Abstract
Socially appropriate behavior requires the concurrent inhibition of actions that are inappropriate in the context. This self‐regulatory function requires an interaction of inhibitory and emotional processes that recruits brain regions beyond those engaged by either processes alone. In this study, we isolated brain activity associated with response inhibition and emotional processing in 24 healthy adults using event‐related functional magnetic resonance imaging (fMRI) and a go/no‐go task that independently manipulated the context preceding no‐go trials (ie, number of go trials) and the valence (ie, happy, sad, and neutral) of the face stimuli used as trial cues. Parallel quadratic trends were seen in correct inhibitions on no‐go trials preceded by increasing numbers of go trials and associated activation for correct no‐go trials in inferior frontal gyrus pars opercularis, pars triangularis, and pars orbitalis, temporoparietal junction, superior parietal lobule, and temporal sensory association cortices. Conversely, the comparison of happy versus neutral faces and sad versus neutral faces revealed valence‐dependent activation in the amygdala, anterior insula cortex, and posterior midcingulate cortex. Further, an interaction between inhibition and emotion was seen in valence‐dependent variations in the quadratic trend in no‐go activation in the right inferior frontal gyrus and left posterior insula cortex. These results suggest that the inhibition of response to emotional cues involves the interaction of partly dissociable limbic and frontoparietal networks that encode emotional cues and use these cues to exert inhibitory control over the motor, attention, and sensory functions needed to perform the task, respectively. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: fMRI, motor inhibition, emotion, response context, prefrontal cortex, amygdala
INTRODUCTION
Socially appropriate behavior requires the integration of information from the context to select the proper action and inhibit competing responses that are inappropriate in the context. The inhibition of inappropriate responses is a fundamental self‐regulatory process that requires the encoding of behavioral cues from the context to guide decisions about which actions to inhibit [Haberman and Whitney, 2007]. In social contexts, facial expressions and body gestures from others convey emotional cues that are important for this decision process [Otta et al., 1994]. However, these emotional cues are not simply encoded, but rather influence the inhibition of behavioral responses [Hare et al., 2005; Maxwell et al., 2005; Schulz et al., 2007]. Thus, responses elicited by facial expressions of happiness that are associated with positive affect [Otta et al., 1994] and approach behavior [Johansson and Ronnberg, 1996], are more difficult to inhibit than responses to nonemotional faces [Hare et al., 2005; Schulz et al., 2007]. This emotional biasing of response inhibition has important implications for adaptive goal‐directed social behavior [Mathews and McLeod, 1994].
The influence of emotional stimuli on the neural substrates for response inhibition is still uncertain. The inhibition of responses to both emotional and nonemotional cues is mediated by a brain network distributed across multiple cortical and subcortical neural ensembles [Buchsbaum et al., 2005; Elliott et al., 2000; Garavan et al., 2006; Shafritz et al., 2006; Simmonds et al., 2008], including basal ganglia‐cortical loops, premotor and supplementary motor areas (SMA) involved in motor programming [Hoshi and Tanji, 2004], and preSMA and cingulate motor areas implicated in response selection [Mostofsky and Simmonds, 2008; Picard and Strick, 2001], as well as the hand areas of the pars opercularis (area 44) and pars triangularis (area 45) of the right inferior frontal gyrus [Rizzolatti et al., 2002]. However, this inhibition‐related inferior frontal activity extended rostroventrally to the pars orbitalis (area 47) when explicitly inhibiting responses to emotional cues [Elliott et al., 2000; Hare et al., 2005] and was centered in the orbitofrontal cortex when the emotional stimuli were incidental to the inhibitory task [Goldstein et al., 2007]. These pars orbitalis and orbitofrontal activations may represent a distinct inhibitory mechanism specialized for emotional stimuli [Mostofsky et al., 2003] or the emotional modulation of activity in the neural substrate for response inhibition [Davidson, 2003], such as the behavioral coding of emotional cues [Sakagami and Pan, 2007].
There is growing interest in the mechanisms by which the limbic substrates for emotional perception influence the inferior frontal inhibitory circuits [Phelps and LeDoux, 2005]. Most of this interest has focused on the amygdala that receives extensive sensory input [Price, 2003], and in turn, has bidirectional functional connections with the prefrontal cortex [Hampton et al., 2007; Herwig et al., 2007]. The amygdala is believed to encode the emotional value of stimuli [Dolan, 2007] and is consistently engaged by affective stimuli [Costafreda et al., 2008; Phan et al., 2002]. Nevertheless, amygdala activity has proven difficult to detect during emotional response inhibition [Elliott et al., 2000; Hare et al., 2005; Shafritz et al., 2006] and may capture the interaction of stimulus processing and response inhibition [Goldstein et al., 2007].
Neural activation related to the inhibition of responses to emotional cues has been more commonly described in the anterior cingulate cortex (ACC) and anterior insula cortex [Elliott et al., 2000; Hare et al., 2005; Shafritz et al., 2006]. These limbic structures may form cortical entry points through which cognitive, sensory, and emotional inputs influence motor functions [Augustine, 1996; Paus, 2001]. Accordingly, the anterior insula was activated during response inhibition, specifically, by emotional stimuli [Elliott et al., 2000; Shafritz et al., 2006], particularly the negative stimuli [Goldstein et al., 2007]. In contrast, activation in the ACC during emotional response inhibition has been linked to motor control [Elliott et al., 2000; Shafritz et al., 2006], emotional processing [Elliott et al., 2000; Hare et al., 2005], and the interaction of these processes [Goldstein et al., 2007; Shafritz et al., 2006] and has varied in location from subgenual [Elliott et al., 2000; Shafritz et al., 2006] to dorsal regions of the ACC [Goldstein et al., 2007; Hare et al., 2005; Shafritz et al., 2006]. Disentangling the functional contributions of these regions to emotional response inhibition will require activation paradigms thatindependently manipulate emotional and inhibitory processes.
The current study isolated brain activity associated with response inhibition and emotional processing in 24 healthy adults using event‐related functional magnetic resonance imaging (fMRI) and a go/no‐go paradigm that independently manipulated the context preceding no‐go trials and the valence of the face stimuli used as trial cues. Happy, sad, and neutral facial expressions were used as trial cues in the go/no‐go task to identify neural activity that varied as a function of emotional valence regardless of trial type. In addition, a parametric manipulation of the number of go trials preceding no‐go trials was incorporated into the task design to isolate activation specific to varying levels of response inhibition irrespective of the emotional valence [Durston et al., 2002]. We predicted that valence‐dependent activation would be seen in amygdala, anterior insula cortex, and ACC, although context‐dependent activation would be found in a frontoparietal network dedicated to response inhibition, including right inferior frontal gyrus pars opercularis, pars triangularis, and pars orbitalis irrespective of stimulus characteristics.
