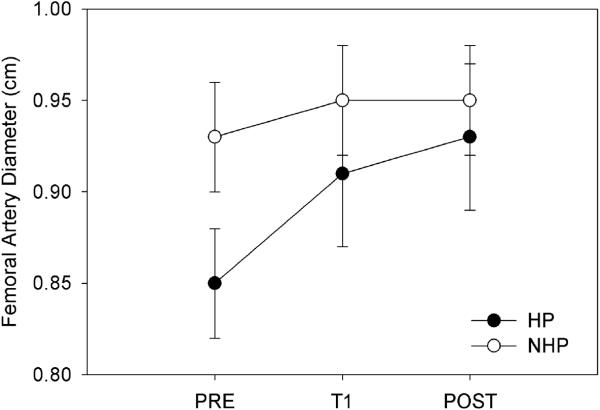
Femoral artery hemodynamics improved in the hemiparetic limb after the 4-week SLE training period while non-significant changes were found in the control limb. Further, no unanticipated or adverse events were reported as a result of the training intervention. Femoral artery blood flow significantly improved after SLE as indicated by an interaction of Side (hemiparetic, less affected) and Time (F(2,22)= 12.12; p < 0.0001, Figure 1). After the training period, a 41.84% increase in blood flow was observed. This resulted in a 4.37% deficit for leg blood flow in the hemiparetic leg when compared to the other side. Furthermore, the significance between the two limbs disappeared after the training period (Table 2). Two-way repeated measures ANOVA with an effect of Side * Time indicated that both femoral artery diameter (F(2,22) =24.76, p < 0.0001; Figure 2) and peak blood flow velocity (F(2,22)= 27.97, p < 0.0001) in the hemiparetic limb significantly improved after SLE. No significant differences were detected in the non-trained limb for femoral artery blood flow (F(2,22) = .905; p= 0.08), diameter (F(2,22) = 0.651, p = 0.53) and peak blood flow velocity (F(2, 22) = 1.28, p = 0.30). Post-hoc analysis suggested a significant improvement in percent change scores for femoral artery diameter (p < 0.001) and peak blood flow velocity (p < 0.001) in the hemiparetic limb. The less affected limb did not demonstrate significant percent change scores for arterial diameter (p = 0.78) or blood flow velocity (p = 0.15). Vascular conductance significantly improved (p< 0.0013) in the trained limb but not the untrained side (p= 0.38).
Figure 1.
Femoral Artery Blood Flow Over the Intervention
*T1 = 2 weeks; Post = 4 weeks
Figure 2.
Femoral Artery Diameter Over the Intervention
*T1 = 2 weeks; Post = 4 weeks
Lean Tissue Composition and the Relationship to Blood Flow
Baseline values for lean tissue mass in the hemiparetic and less affected limb were significantly different (p = 0.05). After the SLE intervention, lean tissue in the hemiparetic leg was not significantly different from baseline (p = 0.56). However, between-limb differences post-intervention approached significance (p = 0.06).
The relationship between the blood flow and lean tissue was also explored after the training intervention. The percent change in blood flow from baseline to post-intervention was not related to lean muscle tissue as evidenced by a weak correlation (r = -0.07; p = 0.83).
Ankle-Brachial Index (ABI)
ABI values were not significantly different after the training intervention (F2,22) = 0.211, p = 0.81. Further analysis of ABIhemi also identified non-significant changes (F2,22) = 0.556, p = 0.58).
Discussion
This study examined the effect of a 4-week single limb exercise (SLE) training protocol on cardiovascular function and femoral artery blood flow in the femoral arteries in people post-stroke. The primary findings were that a SLE training intervention that focused on the hemiparetic leg resulted in structural vascular adaptations, with improved arterial diameter and blood flow to the trained limb.
Femoral Artery Characteristics and Blood Flow Prior to SLE
After stroke, vascular remodeling may occur if the hemiparetic leg has a reduced metabolic demand due to the lack of physical activity or exercise5, 6, 10 As demonstrated in our previous work17 and by others,5, 6 resting femoral artery blood flow, diameter and peak blood flow velocity in the hemiparetic limb is significantly lower than the less affected side. This study supports previous work that lean muscle tissue is significantly lower in the hemiparetic limb when compared to the less affected side.24 Similar to the work by Ivey and colleagues,5 a weak, non-significant relationship was found between resting blood flow and lean muscle tissue. Therefore, the difference in blood flow may, in fact, be due to lower oxygen demand in the hemiparetic limb.10
Effect of SLE Training
Results from this study suggest that after 4-weeks of SLE, vascular adaptations in hemiparetic limb can be improved to reflect values of the control limb. After the exercise period, a 4.37% deficit, which was non-significant remained between the two limbs for femoral artery blood flow. This is not surprising since exercise is a potent stimulus for inducing vascular changes. Miyachi and colleagues,25 reported that healthy adults participating in a 6-week SLE training protocol induced femoral artery remodeling that resulted in a significant increase in the cross-sectional area (CSA) of the artery. No significant changes were found in the non-trained limb. They concluded that exercise-induced blood flow changes during SLE facilitated arterial diameter expansion and may be the mechanistic factor driving vascular adaptations.
