Abstract
IL-17 and IL-22 are typical cytokines produced by the Th17 T cell subset, but it is unclear if Th17 cytokines can be produced by other cell types. We demonstrate that IL-10-deficient and IL-10R-deficient macrophages stimulated with LPS produce high levels of IL-17 and IL-22. Addition of exogenous IL-10 to IL-10-deficient macrophages abolished IL-17 production. When IL-10-deficient and IL-10R-deficient splenocytes were cultured under Th17 polarizing conditions the population of IL-17 producing cells was increased and the cultures produced significantly higher levels of IL-17 and IL-22. The addition of recombinant IL-10 to IL-10-deficient splenocytes significantly decreased the percentage of IL-17-producing CD4+ T cells. Finally, the mRNA for the Th17 transcription factor RORγt was significantly elevated in IL-10-deficient spleen cells and macrophages. These data demonstrate that Th17 cytokines and RORγt are also expressed in macrophages and that IL-10 negatively regulates the expression of Th17 cytokines and RORγt by both macrophages and T cells.
Keywords: T cell, Macrophage, Cytokines, Cell Differentiation, Gene Regulation
INTRODUCTION
Th17 cells, which secrete interleukin 17 (IL-17) and IL-22, comprise a recently identified subset of CD4+ T cells that is distinct from Th1 and Th2 subsets [1–4]. The family of IL-17-related cytokines includes IL-17A-F. IL-17A, usually referred to as IL-17, shares a similar structure, chromosomal location, and receptor usage with IL-17F [5]. The expression of IL-17A and IL-17F was originally believed to be regulated by IL-23, a member of the IL-12 family that shares a common p40 subunit with IL-12 and binds to a common receptor, IL-12Rβ1 [6, 7]. However, recent evidence suggests that IL-23 promotes the survival and expansion