METHODS
Participants
Participants were 24 healthy right‐handed adults (16 males, aged 18–35 years) with normal or corrected‐to‐normal vision. All subjects were screened for current or past psychiatric, neurological, or systemic medical illness and completed the Conners Adult ADHD Rating Scale‐Self‐Report: Long Version (CAARS) [Conners, 1997] and Beck Depression Inventory‐II (BDI‐II) [Steer et al., 1999]. A total score ≥6 on the BDI‐II and a T‐score of 1 SD above age and gender means (ie, >60) on the CAARS total ADHD symptoms index were used to screen for clinically significant mood disturbances and attention problems that might impact on emotional processing and/or response inhibition. Mean BDI‐II total score was 2.4 ± 2.9 (Mean ± SD) and mean CAARS total ADHD symptoms T‐score was 41.9 ± 9.4. The sample was 37.5% African‐American, 29.2% Caucasian, 20.8% Hispanic, and 12.5% Asian. Data from one additional subject had to be discarded due to technical problems with the scanner. All subjects provided written informed consent for participation. The study was approved by the Institutional Review Boards of Queens College and The Mount Sinai School of Medicine.
Emotional Go/No‐Go Task
The emotional go/no‐go task and a training program were compiled and were run using E‐Prime software (Psychology Software Tools, Pittsburgh, PA). All subjects initially completed a training session in a quiet testing room to check simple face perception and to familiarize subjects with the emotional go/no‐go task. The training session started with an untimed emotional perception task in which all the 54 face stimuli used in the go/no‐go task were randomly presented one at a time in the center of the screen. Subjects had to make a key press to indicate face valence. Face stimuli consisted of digitized gray‐scaled happy, sad, and neutral facial expressions with closed mouths from 18 individuals (9 female and 9 male) selected from the MacBrain Face Stimulus Set available at http://www.macbrain.org. Six models (3 female and 3 male) were used from each of the following races: African American, Asian, and Caucasian. The images were normalized for size and luminance, morphed to exclude hair, and cropped into a black square, which was presented against a black background. The subjects all demonstrated 90% accuracy on the emotional perception task. Then, the subjects performed one block randomly selected from the emotional go/no‐go task described later, to familiarize themselves with the response demands and speeded nature of the task.
The emotional go/no‐go task described in Schulz et al. [2007] was modified in two ways for the current study. First, neutral facial expressions were added to the happy and sad faces used as go and no‐go trial cues, resulting in six blocks with the following trials: (1) happy go/sad no‐go; (2) sad go/neutral no‐go; (3) neutral go/happy no‐go; (4) happy go/neutral no‐go; (5) sad go/happy no‐go; and (6) neutral go/sad no‐go. Second, 30‐second periods of fixation were added to the beginning and end of each block to establish a baseline of neural activation to model‐out low‐signal drift. Otherwise, the original structure, timing parameters, trial order, and response demands were maintained in the current task, as described later.
The emotional go/no‐go task required subjects to monitor six series of stimuli presented individually in the center of the screen and respond as rapidly as possible by pressing the “go” cues and withholding responses to “no‐go” cues. The task consisted of six 252‐sec blocks. Each block contained 72 (75%) go cues and 24 (25%) no‐go cues, resulting in a total of 432 go cues and 144 no‐go cues in the task. Trial order was determined by counterbalancing across all conditions in the task (eg, trial type, facial expression, face ethnicity, face gender, face) to ensure that each trial type followed every other trial type equally often. This counterbalancing also embedded the parametric manipulation of the number of go cues preceding no‐go cues (ie, from 0 to 11 go cues) in the trial order. Stimuli were presented in the center of the screen for 500 msec each. The interstimulus interval was pseudorandomized from 1,250 to 1,750 msec (mean per block = 1,500 msec) to discourage anticipatory responses. Instructions were displayed on the computer screen at the beginning of each block. Participants responded with the right index finger using the BrainLogics fiber optic button system (Psychology Software Tools, Pittsburgh, PA). Responses were recorded on a desktop computer and provided measures of reaction time and accuracy.
Image Acquisition
All subjects were scanned on the same 3.0‐Tesla Siemens Allegra (Siemens Medical Systems, Erlangen, Germany) head‐dedicated MRI scanner using a high‐performance head gradient system that was designed especially for functional brain imaging. Participants were fitted with headphones and their heads were stabilized with firm foam padding. Stimuli were projected via an SVGA projector system onto a rear‐projection screen mounted at the head of the magnet bore. Subjects viewed the stimuli through a mirror on the head coil positioned above their eyes.
Scan sessions began with shimming and sagittal localization. Next, a high‐resolution T2‐weighted anatomical volume of the brain was acquired with a turbo spin‐echo (TSE) pulse sequence with TR = 4,500 msec, TE = 99 msec, flip angle = 170°, FOV = 21 cm, and a matrix of 512 × 336. Forty‐two axial slices were acquired at a thickness of 3 mm with a skip of 1 mm and an in‐plane resolution of 0.41 × 0.41 mm2. Functional T2*‐weighted images depicting the blood oxygenation level‐dependent (BOLD) signal were then acquired at the same 42 slice locations using gradient‐echo echo‐planar images with TR = 3,000 msec, TE = 27 msec, flip angle = 85°, FOV = 21 cm, and an acquisition matrix of 64 × 64. Each functional image comprised a brain volume of 42 axial slices with a thickness of 3 mm, a skip of 1 mm, and an in‐plane resolution of 3.75 × 3.75 mm2. The TR was a trade‐off for whole‐brain coverage with thinner slices that minimized distortions and increased sensitivity in regions of interest (eg, amygdala). All images were acquired with slices positioned parallel to the anterior AC‐PC commissure line. Each participant completed six runs of 252 sec each, yielding 84 time points per participant.
Data Analysis
Percent correct inhibitions on no‐go trials served as the primary measure of response inhibition on the emotional go/no‐go task. Percent correct inhibitions were calculated for: (1) all face trials (regardless of expression) and separately for trials with happy, sad, and neutral cues; and (2) each level of the parametric manipulation (ie, from 0 to 11 preceding go trials). The 12 levels of the parametric manipulation were then collapsed into the four levels used by Schulz et al. [2007], that is, correct inhibitions on no‐go trials following: (1) 0 go trials; (2) 1 or 2 go trials; (3) 3 or 4 go trials; and (4) 5 or more go trials. Reaction time (RT) and percent correct responses on go trials and signal detection measures of perceptual sensitivity (d′) and response biases (β) were also calculated. The effects of emotional valence on behavioral measures were tested with repeated measures of analyses of variance (ANOVA) with the three face emotion valences as the within‐subject factors. Separate repeated measures of ANOVA with orthogonal contrasts were used to test for linear, quadratic, and cubic trends in percent correct inhibitions on no‐go trials following increasing numbers of go trials. Statistical significance was set at the 0.05 level for these analyses. All probabilities were based on two‐tailed tests.
Event‐related analyses of the fMRI data were conducted using statistical parametric mapping (SPM2; Wellcome Department of Imaging Neuroscience, London, UK). Preprocessing of the six functional time series was performed individually for each subject. The functional scans were slice scan time corrected, realigned to the first volume to correct for inter‐scan motion, coregistered to the T2 image, normalized to a standard template (Montreal Neurological Institute), and spatially smoothed with an 8 × 8 × 8 mm3 full width at half‐maximum (FWHM) Gaussian kernel.