In the present study, we report similar vascular adaptations after the SLE training intervention. Femoral artery blood flow and diameter in the hemiparetic limb significantly improved after SLE while the untrained limb demonstrated non-significant changes. The percent change in blood flow from baseline to post-intervention was not related to increased lean muscle tissue. Therefore, vascular remodeling may be related more to metabolic activity25 rather than tissue composition. 5, 20 A peripheral “feedback mechanism”26 known as the flow-diameter relationship 27 may also provide information regarding the vascular adaptations. With increased blood flow, greater sheer stress is placed on the arterial wall. The endothelial cells detect changes in wall tension and undergo structural modification to increase vessel diameter 10, 28
The intrinsic nature of the flow-diameter relationship would support the findings that vascular conductance improved after SLE. Vascular conductance is the product of mean arterial pressure and blood flow. The individuals in this study did not demonstrate a significant improvement in mean arterial pressure from baseline to post-training but blood flow significantly increased. Therefore, the improvement in vascular conductance likely resulted from peripheral rather than central adaptations.25 Using the less affected limb as the control limb, we were able to compare peripheral vascular changes that occurred over time in the hemiparetic limb as a result of the training intervention. Although a control group consisting of bilateral exercise would strengthen the study design, this work was an initial step to identify whether or not vascular changes could occur in the hemiparetic limb after an intense training period. Individuals engaging in stroke rehabilitation may benefit from encouraged use such as SLE to minimize peripheral vascular alterations that appear to be observed in people post-stroke.
Despite the vascular adaptations that occurred for femoral artery blood flow and conductance, ABI values post-training did not support improved endothelial function as suggested by Andreozzi and colleagues.29 In the present study, ABI values less than 0.90 were reported for only 4 individuals. Since 67% of the individuals were already considered in the normal range for ABI,14, 22 a ceiling effect is likely.
Summary
The findings from this study support the hypotheses that a 4-week SLE training program that encourages use of the hemiparetic limb improves femoral artery blood flow, diameter, peak velocity and vascular conductance. These peripheral vascular changes result from an exercise-induced stimulus that directly affects the flow-diameter relationship. Simply, if the arterial wall is chronically exposed to increased blood flow (i.e. exercise) then the diameter expands to accommodate a larger volume of flow. However, systemic vascular function using ABI did not significantly improve after the intervention. Future research is needed to examine the role of exercise in the peripheral mechanisms (ie nitric oxide dependent vasodilation) behind vascular remodeling in the hemiparetic limb after stroke.
Acknowledgements and Funding Page
We thank Sonosite, Inc for the Doppler ultrasound equipment loan and the study participants for their dedication and hard work.
This research project was supported by Grant Number M01 RR023940 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
References
- 1.Schmidt-Trucksass A, Grathwohl D, Schmid A, Boragk R, Upmeier C, Keul J, Huonker M. Structural, functional, and hemodynamic changes of the common carotid artery with age in male subjects. Arterioscler Thromb Vasc Biol. 1999;19:1091–1097. doi: 10.1161/01.atv.19.4.1091. [DOI] [PubMed] [Google Scholar]
- 2.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: Relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- 3.Maiorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001;38:860–866. doi: 10.1016/s0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- 4.De Groot PC, Van Kuppevelt DH, Pons C, Snoek G, Van Der Woude LH, Hopman MT. Time course of arterial vascular adaptations to inactivity and paralyses in humans. Med Sci Sports Exerc. 2003;35:1977–1985. doi: 10.1249/01.MSS.0000099088.21547.67. [DOI] [PubMed] [Google Scholar]
- 5.Ivey FM, Gardner AW, Dobrovolny CL, Macko RF. Unilateral impairment of leg blood flow in chronic stroke patients. Cerebrovasc Dis. 2004;18:283–289. doi: 10.1159/000080353. [DOI] [PubMed] [Google Scholar]
- 6.Landin S, Hagenfeldt L, Saltin B, Wahren J. Muscle metabolism during exercise in hemiparetic patients. Clin Sci Mol Med. 1977;53:257–269. doi: 10.1042/cs0530257. [DOI] [PubMed] [Google Scholar]
- 7.Macko RF, DeSouza CA, Tretter LD, Silver KH, Smith GV, Anderson PA, Tomoyasu N, Gorman P, Dengel DR. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. A preliminary report. Stroke. 1997;28:326–330. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 8.Mackay-Lyons MJ, Makrides L. Exercise capacity early after stroke. Arch Phys Med Rehabil. 2002;83:1697–1702. doi: 10.1053/apmr.2002.36395. [DOI] [PubMed] [Google Scholar]