of Th17 cells, and that IL-6 and TGF-β in concert induce the differentiation in vitro of Th17 cells from naïve CD4+ T cells [8, 9]. Recently the orphan nuclear receptor RORγt has been identified as the key transcription factor involved in Th17 cell differentiation [10]. Although it is believed that IL-17 and IL-22 produced by Th17 cells contribute to pathological inflammation, the contribution of other cell types to IL-17 and IL-22 synthesis has not been examined.
IL-10, a 37 kD homodimer first described as a factor inhibiting cytokine synthesis by Th2 cell clones, inhibits interferon-γ (IFN-γ) expression in Th1 cells [11]. Various cell types produce IL-10, including Th2 and Tr1 T cell subsets, monocytes, and macrophages [12]. IL-10 signals through the IL-10 receptor (IL-10R), which is expressed on a variety of cells, especially of immune origin. The IL-10R is composed of two chains, IL-10R1 and IL-10R2. Interaction of IL-10 with the IL-10R activates phosphorylation and activation of the receptor-associated Janus tyrosine kinases, resulting in STAT3-mediated signal transduction [12]. IL-10 inhibits Th1 differentiation and macrophage production of IL-12. IL-10 also inhibits cytokine production by Th1 cells but does not directly inhibit CD8+ T cell function. IL-10-deficient mice spontaneously develop colitis due to an enhanced Th1 immune response. However, recent studies suggest that IL-17 may contribute to the development of colitis in IL-10-deficient mice [13]. Although it is clear that IL-10 plays a negative role in Th1 cell immune responses, the importance of IL-10 in the regulation of the Th17 response has not been examined.
RESULTS AND DISCUSSION
IL-17 and IL-22 expression was enhanced in IL-10−/− or IL-10R−/− T cells
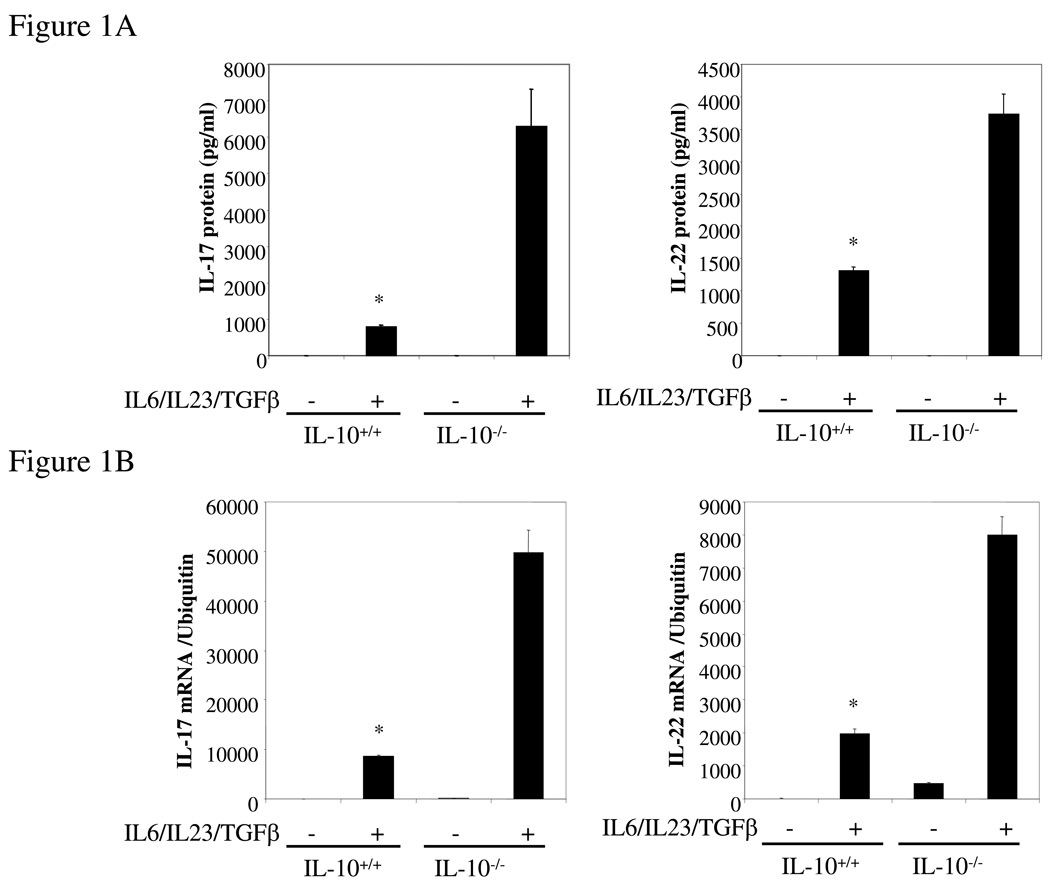