First‐level individual analyses were performed twice for each subject to separately test the effects of face emotion and preceding context on response inhibition. Separate general linear models (GLM) were conducted to determine the relationship between observed event‐related BOLD signals and regressors that represented expected neural responses to events for emotion and preceding context. Regressors were created by convolving a train of delta functions that represented the individual trial events with the default SPM basis function that consisted of a synthetic hemodynamic response function, composed of two gamma functions and their derivatives [Friston et al., 1998]. There were four inhibition‐related regressors in the first GLM: (1) correct no‐go; (2) correct go; (3) incorrect no‐go; and (4) incorrect go events. Six preceding context‐related regressors were created in the second GLM: correct no‐go events after (1) 0 go, (2) 1–2 go, (3) 3–4 go, and (4) ≥ 5 go events; (5) incorrect no‐go events; and (6) all go events. The same regressors were included for all six blocks of the task, the combination of which effectively collapsed the effects of interest over face valence. At the same time, the variation of face emotion across the six blocks, as described above, enabled the comparison of face emotion between blocks. Finally, the interaction of face emotion and preceding context on response inhibition was tested by comparing the six context‐related regressors between blocks with different face emotions. The six parameters (x, y, and z translations and rotations) generated during motion correction were entered as covariates of no interest in the GLM [Johnstone et al., 2006].
The general effect of emotional faces on response inhibition was tested in the first GLM by applying appropriate linear contrasts to the parameter estimates for correct no‐go events minus correct go events collapsed over face valence. The specific neural effects of face valence were also tested in the first GLM by separately contrasting happy versus neutral faces and sad versus neutral faces, both collapsed over trial type. These analyses resulted in three contrast maps for each participant. Testing of the neural effect of parametrically manipulating preceding context on response inhibition was informed by the behavioral results. The specific effects of preceding context were tested in the second GLM by applying either linear, quadratic, or cubic contrasts to the parameter estimates for the four parametric levels of correct no‐go events, resulting in a single contrast map for each subject. The interactive effects of face emotion and preceding context on response inhibition were tested in the second GLM by separately contrasting the four parametric levels of correct no‐go events in blocks with happy versus neutral faces and in blocks with sad versus neutral faces, resulting in two contrast maps for each subject.
The images of contrast estimates for all participants were entered into second‐level group analyses conducted with separate random‐effects statistical models that account for intersubject variability and permit population‐based inferences. The resultant voxel‐wise statistical maps were thresholded for significance using a cluster‐size algorithm that protects against false‐positive results in spatially extended continuous data [Hayasaka et al., 2004]. The height (intensity) threshold of each activated voxel was set at an uncorrected P value of 0.01. A Monte Carlo simulation of the brain volume in the current study that assumed an individual voxel type I error of P < 0.01 established that a cluster extent of 100 contiguous resampled voxels (2 × 2 × 2 mm3) was necessary to correct for multiple voxel comparisons at P < 0.05 [Slotnick and Schacter, 2004]. Coordinates of activation were converted to Talairach and Tournoux [1988] coordinates using a nonlinear transformation (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html) to designate the Brodmann area. Cytoarchitectural areas in the cingulate gyrus were designated according to the conventions of Vogt et al. [1995].
RESULTS
Behavioral Results
Performance measures for happy, sad, and neutral face trials on the emotional go/no‐go task are presented in Table I. There was a trend toward an effect of face emotional valence on correct inhibitions on no‐go trials, F (2, 46) = 2.73, P = 0.08, which accounted for a limited amount of the variance in performance, η2 = 0.11. In contrast, there was a significant effect of face emotion on percent correct responses on go trials, F (2, 46) = 3.28, P < 0.05. Post hoc comparisons using Least Significant Difference tests revealed significantly more correct responses on neutral face go trials than either happy face trials, P < 0.05, or sad face trials, P < 0.05, which did not differ from each other, P > 0.10. Face emotional valence had no significant effects on RT and there was no difference in perceptual sensitivity (d') or bias (β) for happy, sad, or neutral faces, all P > 0.10.
Table I.
Behavioral measures of performance on the emotional go/no‐go task
| Measure | Happy faces | Sad faces | Neutral faces |
|---|---|---|---|
| Correct inhibitions (%) | 89.3 ± 0.2 | 89.1 ± 0.2 | 82.7 ± 0.4 |
| Correct responses (%) | 85.7 ± 0.4 | 88.0 ± 0.3 | 93.8 ± 0.2 |
| Reaction time (ms) | 486.0 ± 20.2 | 497.0 ± 18.2 | 505.0 ± 21.6 |
| Perceptual sensitivity (d′) | 3.2 ± 0.2 | 2.9 ± 0.8 | 3.1 ± 0.2 |
| Criterion (β) | 0.7 ± 0.1 | 1.1 ± 0.2 | 0.7 ± 0.2 |
Values presented as Mean ± SEM.
The effect of parametrically manipulating the number of preceding go trials on percent of correct inhibitions on no‐go trials is shown in Figure 1. There was a significant quadratic trend in percent correct inhibitions on no‐go trials following increasing numbers of go trials, F (1, 23) = 14.11, P = 0.001. Post hoc comparisons using Least Significant Difference tests revealed that the quadratic trend was due to significantly fewer correct inhibitions following: (1) 0 go trials than 1–2 go trials, P < 0.05; and (2) 5 or more go trials than either 1 or 2 go trials, P < 0.05, or 3–4 go trials, P < 0.05.
Figure 1.
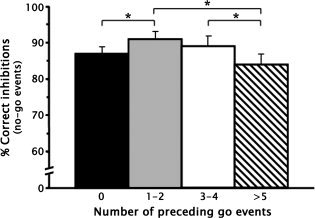
The parametric manipulation of the context preceding response inhibition. There was a significant quadratic trend in the percent correct inhibitions on no‐go trials after 0, 1–2, 3–4, and 5 or more go events, F (1, 23) = 14.11, P = 0.001. Error bars denote the standard error of the mean. *P < 0.05.
fMRI Results
Inhibition of responses to emotional faces
The contrast of correct no‐go events minus correct go events collapsed over face valence identified a distributed fronto‐temporo‐limbic network that is depicted in Figure 2 and detailed in Table II. Specifically, prominent BOLD signal increases were seen in regions associated with response inhibition, including right pars opercularis (area 44) and bilateral pars triangularis (area 45) of the inferior frontal gyrus, with both extending inferomedially to anterior insula cortex, as well as in right anterior midcingulate cortex (MCC; area 24c′/32′), which has been more closely linked to response selection. Another cluster of activation was centered ∼2 mm lateral to the anterior extent of the left amygdala. Robust BOLD signal increases were also seen along the intraparietal sulcus of right superior parietal lobule (area 7) and bilaterally in temporo‐occipital association cortices, with separate peaks in right middle temporal (area 37) and inferior occipital gyri (area 19) and left fusiform face area (area 19) and inferior temporal gyrus (area 37).
Figure 2.
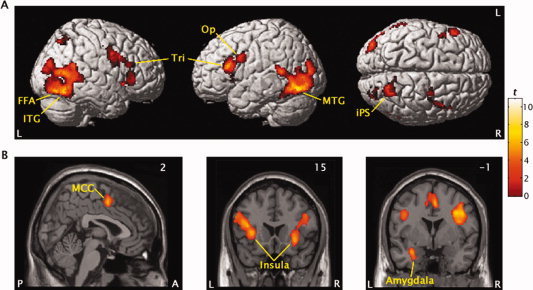
Regional activation for the inhibition of responses to emotional faces collapsed over valence shown on left, right, and superior brain surfaces (upper panels) and on coronal and sagital sections (lower panels). The pars opercularis (Op) and pars triangularis (Tri) of the inferior frontal gyrus, inferior (ITG) and middle temporal gyrus (MTG), fusiform face area (FFA), superior parietal lobule along the intraparietal sulcus (iPS), insula, amygdala, and midcingulate gyrus (MCC) showed greater signal increase in the correct no‐go versus correct go events contrast. Values in corner of sections refer to Talairach coordinates.