- 9.Watts K, Beye P, Siafarikas A, Davis EA, Jones TW, O'Driscoll G, Green DJ. Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol. 2004;43:1823–1827. doi: 10.1016/j.jacc.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Gerrits HL, de Haan A, Sargeant AJ, van Langen H, Hopman MT. Peripheral vascular changes after electrically stimulated cycle training in people with spinal cord injury. Arch Phys Med Rehabil. 2001;82:832–839. doi: 10.1053/apmr.2001.23305. [DOI] [PubMed] [Google Scholar]
- 11.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 12.Sibley KM, Tang A, Brooks D, Brown DA, McIlroy WE. Feasibility of adapted aerobic cycle ergometry tasks to encourage paretic limb use after stroke: A case series. J Neurol Phys Ther. 2008;32:80–87. doi: 10.1097/NPT.0b013e318176b466. [DOI] [PubMed] [Google Scholar]
- 13.Chen HY, Chen SC, Chen JJ, Fu LL, Wang YL. Kinesiological and kinematical analysis for stroke subjects with asymmetrical cycling movement patterns. J Electromyogr Kinesiol. 2005;15:587–595. doi: 10.1016/j.jelekin.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: The strong heart study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 15.Daly JJ, Roenigk K, Holcomb J, Rogers JM, Butler K, Gansen J, McCabe J, Fredrickson E, Marsolais EB, Ruff RL. A randomized controlled trial of functional neuromuscular stimulation in chronic stroke subjects. Stroke. 2006;37:172–178. doi: 10.1161/01.STR.0000195129.95220.77. [DOI] [PubMed] [Google Scholar]
- 16.Anton MM, Cortez-Cooper MY, DeVan AE, Neidre DB, Cook JN, Tanaka H. Resistance training increases basal limb blood flow and vascular conductance in aging humans. J Appl Physiol. 2006;101:1351–1355. doi: 10.1152/japplphysiol.00497.2006. [DOI] [PubMed] [Google Scholar]
- 17.Billinger SAaK. P.M. Use of doppler ultrasound to assess femoral artery adaptations in the hemiparetic limb in chronic stroke. Cerebrovascular Diseases. doi: 10.1159/000214218. in press. [DOI] [PubMed] [Google Scholar]
- 18.Peiffer JC, Abbiss CR, Laursen PB, NoSaka K. Reliability of femoral blood vessel diameter measurement by b-mode ultrasonography. J Exerc Physiol (online) 2007;10:10–16. [Google Scholar]
- 19.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Herr MD, Proctor DN. Sex differences in leg vasodilation during graded knee extensor exercise in young adults. J Appl Physiol. 2007;103:1583–1591. doi: 10.1152/japplphysiol.00662.2007. [DOI] [PubMed] [Google Scholar]
- 20.Radegran G, Saltin B. Human femoral artery diameter in relation to knee extensor muscle mass, peak blood flow, and oxygen uptake. Am J Physiol Heart Circ Physiol. 2000;278:H162–167. doi: 10.1152/ajpheart.2000.278.1.H162. [DOI] [PubMed] [Google Scholar]
- 21.Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol. 2004;556:1001–1011. doi: 10.1113/jphysiol.2003.059717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDermott MM, Ferrucci L, Simonsick EM, Balfour J, Fried L, Ling S, Gibson D, Guralnik JM. The ankle brachial index and change in lower extremity functioning over time: The women's health and aging study. J Am Geriatr Soc. 2002;50:238–246. doi: 10.1046/j.1532-5415.2002.50054.x. [DOI] [PubMed] [Google Scholar]
- 23.Breslow NEaC. D. G. Approximate inference in generalized linear mixed model. Journal of the American Statistical Association. 1993;88:9–25. [Google Scholar]
- 24.De Deyne PG, Hafer-Macko CE, Ivey FM, Ryan AS, Macko RF. Muscle molecular phenotype after stroke is associated with gait speed. Muscle Nerve. 2004;30:209–215. doi: 10.1002/mus.20085. [DOI] [PubMed] [Google Scholar]
- 25.Miyachi M, Tanaka H, Yamamoto K, Yoshioka A, Takahashi K, Onodera S. Effects of one-legged endurance training on femoral arterial and venous size in healthy humans. J Appl Physiol. 2001;90:2439–2444. doi: 10.1152/jappl.2001.90.6.2439. [DOI] [PubMed] [Google Scholar]
- 26.Kamiya A, Ando J, Shibata M, Masuda H. Roles of fluid shear stress in physiological regulation of vascular structure and function. Biorheology. 1988;25:271–278. doi: 10.3233/bir-1988-251-236. [DOI] [PubMed] [Google Scholar]
- 27.Ono O, Ando J, Kamiya A, Kuboki Y, Yasuda H. Flow effects on cultured vascular endothelial and smooth muscle cell functions. Cell Struct Funct. 1991;16:365–374. doi: 10.1247/csf.16.365. [DOI] [PubMed] [Google Scholar]
- 28.Prior BM, Lloyd PG, Yang HT, Terjung RL. Exercise-induced vascular remodeling. Exerc Sport Sci Rev. 2003;31:26–33. doi: 10.1097/00003677-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Andreozzi GM, Leone A, Laudani R, Deinite G, Martini R. Acute impairment of the endothelial function by maximal treadmill exercise in patients with intermittent claudication, and its improvement after supervised physical training. Int Angiol. 2007;26:12–17. [PubMed] [Google Scholar]