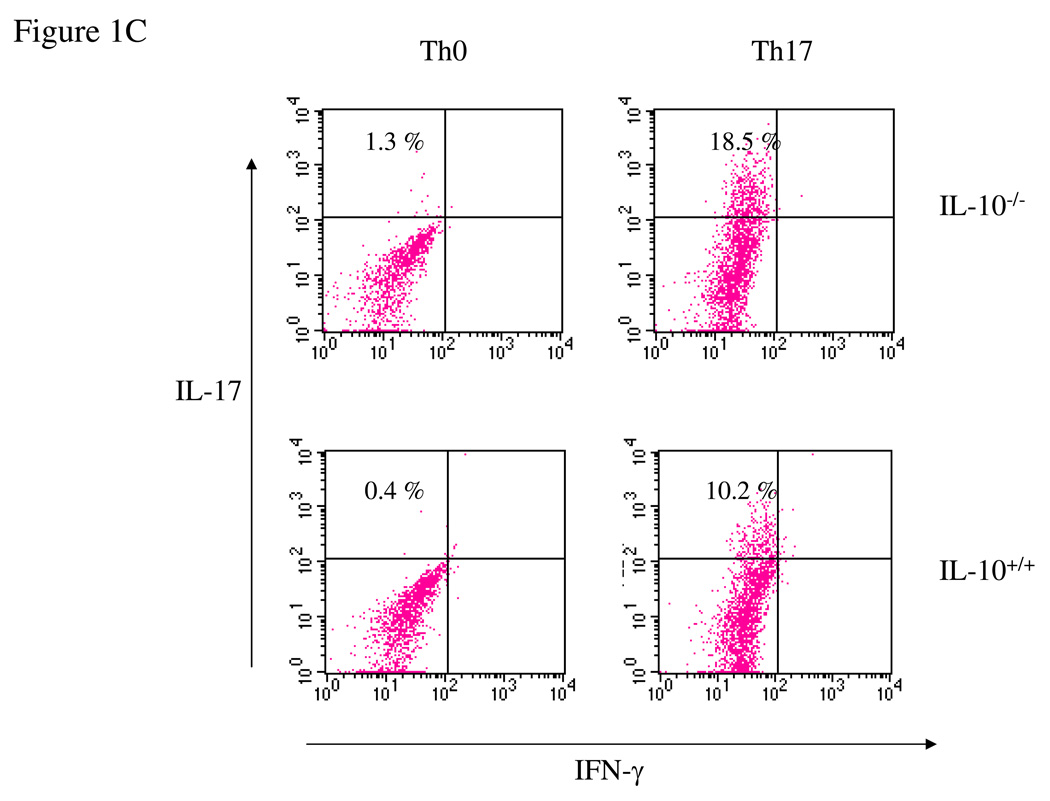
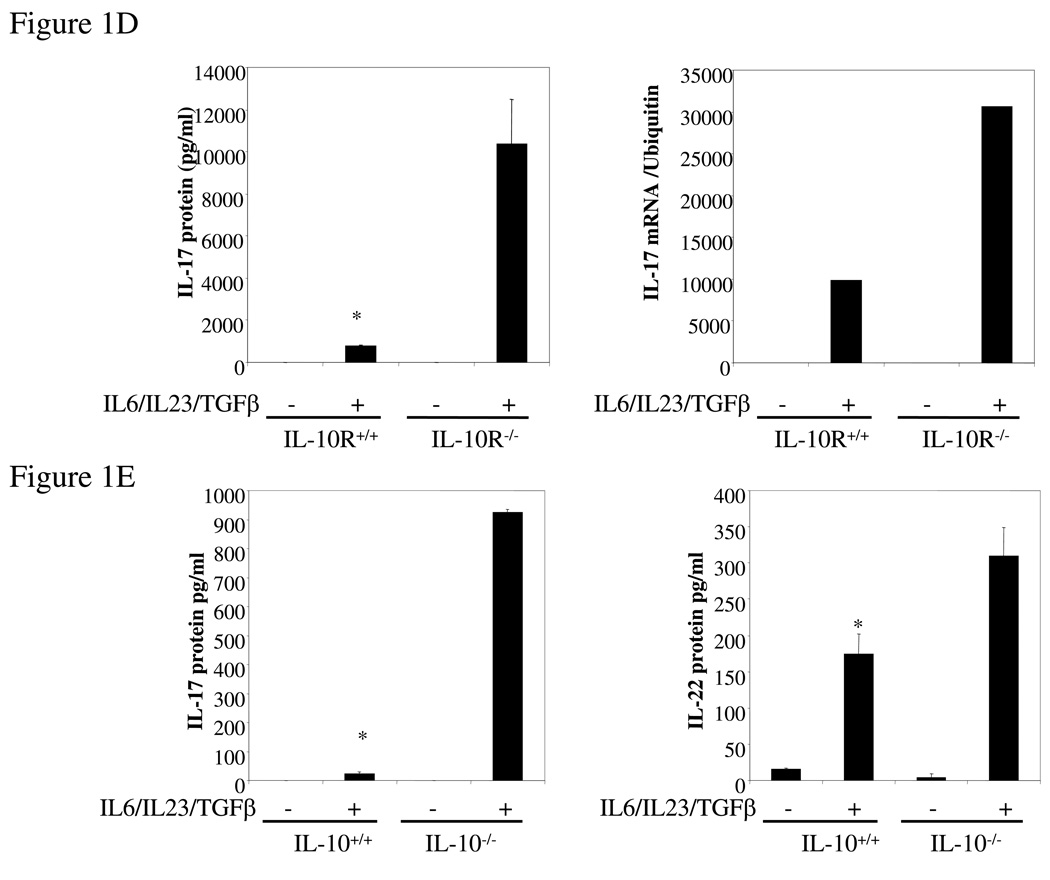
To analyze the effect of IL-10 on the Th17 immune response, spleen cells from wild type and IL-10-deficient mice were incubated in vitro with IL-23, IL-6 and TGF-β to force Th17 differentiation. We found that IL-10−/− splenocytes produced significantly higher levels of both IL-17 and IL-22 protein (Figure 1A) and mRNA (Figure 1B). We then quantified by FACS intracellular IL-17 in CD4+ T cells differentiated under Th0 or Th17 conditions from wt and IL-10-deficient mice. IL-10 deficiency did not significantly alter the percentage of IL-17-producing Th0 cells. However, when the cells were differentiated under Th17 polarizing conditions, 18.5% of the IL-10-deficient CD4+ T cells were IL-17-positive compared to 10.2% of the wild type cells (Figure 1C). We also found that the percentage of IL-17-producing CD8+ T cells was also significantly increased (data not shown). Since IL-10 acts through the IL-10R, IL-10R-deficient cells should behave similarly with respect to Th17 induction. As predicted, IL-10R−/− spleen cells polarized under Th17 conditions secreted 10-fold more IL-17 than wild type cells (Figure 1D) and the level of IL-17 mRNA was similarly elevated (Figure 1D). We purified lamina propria lymphocytes (LPL) from both wild type and IL-10-deficient mice, and found that Th17-polarized LPL from IL-10-deficient mice produced significantly higher levels of IL-17 and IL-22 than LPL from wild type mice (Figure 1E). In summary, in the absence of IL-10 or IL-10R the expression of IL-17 and IL-22 and the differentiation of Th17 cells were both significantly enhanced.
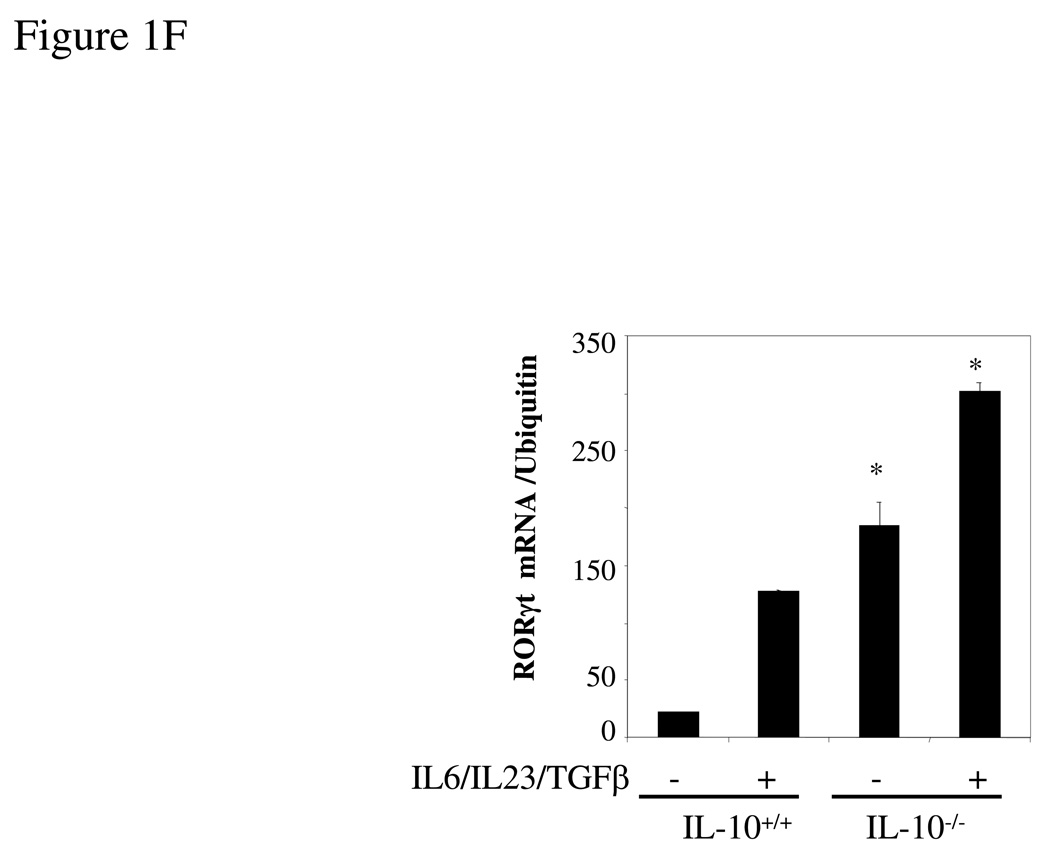
Figure 1. IL-17 and IL-22 expression is elevated in IL-10−/− and IL-10R−/− Th17 cells.