Table II.
Regional activation for the inhibition of responses to emotional faces collapsed over valence
| Region | Side | BA | Talairach coordinates | Cluster size (mm3) | t | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| IFG pars triangularis | R | 45 | 40 | 30 | 8 | 304 | 4.75 |
| IFG pars triangularis | L | 45 | −40 | 20 | 12 | 1,458a | 7.12 |
| Anterior insula cortex | L | – | −34 | 18 | 3 | 5.87 | |
| IFG pars opercularis | R | 44 | 42 | 7 | 29 | 2,062a | 8.29 |
| Anterior insula cortex | R | – | 30 | 15 | −4 | 5.53 | |
| Anterior midcingulate cortex | R | 24c′/32′ | 2 | 4 | 44 | 730 | 5.68 |
| Intraparietal sulcus area | R | 7 | 24 | −60 | 47 | 942 | 9.32 |
| Middle temporal gyrus | R | 37 | 46 | −61 | 10 | 5,810a | 7.92 |
| Inferior occipital gyrus | R | 19 | 38 | −82 | −4 | 6.26 | |
| Fusiform face area | L | 19 | −40 | −64 | −7 | 4,632a | 10.91 |
| Inferior temporal gyrus | L | 37 | −46 | −55 | −7 | 8.21 | |
| Amygdala | L | – | −32 | −1 | −18 | 238 | 5.27 |
The volume consisted of two separate peaks.
BA, Brodmann area; IFG, inferior frontal gyrus; L, left; R, right.
Parametric manipulation of the context preceding response inhibition
The neural effects of parametrically manipulating the context preceding response inhibition were tested by applying appropriate quadratic contrasts (ie, 1, −1, −1, 1) to the parameter estimates for the four parametric levels of correct no‐go events collapsed across emotional valence. This contrast isolated a predominantly right‐lateralized fronto‐temporo‐parietal network that demonstrated a quadratic trend in BOLD signal across the four parametric levels, as shown in Figure 3 and listed in Table III. As predicted, this network not only included the pars opercularis (area 44) and pars triangularis (area 45) of the right inferior frontal gyrus but also comprised bilateral pars orbitalis (area 47), as well as parietal attention control regions along the right temporoparietal junction (TPJ; area 40) and in right superior parietal lobule (area 7). The quadratic trends in BOLD signal increases in bilateral pars orbitalis are illustrated in Figure 3 B. Most surprisingly, quadratic trends in BOLD signal across the four no‐go levels were also seen in temporal sensory association cortices, specifically along the inferior bank of the left anterior sylvan sulcus (area 22) and bilaterally in the inferior temporal gyrus (area 37).
Figure 3.
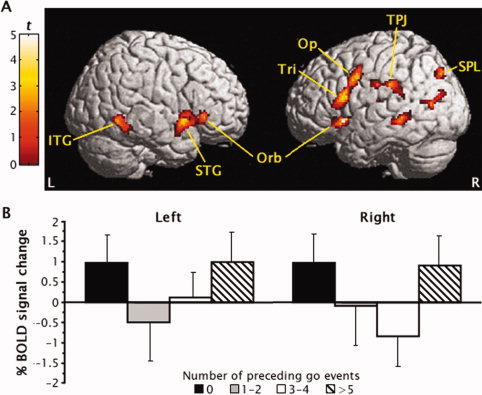
(A) Regional activation generated by the parametric manipulation of the context preceding response inhibition shown on right and left brain surfaces. The pars opercularis (Op), pars triangularis (Tri), and pars orbitalis (Orb) of the inferior frontal gyrus, inferior (ITG) and superior temporal gyri (STG), temporoparietal junction (TPJ) and intraparietal sulcus area (SPL) demonstrated quadratic trends in activation for correct no‐go events after 0, 1 to 2, 3–4, and 5 or more go events. (B) The quadratic trends in BOLD signal increases for no‐go events across the four parametric levels are shown for left and right inferior frontal gyrus pars orbitalis. Error bars denote the standard error of the mean.
Table III.
Regional activation generated by the parametric manipulation of the context preceding response inhibition
| Region | Side | BA | Talairach coordinates | Cluster size (mm3) | t | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| IFG pars orbitalis | R | 47 | 38 | 23 | −10 | 524 | 4.28 |
| IFG pars orbitalis | L | 47 | −42 | 25 | −5 | 216 | 3.51 |
| IFG pars triangularis | R | 45 | 48 | 20 | 14 | 1,438a | 4.30 |
| IFG pars opercularis | R | 44 | 50 | 9 | 33 | 4.97 | |
| Temporoparietal junction | R | 40 | 53 | −26 | 29 | 452 | 3.19 |
| Superior parietal lobule | R | 7 | 28 | −74 | 44 | 310 | 3.75 |
| Superior temporal gyrus | L | 22 | −53 | 9 | −9 | 604 | 4.17 |
| Inferior temporal gyrus | L | 37 | −51 | −47 | −8 | 478 | 3.84 |
The volume consisted of two separate peaks.
BA, Brodmann area; IFG, inferior frontal gyrus; L, left; R, right.
Manipulation of emotional face expression valence
Limbic and paralimbic regions identified by separately contrasting happy and sad face events to neutral face events collapsed over trial types are shown in Figure 4 and listed in Table IV. There was a lateralization of activation for emotional face events in anterior insula cortex; significant BOLD signal increases were predominantly left lateralized for happy faces and right lateralized for sad faces. Happy faces also generated a cluster of activation in left posterior MCC (area 32′) about 15–20 mm dorsal to the MCC activation described earlier for the correct no‐go versus go events contrast. In contrast, sad faces activated a basolateral region of the left amygdala dorsomedial to the activation seen in the no‐go minus go events contrast. Figure4 B shows that all four of these regions also displayed a nonsignificant activation for emotional faces of the opposite valence. Post hoc analyses revealed no differences in these BOLD signal increases across trial types (ie, no‐go vs. go events).
Figure 4.
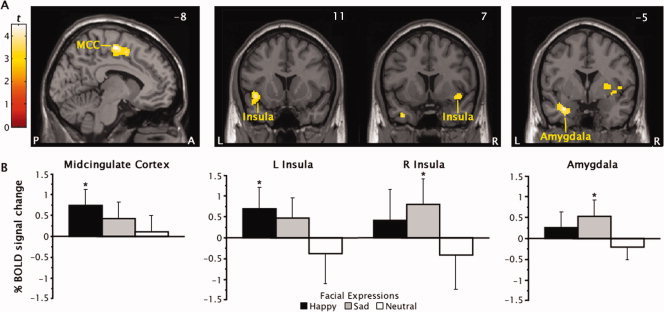
(A) Regional activation produced by the manipulation of emotional face expression valence shown on sagittal and coronal sections. The posterior midcingulate cortex (MCC), anterior insula cortex, and amygdala showed greater signal increases in either the happy face minus neutral face or sad face minus neutral face contrasts. Values in corner of sections refer to Talairach coordinates. (B) Percent change in blood oxygen level‐dependent (BOLD) signal plotted separately for happy, sad, and neutral face events. Error bars denote the standard error of the mean. *Different from neutral faces, P < 0.01.