(A) Wild type or IL-10−/− splenocytes was differentiated under Th17 conditions for 4 days and the IL-17 and IL-22 in supernatants quantified by ELISA. *, p <0.01 vs IL-10-deficient cells. (B) Total RNA was extracted from cells as in A and analyzed by q-PCR for IL-17 and IL-22 mRNA. *, p < 0.01 vs IL-10-deficient cells. (C) Wild type or IL-10-deficient splenocytes were differentiated under Th0 or Th17 conditions for 4 days. Cells were then harvested and stained for intracellular IL-17, IFN-γ, and CD4. Cells were then analyzed by FACS for IL-17 and IFN-γ, gated on CD4+ T cells. (D) Wild type or IL-10R-deficient cells were differentiated under Th17 conditions for 4 days and IL-17 in supernatants quantified by ELISA. Total RNA was extracted and analyzed by q-PCR for IL-17 mRNA. *, p< 0.01 vs IL-10R-deficient cells. (E) Wild type or IL-10-deficient LPL were differentiated under Th17 conditions for 4 days. IL-17 in supernatants was quantified by ELISA and total RNA was analyzed for IL-17 mRNA by q-PCR. *, p <0.01 vs IL-10-deficient cells. (F) Wild type or IL-10 deficient cells were differentiated under Th17 conditions for 4 days and total RNA was extracted and analyzed by q-PCR for RORγt mRNA. *, p< 0.01 vs wild type mice.
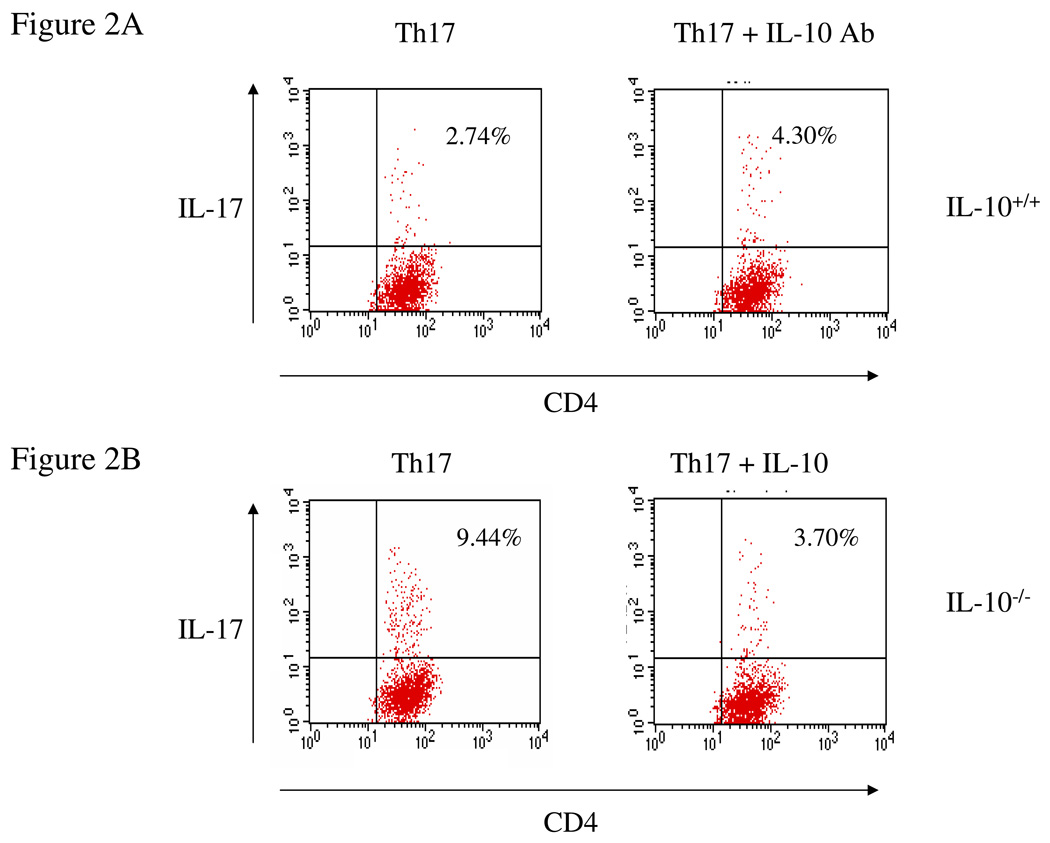
To demonstrate that IL-10 inhibits IL-17 production from CD4+ T cells, spleen cells from wild type and IL-10-deficient mice were incubated in vitro under Th17 differentiation conditions in the presence of neutralizing anti-IL-10 antibody or recombinant IL-10. We found that addition of anti-IL-10 antibody significantly increased the percentage of IL-17-producing cells in wild type mice (Figure 2A), while addition of recombinant IL-10 had the opposite effect in IL-10 deficient mice (Figure 2B). Thus, IL-10 negatively regulates Th17 cell differentiation.
Figure 2. IL-10 inhibits IL-17 expression.