Table IV.
Regional activation produced by the manipulation of emotional face expression valence
| Contrast and region | Side | BA | Talairach coordinates | Cluster size (mm3) | t | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Happy vs. Neutral | |||||||
| Posterior midcingulate cortex | L | 32′ | −8 | −11 | 52 | 926 | 4.24 |
| Anterior insula cortex | L | – | −43 | 11 | −7 | 302 | 3.72 |
| Sad vs. Neutral | |||||||
| Anterior insula cortex | R | – | 40 | 7 | −9 | 760 | 4.52 |
| Amygdala | L | – | −26 | −5 | −22 | 350 | 4.32 |
BA, Brodmann area; IFG, inferior frontal gyrus; L, left; R, right
Interaction of emotional face valence and context preceding response inhibition
Contrasting quadratic trends in the BOLD signal across the four parametric levels separately for sad and happy no‐go events versus neutral no‐go events identified significant interaction effects in the left insula cortex and right inferior frontal gyrus right pars opercularis (area 44) and pars triangularis (area 45), as shown in Figure 5 and detailed in Table V. Figure 5 B illustrates that the quadratic trends in BOLD signal increases seen in the inferior frontal gyrus for happy faces and in the left insula cortex for sad faces were inverted for neutral face events. These inferior frontal regions corresponded to those identified by the contrasts for both the quadratic trends and no‐go events minus go events collapsed over face valence. In contrast, the interaction effects in the left insula were contralateral and ≥9 mm dorsal to the insula activation identified by contrasting sad to neutral face events collapsed over trial type. No other regions showed a significant interaction effect.
Figure 5.
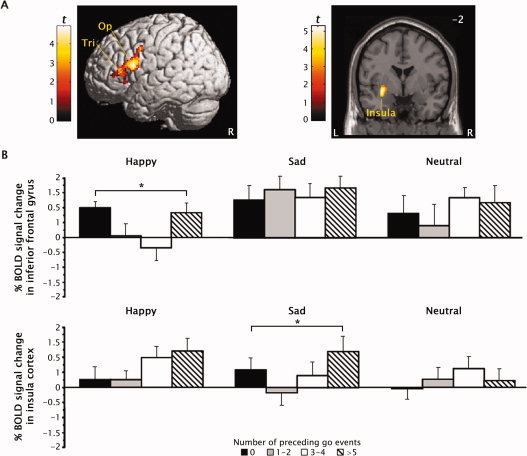
(A) Regional activation in the pars opercularis (Op) and pars triangularis (Tri) of the right inferior frontal gyrus and the left insula showed an interaction between the manipulation of the context preceding response inhibition and the emotional face expression valence. Values in corner of sections refer to Talairach coordinates. (B) Quadratic trends in BOLD signal increases for correct no‐go events after 0, 1–2, 3–4, and 5 or more go events were seen in the inferior frontal gyrus pars opercularis for happy face but not for neutral faces (upper panel) and in the left insula for sad but neutral faces (lower panel). Error bars denote the standard error of the mean. *Different from neutral faces, P < 0.01.
Table V.
Regional activation produced by the interaction of the context preceding response inhibition and emotional face expression valence
| Contrast and region | Side | BA | Talairach coordinates | Cluster size (mm3) | t | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Happy vs. Neutral faces | |||||||
| IFG pars opercularis | R | 44 | 51 | 14 | 16 | 1,588a | 4.90 |
| IFG pars triangularis | R | 45 | 36 | 30 | 8 | 3.91 | |
| Sad vs. Neutral faces | |||||||
| Insula cortex | L | – | −32 | −2 | −7 | 766 | 4.19 |
The volume consisted of two separate peaks.
BA, Brodmann area; IFG, inferior frontal gyrus; L, left; R, right.
DISCUSSION
These results indicate that the behavioral encoding of emotional stimuli to successfully guide response inhibition in social contexts entailed the cooperation and interaction of previously identified neural substrates for response inhibition and emotional processing. Response inhibition cued by emotionally salient stimuli activated the same prefrontal inhibitory circuits [Rizzolatti et al., 2002], cingulate substrates for response selection [Picard and Strick, 2001], and superior parietal attention areas [Corbetta and Shulman, 2002] that have previously been linked to inhibition in nonemotional contexts [Garavan et al., 2006; Simmonds et al., 2008]. These regions may constitute a core network that mediates response inhibition across contexts and task demands. In addition, successful inhibition in the current context required sensory processing of face stimuli by temporo‐occipital association cortex [Kanwisher et al., 1997], as well as encoding of emotional cues from the context by the amygdala and anterior paralimbic cortex [Dolan, 2007]. This pattern of activation is broadly consistent with findings from previous studies [Elliott et al., 2000; Shafritz et al., 2006]. The independent manipulation of the context preceding response inhibition and emotional valence of the face stimuli in the current task provided additional clues to the functional significance of these activations.
Manipulation of context preceding response inhibition
Parametric manipulation of the context preceding response inhibition had similar effects on behavioral performance and activation in a right‐lateralized frontoparietal network that is specialized to use contextual information in inhibitory control. Subjects had greater difficulty inhibiting responses on no‐go trials preceded by another no‐go trial (ie, 0 go trials) and five or more go trials than no‐go trials preceded by one to four go trials. In contrast, greater difficulty in inhibiting responses was only found following five or more go trials in a large sample of college students [Schulz et al., 2007]. This discrepancy in the effect of preceding context on response inhibition may reflect either the vast differences in the testing environment between the MRI scanner in this study and the quiet room used in the previous study or the difference in the size of the samples in the two studies. It is noteworthy that the two studies differed only in the inhibition of responses following another no‐go trial.
Inverse context‐related variations in the magnitude of activation for no‐go events were seen in several regions engaged by emotional response inhibition, as well as in novel areas in pars orbitalis inferior frontal gyrus, right temporoparietal junction, and left anterior temporal association cortex. Neural activation was greater when subjects made fewer correct inhibitions on no‐go trials, specifically on no‐go trials that followed other no‐go trials or five or more go trials. These results are only partially consistent with the findings of previous studies that used emotional [Elliott et al., 2000; Hare et al., 2005] and nonemotional go/no‐go tasks [Durston et al., 2002]. The quadratic trend in inferior frontal opercular no‐go activation in the present study is analogous to a prior finding of linear increases in both errors and frontal operculum activity across no‐go trials preceded by one to five go trials [Durston et al., 2002]. This task did not include no‐go trials preceded by other no‐go trials. However, the present study did not replicate the previous reports of activation in the inferior frontal pars orbitalis during the inhibition of responses to emotional cues [Elliott et al., 2000; Hare et al., 2005]. Rather, pars orbitalis activation was only detected when no‐go trials were contrasted across the different contexts preceding no‐go trials. None of these studies found variations in pars orbitalis activity as a function of stimulus valence. These results suggest that the pars orbitalis may be involved in the coding of the behavioral significance of emotional stimuli.