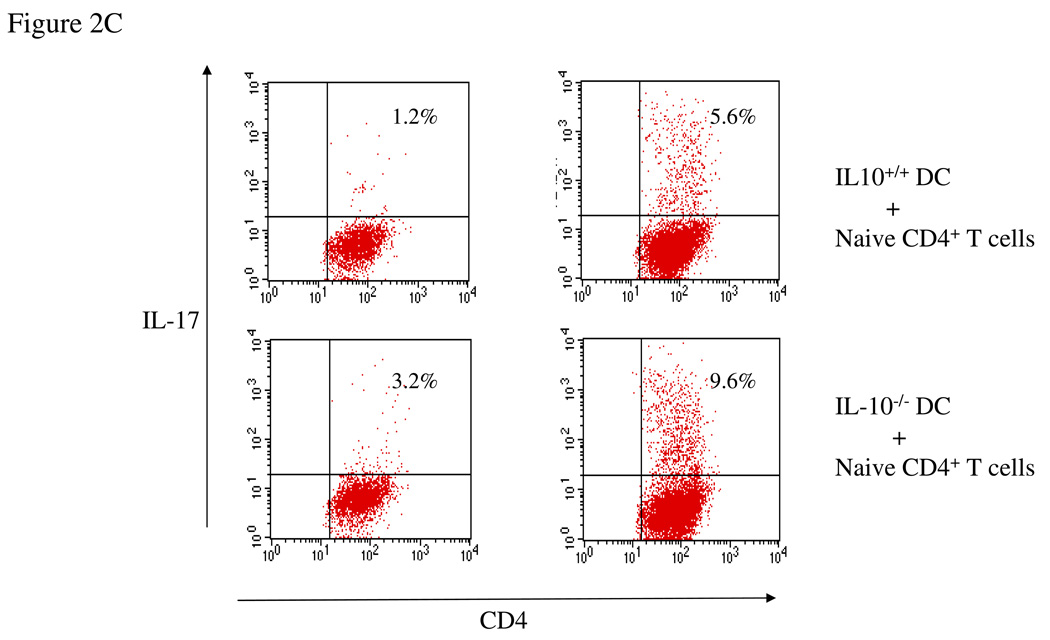
(A) Wild type splenocytes were differentiated under Th17 conditions in the presence of neutralizing IL-10 antibody (1 µg/ml) for 4 days. Cells were then harvested and stained for intracellular IL-17. (B) IL-10-deficient splenocytes were differentiated under Th17 conditions in the presence of recombinant IL-10 (10 ng/ml) for 4 days. Cells were then harvested and stained for intracellular IL-17. (C) Bone-marrow-derived dendritic cells were co-cultured with naïve CD4+ T cells from OT-II mice under Th17 conditions for 4 days. Cells were then harvested and stained for intracellular IL-17.
Our results demonstrate that IL-10 negatively regulates Th17 cell differentiation. However, it is not clear if this inhibition is due to CD4+ T cells or antigen presenting cells. To address this question, we co-cultured dendritic cells from wild type and IL-10−/− mice with CD4+ T cells from OT-II mice under Th17 polarization conditions in the presence of OVA. We found that the population of Th17 cells was significantly increased in the co-culture with IL-10−/− DC compared to wild type DC (Figure 2C), showing that antigen-presenting cells contribute to the negative regulation of Th17 cell differentiation by IL-10.
IL-17 and IL-22 expression was enhanced in IL-10−/− or IL-10R−/− macrophages
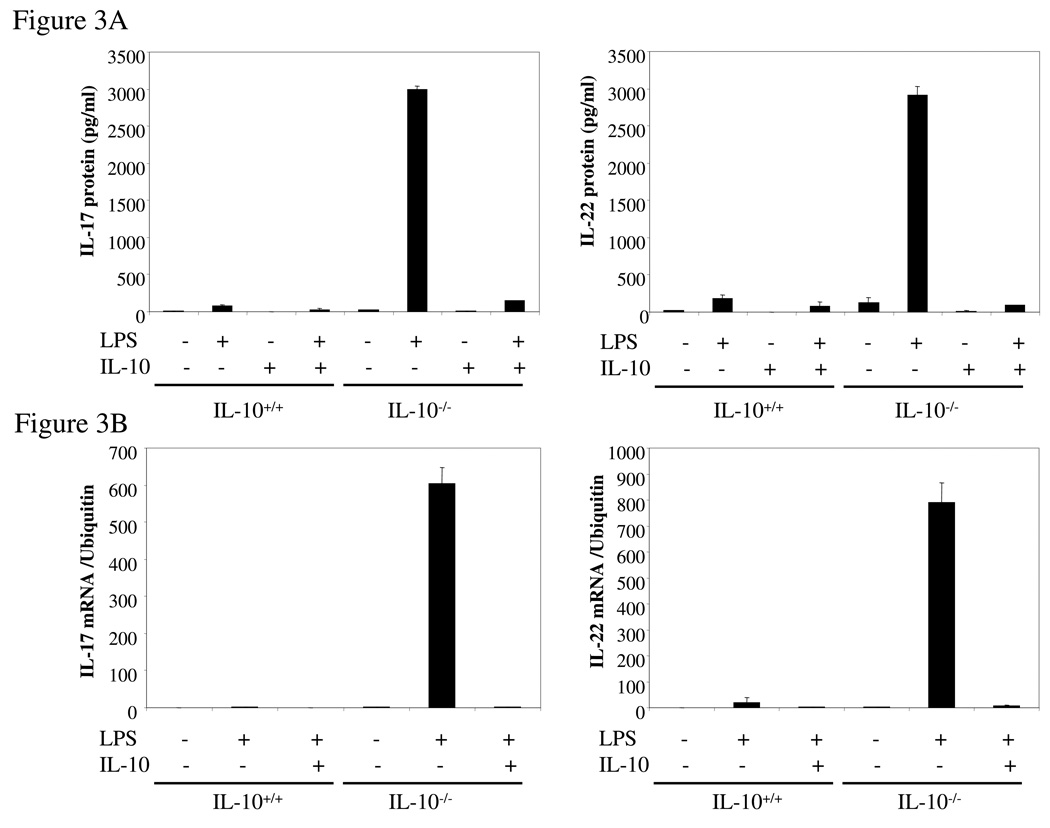
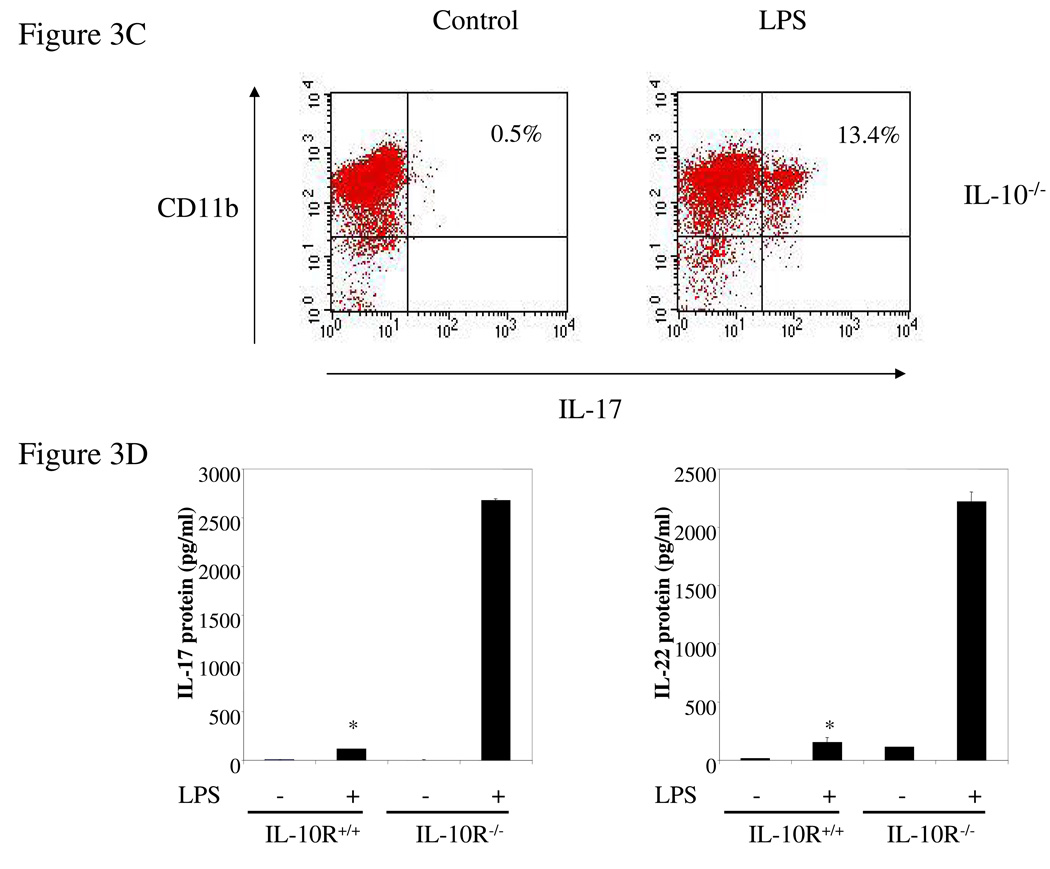
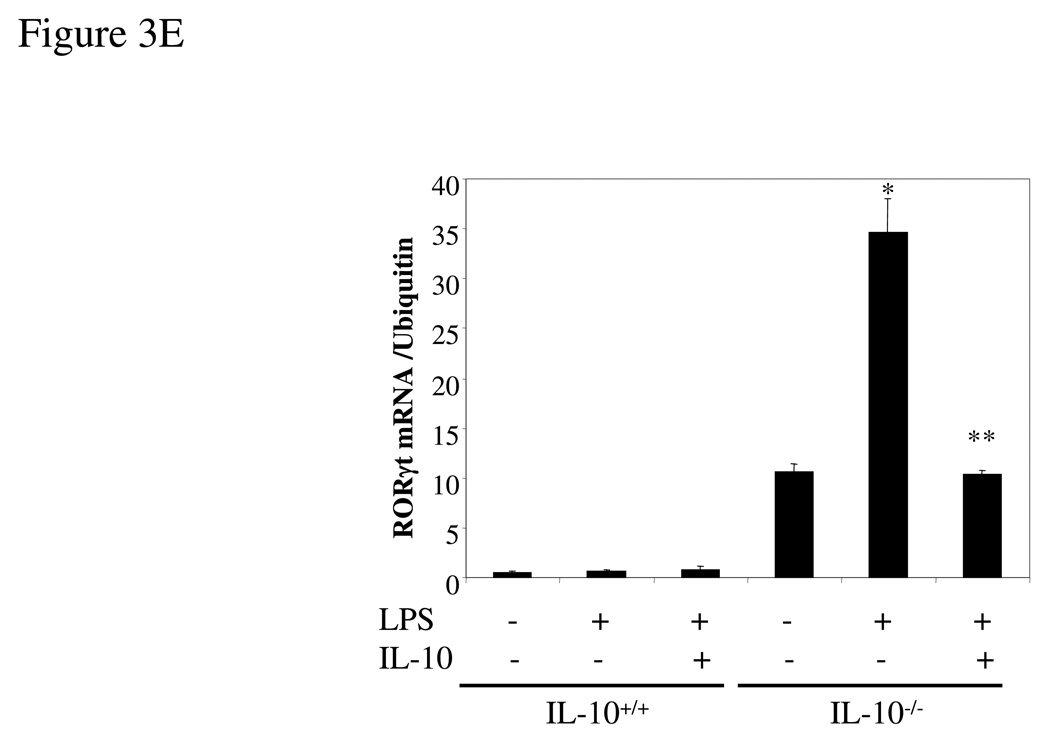
Intrigued by the dramatic increase in the expression of IL-17 from IL-10−/− spleen cells compared to the modest increase in IL-17-producing CD4+ T cells under the same conditions, we examined the possibility that other cell types such as macrophages produce IL-17. Thioglycollate-elicited peritoneal macrophages from IL-10−/− and wild type mice were activated for 24 hrs with LPS (1 µg/ml) and IL-17 secretion quantified by ELISA. Surprisingly, IL-10-deficient macrophages produced very high levels of IL-17 whereas wild type cells produced almost undetectable IL-17 (Figure 3A), and decent amount of IL-10 (data not shown). These results were confirmed by q-PCR analysis of IL-17 mRNA (Figure 3B). IL-22 production and mRNA expression were also strongly induced by LPS from IL-10−/− but not wild type macrophages (Figure 3A, B). Remarkably, addition of IL-10 to cultures of the IL-10 deficient macrophages nearly totally suppressed synthesis of IL-17 and IL-22, both at the protein and mRNA levels (Figure 3A, B). FACS analysis showed that LPS stimulation of IL-10 deficient macrophages resulted in the induction of intracellular IL-17 in a subpopulation (13.4%) of CD11b+ thioglycollate-elicited cells, compared to 0.5% for un-stimulated macrophages (Figure 3C). Control wild type CD11b+ cells were not positive in the presence or absence of LPS (data not shown). As discussed above, IL-10R-deficient mice should have the same phenotype as IL-10-deficient mice. Indeed, we found that high levels of IL-17 and IL-22 were produced by thioglycollate-elicited macrophages from IL-10R-deficient mice after cells were stimulated with LPS while wild type macrophages produced very low amounts of IL-17 and IL-22 (Figure 3D). The macrophages in the IL-10-deficient and IL-10R-deficent mice seem normal, at least by the criteria of expression of CD11b and CD86 (data not shown). The results show that endogenous IL-10 induced by LPS suppresses IL-17 production in macrophages.