The inferior frontal gyrus pars orbitalis plays a critical role as an interface between sensory events, motor function, and contextual information in the frontoparietal substrate for inhibitory control [Sakagami and Pan, 2007]. The pars orbitalis receives contextual input from inferotemporal cortex [Ungerleider et al., 1989] and incentive‐valence input from orbitofrontal cortex and amygdala [Petrides and Pandya, 2002], and is unique among prefrontal areas in that it contains neurons that encode sensory cues that signal the inhibition of responses based on the behavioral significance of the cue stimulus [Sakagami et al., 2001]. These neurons fire selectively for no‐go cues in go/no‐go tasks. This cue‐selective activity precedes response execution [Sakagami et al., 2001] and partially reflects interference from past stimulus‐response associations [Lauwereyns et al., 2001]. Thus, no‐go neurons convert sensory and contextual inputs into behavioral codes to guide inhibition rather than actually inhibit responses [Sakagami and Pan, 2007]. Regrettably, we were unable to determine whether the variation in pars orbitalis no‐go activation across the contexts preceding inhibition reflected the encoding of these context differences or the differences in the difficulty inhibiting responses across the contexts, as evidenced by the parallel variations in commission errors.
The pars orbitalis may influence response inhibition through dense connections with the opercular and triangular areas of inferior frontal gyrus [Petrides and Pandya, 2002]. These inferior frontal regions correspond to Broca's speech motor area on the dominant side [Amunts et al., 1999]. However, evidence from neuroimaging studies suggest that the opercular and triangular areas could also be regarded as rostral extensions of ventral premotor cortex, involved in complex sensory guided motor acts, possibly by storing motor representations of goal‐directed hand actions [Iacoboni and Wilson, 2006]. These ventral motor cortical areas can directly influence motor functions through extensive connections with the primary motor cortex [Miyachi et al., 2005]. Meta‐analyses of go/no‐go and stop tasks have implicated these regions as the neural effectors for response inhibition [Garavan et al., 2006; Simmonds et al., 2008]. The variation in the quadratic trend in no‐go activation in the pars opercularis and triangularis as a function of stimulus valence in the current study suggests that these regions also encode the cognitive (ie, preceding events) and emotional or motivational contexts of the stimulus [Watanabe and Sakagami, 2007]. These variations may have reflected differences in the difficulty of inhibiting responses across emotional contexts, with the quadratic trends in no‐go activation reflecting the marginally better inhibition of responses to happy faces than neutral faces. Thus, these inferior frontal areas may form one cortical entry point through which emotional inputs influence motor functions.
No‐go output from the pars orbitalis may also exert top‐down inhibitory control over attention regulation and sensory processing [Sakagami and Pan, 2007]. Comparable context‐dependent activations were found in right temporoparietal junction and superior parietal areas that have been implicated in target detection [Husain and Nachev, 2007] and attention control [Corbetta and Shulman, 2002], respectively. The increased activation for no‐go events preceded by zero and five or more go events may reflect greater salience [Husain and Nachev, 2007], top‐down allocation of attention [Adler et al., 2001], and/or interference resulting in more errors [Mecklinger et al., 2003]. Elevated top‐down attention for these events could also account for the context‐dependent pattern of activation in temporal sensory association cortex [Reddy et al., 2007].
Overall, the results indicate that emotionally guided response inhibition involved a right frontoparietal network that used contextual cues to exert inhibitory control over perception, attention, and behavior. It is noteworthy that despite input from neural substrates of emotional processing, this network did not respond to emotional stimuli per se, but rather coded the behavioral significance of these stimuli according to both the cognitive and emotional contexts. However, these findings cannot clarify whether the context‐dependent activation reflected some stimulus feature (eg, salience) or increased interference for these events.
Manipulation of emotional face expression valence
Manipulation of the emotional valence of the face stimuli used as trial cues in the current study identified a limbic and paralimbic brain circuit that integrates ongoing behavior with emotion and internal states [Phillips et al., 2003]. Valence‐dependent activation was seen in left amygdala, bilateral anterior insula cortex, and left posterior MCC in the current study. The behavioral results suggest that activity in this circuit may have biased performance on the go/no‐go task. The greater number of missed responses to happy and sad faces than neutral faces suggest that stimulus valence had a detrimental effect on response execution processes. In contrast, stimulus valence produced a marginal improvement in response inhibition. However, there was no evidence of the previously reported difficulty with inhibiting responses to happy faces when compared with sad faces [Schulz et al., 2007]. This discrepancy may reflect the addition of neutral faces to the current study. Neutral expressions tend to be mistakenly evaluated as happy or sad faces [Lee et al., 2008; Russell and Fehr, 1987]. This difficulty with face emotion discrimination may have been compounded by the use of faces with closed mouths in this study, which reduced the advantage in detection and salience that smiling mouths confer to happy faces [Calvo and Nummenmaa, 2008].
The pattern of valence‐dependent activation is broadly consistent with the results of previous studies that used emotional go/no‐go tasks [Elliott et al., 2000; Goldstein et al., 2007; Hare et al., 2005; Shafritz et al., 2006]. The significant amygdala activation for sad but not happy faces in the current study is consistent with a previous finding of amygdala responses to fearful faces in a go/no‐go task [Hare et al., 2005], and together with a prior report of greater amygdala activation for facial expressions of surprise in the context of sad than happy words [Kim et al., 2004], suggests that amygdala activity may code for stimulus valence, particularly negative valence [Straube et al., 2008]. However, accumulating evidence indicates that the amygdala activity reflects the representation of the salience rather than valence of emotional stimuli [Gerber et al., 2008; Kim et al., 2004]. From this perspective, the current findings suggest that happy faces were less salient than sad faces, which may also be attributable to the use of faces with closed mouths [Calvo and Nummenmaa, 2008]. In turn, this amygdala activity may have influenced response inhibition indirectly through connections with the inferior frontal gyrus [Petrides and Pandya, 2002] and the anterior insula [Mesulam and Mufson, 1982].
The valence‐dependent activation of the anterior insula cortex seen in the current study is consistent with the model of this region as a cortical entry point through which visceral, sensory, and autonomic inputs influence emotional processing and motor functions [Augustine, 1996]. The anterior insula is reciprocally connected with the amygdala and receives primary olfactory, gustatory, and autonomic input, and contextual‐ and reward‐related information from inferotemporal and orbitofrontal cortices [Mesulam and Mufson, 1982]. This region has been selectively activated by emotional stimuli during response inhibition [[Elliott et al., 2000; Shafritz et al., 2006] and other cognitive functions [Phan et al., 2002], but there is little evidence of the lateralization of emotional processing by valence found in this study [Wager et al., 2003]. The anterior insula is ideally positioned to integrate stimulus‐evoked internal physiological states with input on stimulus context and reward value [Augustine, 1996], but valence‐dependent variations in the quadratic trend in no‐go activation indicative of these integrative functions were localized more dorsally in the insula, more proximal to the opercular, premotor, and cingulate motor areas through which the insula influences motor functions [Shi and Cassell, 1998]. These variations may have also reflected the differences in inhibiting responses across emotional contexts. The quadratic trends in no‐go activation may have represented the marginally better inhibition of responses to sad faces than neutral faces.
The conditional recruitment of the ACC found in this study is among the most commonly reported finding among studies that used emotional go/no‐go tasks [Elliott et al., 2000; Goldstein et al., 2007; Hare et al., 2005; Shafritz et al., 2006] and other cognitive tasks with emotional stimuli [Phan et al., 2002]. The inhibition of responses to emotional stimuli regardless of valence has been found to reliably engage a perigenual region of the ACC implicated in conflict monitoring and error processing [Bush et al., 2000], possibly reflecting the integration of emotional cues into the conflict resolution process [Cardinal et al., 2002]. Most studies also reported greater ACC activation for negatively than positively valenced stimuli, but the localization of this activity varied from more emotional areas in the subgenual ACC [Shafritz et al., 2006] to more cognitive regions in the anterior MCC [Elliott et al., 2000; Goldstein et al., 2007; Hare et al., 2005]. The current results provide little help in disentangling the role of the ACC in emotional response inhibition. The valence‐independent inhibition of responses to emotional stimuli engaged the same approximate region of the anterior MCC that was selectively activated by sad stimuli in other studies [Elliott et al., 2000; Goldstein et al., 2007; Hare et al., 2005], possibly reflecting conflicts in the selection of responses elicited by cognitive and emotional cues [Picard and Strick, 2001]. More incongruously, the current study localized valence‐dependent activation for sad faces in cingulate motor areas in the posterior MCC [Picard and Strick, 2001]. The lack of preceding context effects on no‐go activation in the ACC suggests that this region subserves executive functions other than response inhibition [Garavan et al., 2006], and that the go/no‐go task may not be the most ideal paradigm to study ACC function.
In summary, the current results provide evidence that the inhibition of responses to emotional facial expressions requires the cooperation and interaction of two dissociable brain networks. The inferior frontal gyrus pars orbitalis formed the apex of a frontoparietal network that was specialized to use contextual information to exert inhibitory control over the motor, attention, and sensory functions needed to perform the task. A second circuit involving the amygdala and paralimbic cortex encoded the emotional cues from the context. Regions in the two networks encoded different stimulus features as reflected in either context‐dependent or valence‐dependent patterns of activation. However, successful response inhibition also clearly involved the interaction of the two networks, with valence‐dependent variations in the quadratic trend in no‐go activation in the right inferior frontal gyrus and left posterior insula cortex identifying these regions as cortical entry points through which emotional inputs influence motor functions. Unfortunately, the analyses were unable to identify the significance of MCC activations seen during emotional response inhibition.
REFERENCES
- Adler CM,Sax KW,Holland SK,Schmithorst V,Rosenberg L,Strakowski SM ( 2001): Changes in neuronal activation with increasing attention demand in healthy volunteers: An fMRI study. Synapse 42: 266–272. [DOI] [PubMed] [Google Scholar]
- Amunts K,Schleicher A,Burgel U,Mohlberg H,Uylings HB,Zilles K ( 1999): Broca's region revisited: Cytoarchitecture and intersubject variability. J Comp Neurol 412: 319–341. [DOI] [PubMed] [Google Scholar]
- Augustine JR ( 1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR,Greer S,Chang WL,Berman KF ( 2005): Meta‐analysis of neuroimaging studies of the Wisconsin card‐sorting task and component processes. Hum Brain Mapp 25: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G,Luu P,Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Calvo MG,Nummenmaa L ( 2008): Detection of emotional faces: Salient physical features guide effective visual search. J Exp Psychol Gen 137: 471–494. [DOI] [PubMed] [Google Scholar]
- Cardinal RN,Parkinson JA,Hall J,Everitt BJ ( 2002): Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26: 321–352. [DOI] [PubMed] [Google Scholar]
- Conners CK ( 1997): Conners' Rating Scales—Revised, Technical Manual. Toronto: Multi‐Health Systems. [Google Scholar]
- Corbetta M,Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Costafreda SG,Brammer MJ,David AS,Fu CH ( 2008): Predictors of amygdala activation during the processing of emotional stimuli: A meta‐analysis of 385 PET and fMRI studies. Brain Res Rev 58: 57–70. [DOI] [PubMed] [Google Scholar]
- Davidson RJ ( 2003): Seven sins in the study of emotion: Correctives from affective neuroscience. Brain Cogn 52: 129–132. [DOI] [PubMed] [Google Scholar]
- Dolan RJ ( 2007): The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos Trans R Soc Lond B Biol Sci 362: 787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S,Thomas KM,Worden MS,Yang Y,Casey BJ ( 2002): The effect of preceding context on inhibition: An event‐related fMRI study. Neuroimage 16: 449–453. [DOI] [PubMed] [Google Scholar]
- Elliott R,Rubinsztein JS,Sahakian BJ,Dolan RJ ( 2000): Selective attention to emotional stimuli in a verbal go/no‐go task: An fMRI study. Neuroreport 11: 1739–1744. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Fletcher P,Josephs O,Holmes A,Rugg MD,Turner R ( 1998): Event‐related fMRI: characterizing differential responses. Neuroimage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Garavan H,Hester R,Murphy K,Fassbender C,Kelly C ( 2006): Individual differences in the functional neuroanatomy of inhibitory control. Brain Res 1105: 130–142. [DOI] [PubMed] [Google Scholar]
- Gerber AJ,Posner J,Gorman D,Colibazzi T,Yu S,Wang Z,Kangarlu A,Zhu H,Russell J,Peterson BS ( 2008): An affective circumplex model of neural systems subserving valence, arousal, and cognitive overlay during the appraisal of emotional faces. Neuropsychologia 46: 2129–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M,Brendel G,Tuescher O,Pan H,Epstein J,Beutel M,Yang Y,Thomas K,Levy K,Silverman M,Clarkin J,Posner M,Kernberg O,Stern E,Silbersweig D ( 2007): Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: An emotional linguistic go/no‐go fMRI study. Neuroimage 36: 1026–1040. [DOI] [PubMed] [Google Scholar]
- Haberman J,Whitney D ( 2007): Rapid extraction of mean emotion and gender from sets of faces. Curr Biol 17: R751–R753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN,Adolphs R,Tyszka MJ,O'Doherty JP ( 2007): Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron 55: 545–555. [DOI] [PubMed] [Google Scholar]
- Hare TA,Tottenham N,Davidson MC,Glover GH,Casey BJ ( 2005): Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry 57: 624–632. [DOI] [PubMed] [Google Scholar]
- Hayasaka S,Phan KL,Liberzon I,Worsley KJ,Nichols TE ( 2004): Nonstationary cluster‐size inference with random field and permutation methods. Neuroimage 22: 676–687. [DOI] [PubMed] [Google Scholar]
- Herwig U,Baumgartner T,Kaffenberger T,Bruhl A,Kottlow M,Schreiter‐Gasser U,Abler B,Jancke L,Rufer M ( 2007): Modulation of anticipatory emotion and perception processing by cognitive control. Neuroimage 37: 652–662. [DOI] [PubMed] [Google Scholar]