Figure 3. IL-17 and IL-22 expression was elevated in IL-10−/− or IL-10R−/− macrophages.
(A) Thioglycollate-elicited peritoneal macrophages from IL-10-deficient and wild type mice were activated with LPS (1 µg/ml) for either 4 hrs (RNA) or 24 hrs (protein release). The supernatants were analyzed for IL-17 and IL-22 by ELISA. (B) Total RNA was extracted and analyzed for IL-17 and IL-22 mRNAs by q-PCR. (C) Thioglycollate-elicited peritoneal macrophages from IL-10-deficient mice were activated with LPS (1 µg/ml) for 16 hrs, brefeldin A was added, and the cells were activated for another 6 hrs. IL-17 was analyzed by intracellular staining. (D) Thioglycollate-elicited peritoneal macrophages from IL-10R−/− and wild type mice were activated with LPS (1 µg/ml) for 24 hrs. The supernatants were analyzed for IL-17 and IL-22 by ELISA. *, p <0.01 vs IL-10R−/− cells. (E) Thioglycollate-elicited peritoneal macrophages from IL-10-deficient and wild type mice were activated with LPS (1 µg/ml) in the presence or absence of IL-10 (10 ng/ml) for 4 hrs and then total RNA was extracted and analyzed by q-PCR for RORγt mRNA. *, p <0.01 vs wild type mice; **, p <0.01 vs LPS stimulation only in IL-10 deficient cells.
RORγt expression was enhanced in IL-10−/− spleen cells and macrophages
Recently, Ivanov et al [10] have demonstrated that the orphan nuclear receptor RORγt is a key transcription factor mediating the differentiation of Th17 cells. RORγt induces transcription of the genes encoding IL-17 and the related cytokine IL-17F in naïve CD4+ T cells. Mice with RORγt-deficient T cells are resistant to induction of autoimmune disease and lack tissue-infiltrating Th17 cells. Since IL-10 inhibits Th17 cytokine expression, we next examined the expression of RORγt in IL-10-deficient mice. As predicted, RORγt mRNA expression was significantly higher in spleen cells from IL-10−/− mice differentiated under Th17 conditions (Figure 1F). IL-10-deficient macrophages, which secrete large amounts of IL-17 after stimulation with LPS, showed a parallel elevation in the expression of RORγt mRNA (Figure 3E). The RORγt mRNA expression enhanced by LPS stimulation was inhibited by addition of exogenous IL-10 (Figure 3E). However, RORγt mRNA expression was much more abundant in Th17 cells than macrophages (data not shown). Thus, IL-10 is a negative regulator of RORγt expression.
This study describes a role of IL-10 as a negative regulator of the Th17 immune response. We demonstrate that IL-10-deficient spleen cells polarized under Th17 conditions produced significantly higher levels of IL-17 and IL-22 than wild type cells. Comparable results were found with IL-10R-deficient cells, further implicating IL-10 as a pivotal negative regulatory cytokine for IL-17 and IL-22 expression. Surprisingly, a subpopulation of IL-10-deficient macrophages produced high levels of the Th17 cytokines IL-17 and IL-22 upon activation with LPS whereas wild type macrophages secrete little IL-17 and IL-22. Thus, IL-10 is a pivotal cytokine controlling IL-17 expression in macrophages. Taken together, these data support IL-10 as a negative regulator of Th17 immune response.
IL-17 secretion characterizes the Th17 cell lineage, which is critical in the pathogenesis of autoimmune and inflammatory diseases including experimental autoimmune encephalitis and murine models of colitis. Several cytokines interfere with the development and/or proliferation of Th17 cells. Neutralization of IFN-γ in vitro increases the number of IL-17-producing cells generated by IL-23 stimulation. The number of Th17 cells is further increased by the addition of an IL-4 neutralizing antibody, suggesting that IFN-γ and IL-4 can suppress the expansion of Th17 cells driven by IL-23 [6, 7]. However, it is not clear if IFN-γ and IL-4 interfere with the differentiation of Th17 cells induced by IL-6 and TGFβ. IL-27, another member of IL-12 family, is reported to be a negative regulator of IL-17-producing cells. The absence of IL-27 mediated signaling exacerbates neuroinflammation, enhances the generation of Th17 cells and increases the number of IL-17-producing T cells in inflamed tissue [14, 15]. In the present study we found that IL-10-deficient cells stimulated with IL-23, IL-6, and TGF-β produced significantly higher amounts of IL-17 and IL-22 than wild type cells, suggesting that IL-10 is an important negative regulator of IL-17-producing T cells.
Recently, Montufar-Solis et al reported that IL-17 was produced at high levels in colons from IL-10−/− with mild to severe colitis, but it was not produced by healthy IL-10−/− mice without disease [16]. In addition, IL-4 and IL-5 remained negative in IL-10−/− mice even with colitis. These results suggest that the negative regulation of cytokine expression by IL-10 is selective, not universal. Therefore, further studies need to explore the molecular mechanism for the selective inhibition of cytokine expression by IL-10.