- Hoshi E,Tanji J ( 2004): Differential roles of neuronal activity in the supplementary and presupplementary motor areas: From information retrieval to motor planning and execution. J Neurophysiol 92: 3482–3499. [DOI] [PubMed] [Google Scholar]
- Husain M,Nachev P ( 2007): Space and the parietal cortex. Trends Cogn Sci 11: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M,Wilson SM ( 2006): Beyond a single area: Motor control and language within a neural architecture encompassing Broca's area. Cortex 42: 503–506. [DOI] [PubMed] [Google Scholar]
- Johansson K,Ronnberg J ( 1996): Speech gestures and facial expression in speechreading. Scand J Psychol 37: 132–139. [DOI] [PubMed] [Google Scholar]
- Johnstone T,Ores Walsh KS,Greischar LL,Alexander AL,Fox AS,Davidson RJ,Oakes TR ( 2006): Motion correction and the use of motion covariates in multiple‐subject fMRI analysis. Hum Brain Mapp 27: 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N,McDermott J,Chun MM ( 1997): The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H,Somerville LH,Johnstone T,Polis S,Alexander AL,Shin LM,Whalen PJ ( 2004): Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci 16: 1730–1745. [DOI] [PubMed] [Google Scholar]
- Lauwereyns J,Sakagami M,Tsutsui K,Kobayashi S,Koizumi M,Hikosaka O ( 2001): Responses to task‐irrelevant visual features by primate prefrontal neurons. J Neurophysiol 86: 2001–2010. [DOI] [PubMed] [Google Scholar]
- Lee E,Kang JI,Park IH,Kim JJ,An SK ( 2008): Is a neutral face really evaluated as being emotionally neutral? Psychiatry Res 157: 77–85. [DOI] [PubMed] [Google Scholar]
- Mathews A,McLeod C ( 1994): Cognitive approaches to emotion and emotional disorders. Annu Rev Psychol 45: 25–50. [DOI] [PubMed] [Google Scholar]
- Maxwell JS,Shackman AJ,Davidson RJ ( 2005): Unattended facial expressions asymmetrically bias the concurrent processing of nonemotional information. J Cogn Neurosci 17: 1386–1395. [DOI] [PubMed] [Google Scholar]
- Mecklinger A,Weber K,Gunter TC,Engle RW ( 2003): Dissociable brain mechanisms for inhibitory control: Effects of interference content and working memory capacity. Brain Res Cogn Brain Res 18: 26–38. [DOI] [PubMed] [Google Scholar]
- Mesulam MM,Mufson EJ ( 1982): Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol 212: 38–52. [DOI] [PubMed] [Google Scholar]
- Miyachi S,Lu X,Inoue S,Iwasaki T,Koike S,Nambu A,Takada M ( 2005): Organization of multisynaptic inputs from prefrontal cortex to primary motor cortex as revealed by retrograde transneuronal transport of rabies virus. J Neurosci 25: 2547–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH,Simmonds DJ ( 2008): Response inhibition and response selection: Two sides of the same coin. J Cogn Neurosci 20: 751–761. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH,Schafer JG,Abrams MT,Goldberg MC,Flower AA,Boyce A,Courtney SM,Calhoun VD,Kraut MA,Denckla MB Pekar JJ ( 2003): fMRI evidence that the neural basis of response inhibition is task‐dependent. Brain Res Cogn Brain Res 17: 419–430. [DOI] [PubMed] [Google Scholar]
- Otta E,Lira BB,Delevati NM,Cesar OP,Pires CS ( 1994): The effect of smiling and of head tilting on person perception. J Psychol 128: 323–331. [DOI] [PubMed] [Google Scholar]
- Paus T ( 2001): Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci 2: 417–424. [DOI] [PubMed] [Google Scholar]
- Petrides M,Pandya DN ( 2002): Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci 16: 291–310. [DOI] [PubMed] [Google Scholar]
- Phan KL,Wager T,Taylor SF,Liberzon I ( 2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Phelps EA,LeDoux JE ( 2005): Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 48: 175–187. [DOI] [PubMed] [Google Scholar]
- Phillips ML,Drevets WC,Rauch SL,Lane R ( 2003): Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry 54: 504–514. [DOI] [PubMed] [Google Scholar]
- Picard N,Strick PL ( 2001): Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672. [DOI] [PubMed] [Google Scholar]
- Price JL ( 2003): Comparative aspects of amygdala connectivity. Ann N Y Acad Sci 985: 50–58. [DOI] [PubMed] [Google Scholar]
- Reddy L,Moradi F,Koch C ( 2007): Top‐down biases win against focal attention in the fusiform face area. Neuroimage 38: 730–739. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G,Fogassi L,Gallese V ( 2002): Motor and cognitive functions of the ventral premotor cortex. Curr Opin Neurobiol 12: 149–154. [DOI] [PubMed] [Google Scholar]
- Russell JA,Fehr B ( 1987): Relativity in the perception of emotion in facial expressions. J Exp Psychol Gen 116: 223–237. [Google Scholar]
- Sakagami M,Pan X ( 2007): Functional role of the ventrolateral prefrontal cortex in decision making. Curr Opin Neurobiol 17: 228–233. [DOI] [PubMed] [Google Scholar]
- Sakagami M,Tsutsui K,Lauwereyns J,Koizumi M,Kobayashi S,Hikosaka O ( 2001): A code for behavioral inhibition on the basis of color, but not motion, in ventrolateral prefrontal cortex of macaque monkey. J Neurosci 21: 4801–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KP,Fan J,Magidina O,Marks DJ,Hahn B,Halperin JM ( 2007): Does the emotional go/no‐go task really measure behavioral inhibition? Convergence with measures on a non‐emotional analog. Arch Clin Neuropsychol 22: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz KM,Collins SH,Blumberg HP ( 2006): The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage 31: 468–475. [DOI] [PubMed] [Google Scholar]
- Shi CJ,Cassell MD ( 1998): Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol 399: 440–468. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ,Pekar JJ,Mostofsky SH ( 2008): Meta‐analysis of Go/No‐go tasks demonstrating that fMRI activation associated with response inhibition is task‐dependent. Neuropsychologia 46: 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD,Schacter DL ( 2004): A sensory signature that distinguishes true from false memories. Nat Neurosci 7: 664–672. [DOI] [PubMed] [Google Scholar]
- Steer RA,Ball R,Ranieri WF,Beck AT ( 1999): Dimensions of the beck depression inventory‐II in clinically depressed outpatients. J Clin Psychol 55: 117–128. [DOI] [PubMed] [Google Scholar]
- Straube T,Pohlack S,Mentzel HJ,Miltner WH ( 2008): Differential amygdala activation to negative and positive emotional pictures during an indirect task. Behav Brain Res 191: 285–288. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux M ( 1988): Co‐planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical. [Google Scholar]
- Ungerleider LG,Gaffan D,Pelak VS ( 1989): Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp Brain Res 76: 473–484. [DOI] [PubMed] [Google Scholar]
- Vogt BA,Nimchinsky EA,Vogt LJ,Hof PR ( 1995): Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J Comp Neurol 359: 490–506. [DOI] [PubMed] [Google Scholar]
- Wager TD,Phan KL,Liberzon I,Taylor SF ( 2003): Valence, gender, and lateralization of functional brain anatomy in emotion: A meta‐analysis of findings from neuroimaging. Neuroimage 19: 513–531. [DOI] [PubMed] [Google Scholar]
- Watanabe M,Sakagami M ( 2007): Integration of cognitive and motivational context information in the primate prefrontal cortex. Cereb Cortex 17 ( Suppl 1): i101–i109. [DOI] [PubMed] [Google Scholar]