IL-17 is widely regarded as a T cell derived cytokine. However, we were surprised to find that LPS stimulation of IL-10−/− macrophages resulted in production of high titers of IL-17 and IL-22 while comparable stimulation of wild type macrophages resulted in little IL-17 and IL-22 synthesis. We propose that LPS stimulation of wild type macrophages induces sufficient IL-10 to suppress IL-17. In support of this, we found that recombinant IL-10 suppresses IL-17 and IL-22 synthesis from IL-1−/− macrophages. RORγt is the key transcription factor that orchestrates the differentiation of Th17 cell lineage. In the present study, we found that RORγt was highly expressed in IL-10-deficient macrophages, suggesting that IL-10 suppresses IL-17 production through RORγt. Clearly, both the production of IL-17 and IL-22 by macrophages under other conditions, and the phenotypic differences between the IL-17 secreting and non-secreting macrophage populations need to be further investigated.
Concluding remarks
Taken together, we demonstrate that macrophages express Th17 cytokines, IL-17 and IL-22, as well as the Th17 cell transcription factor RORγt. Furthermore, we demonstrate that IL-10 negatively regulates the expression of IL-17, IL-22 and RORγt in both macrophages and T cells. IL-10, acting on both macrophages and T cells, thus plays a critical role in immunity and autoimmune diseases, and thus recombinant IL-10 may be of significant therapeutic benefit for several autoimmune diseases.
MATERIALS AND METHODS
Mice
C57BL/6 and OT-II mice were purchased from Jackson Laboratories (Bar Harbor, ME). IL-10−/− mice were purchased from Jackson Laboratories, and maintained in the barrier facility at Mount Sinai School of Medicine according to IACUC guidelines. IL-10 receptor (IL-10R)-deficient mice were established as described [17].
T cell differentiation
Spleen cells from wild type, IL-10−/− or IL-10R−/− mice were suspended (5 × 106 cells/ml) in DMEM containing 10% FBS. IL-6 (20 ng/ml), human TGFβ (3 ng/ml), and IL-23 (6 ng/ml) (all from R&D systems, Minneapolis, MN) were added to the culture for 4 days to drive the differentiation of Th17 cells. No additional cytokines were added for Th0 development. Cells were then re-stimulated with plate-bound anti-CD3 for 24 hrs or with PMA and ionomycin for 6 hrs.
Preparation of peritoneal macrophages
Wild type, IL-10-deficient and IL-10R-deficient mice were injected i.p. with 2 ml of 5 % thioglycollate medium for three days. Mice were then sacrificed and peritoneal macrophages were isolated by lavage with PBS. Cells were cultured in DMEM containing 10% FBS and antibiotics for 2 hours and the monolayer washed to remove non-adherent cells before stimulation.
Preparation of bone marrow-derived dendritic cells
Mouse bone marrow-derived dendritic cells were generated from marrow stem cells obtained from the femurs of the mice as described previously [18]. After lysis of the red blood cells 1 × 106 bone marrow cells were cultured in each well of 24 well plates with complete DMEM in the presence of GF-CSF (10 ng/ml) for 7 days.
ELISA
The cytokine titers in the supernatants were analyzed using ELISA kits from eBioscience and R&D systems.
Real-time RT-PCR analysis
Total RNA was extracted using the RNeasy Plus Kit (QIAGEN, Valencia, CA) and the cDNA was generated with an oligo (dT) primer followed by analysis using iCycler PCR with SYBR Green PCR Master Mix (A &B Applied Biosystems). Results were normalized based on the expression of ubiquitin. The following primer pairs were used: IL-17 sense, 5’-CTCCAGAAGGCCCTCAGACTAC-3’, IL-17 anti-sense, 5’AGCTTTCCCTCCGCATTGACACAG-3’; IL-22 sense, 5’-CATGCAGGAGGTGGTACCTT-3’, IL-22 anti-sense, 5’-CAGACGCAAGCATTTCTCAG-3’; RORγt sense, 5’-CCGCTGAGAGGGCTTCA-3’, RORγt anti-sense, TGCAGGAGTAGGCCACATTACA-3’C; ubiquitin sense, 5’-TGGCTATTAATTATTCGGTCTGCA-3’, ubiquitin anti-sense, 5’-GCAAGTGGCTAGAGTGCAGAGTAA-3’.
Intracellular staining and flow cytometry
Spleen cells were stimulated with PMA and ionomycin overnight, after which brefeldin A was added into the culture for an additional two hours prior to intracellular staining. Cells were fixed with IC Fixation Buffer (eBioscience), incubated with permeabilization buffer, and stained with PE-anti-mouse IL-17, FITC-anti-IFN-γ, and Cy5-anti-mouse CD4 antibodies (eBioscience). For macrophage intracellular staining, thioglycollate-elicited peritoneal macrophages were activated with LPS for 16 hrs, brefeldin A was added, and cells were incubated for another 6 hrs, fixed, permeabilized, and stained as above. Flow cytometry was performed on a FACscan.
Statistical analysis
Statistical analysis was performed using Student’s t-Test. P values <0.05 were considered significant.
ACKNOWLEDGMENTS
We are extremely grateful to Dr. Shu Zhang for technical support. Dr. Huabao Xiong was supported by NIH grant P01 DK072201, Crohn’s and Colitis Foundation of America, and the Eli and Edythe L. Broad Foundation.
Footnotes
Conflict of interest: Jianfei Yang is an employee of Boehringer Ingelheim Pharmaceuticals, Inc.
REFERENCES
- 1.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 2.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 4.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 8.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 9.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 13.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 15.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 16.Montufar-Solis D, Schaefer J, Hicks MJ, Klein JR. Massive but selective cytokine dysregulation in the colon of IL-10−/− mice revealed by multiplex analysis. Int Immunol. 2008;20:141–154. doi: 10.1093/intimm/dxm126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, Aguet M. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J Exp Med. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]