Summary
The Duffy antigen receptor for chemokines (DARC) has a high affinity for CC and CXC chemokines. However, it lacks the ability to induce cell responses that are typical for classical chemokine receptors. The role of DARC in inflammatory conditions remains to be elucidated. We studied the role of DARC in a murine model of acute lung injury. We found that in Darc gene deficient (Darc−/−) mice, LPS-induced PMN migration into the alveolar space was elevated more than 2-fold. In contrast, PMN adhesion to endothelial cells and within the interstitial space was reduced in Darc−/− mice. Darc−/− mice also exhibited increased microvascular permeability. Elevated PMN migration in Darc−/− mice was associated with increased concentrations of two essential CXCR2 ligands, CXCL1 and CXCL2/3 in the alveolar space. In the blood, CXCL1 was mostly associated with red blood cells in wild-type mice and with plasma in Darc−/− mice. We found that DARC on red blood cells prevented excessive PMN migration into the alveolar space. In contrast, DARC on non-hematopoietic cells appeared to have only minor effects on leukocyte trafficking in this model. These findings show how DARC regulates lung inflammation by controlling the distribution and presentation of chemokines that bind CXCR2.
Keywords: Acute lung injury, Polymorphonuclear Leukocytes, Transmigration, Chemokines, Inflammation
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) remain major challenges in modern intensive care medicine that are still associated with high mortality and morbidity [1]. ALI and ARDS are characterized by excessive infiltration of polymorphonuclear leukocytes (PMNs) into the lung that can disrupt lung integrity and threaten pulmonary gas exchange. Although the pivotal role of PMNs in lung injury is unchallenged [2–4], molecular mechanisms underlying PMN trafficking in the lung remain obscure. Several targets to control PMN infiltration have been identified and some drug candidates are in clinical trials, but no specific drug is available for treatment of ALI patients today.
Leukocyte recruitment to the lung is initiated by the release of chemokines into the alveolar space. Chemokines are small proteins that are classified according to the number of amino acids between the first and second cysteine residue at the NH2-terminal end [5]. Chemokines exert their biological function through specific G protein-coupled receptors. Signaling involves dissociation of the βγ-subunit from the Gαi protein, leading to a variety of cellular responses including actin polymerization and cell migration [6–8]. Among chemokines that are found in the bronchoalveolar fluid (BAL) of mice, CXCL1 (keratinocyte-derived chemokine, KC) and CXCL2/3 (macrophage inflammatory protein 2, MIP-2) are functional homologues of human growth-regulated oncogenes (GROα, β, and γ) [9–11]. CXCL1 and 2/3 are produced by alveolar macrophages and Type II pneumocytes [12] and contribute to the generation of a chemotactic gradient that attracts chemokine receptor CXC 2 (CXCR2)-expressing leukocytes. Depletion of alveolar macrophages attenuates leukocyte recruitment to the lung and reduces organ damage [13;14]. Accordingly, pulmonary overexpression of CXCL1 induces a substantial increase in PMN influx to the lung [15].
In addition to their local effects, CXC chemokines are selectively transported out of the alveolar space into the systemic circulation [16] where they activate CXCR2-expressing leukocytes, thereby altering their mechanical properties [17] and enhancing their migratory activity [18]. Furthermore, chemokines can be presented on the pulmonary endothelium where they cause PMNs to adhere and to transmigrate and induce cytoskeletal changes in endothelial cells that promote the influx of edema fluid [19].
The Duffy antigen receptor for chemokines (DARC), originally described as blood group antigen and binding site for Plasmodium vivax et knowlesi [20], is a receptor that has a broad chemokine binding profile and binds most inflammatory CC and CXC chemokines [21]. However, failure to couple with G proteins causes DARC to function as a decoy chemokine receptor, i.e. chemokine binding does not induce any known cell responses beyond chemokine internalization [22]. Recently, DARC has attracted attention for its ability to modulate HIV infection by controlling plasma levels of HIV-suppressive chemokine CCL5 (RANTES) [23]. DARC is expressed on red blood cells, on endothelial cells of capillaries and postcapillary venules, and on various other cell types including epithelial cells in kidney and lung. DARC on red blood cells may serve as a chemokine sink, thereby limiting activation and subsequent desensitization of circulating leukocytes [24]. Consistent with this hypothesis, Darc-deficient mice exhibited increased numbers of PMNs in lungs and liver after an intraperitoneal LPS challenge [25]. However, another group found less PMN infiltration into the lung in response to i.p. LPS [26]. Neither study differentiated LPS-induced PMN accumulation in the different compartments of the lung or determined the contribution of endothelial versus RBC DARC.
Endothelial DARC has been implicated in the inhibition of chemokine-induced angiogenesis in experimental models [27;28], most likely by preventing chemokine binding to endothelial CXCR2. Under inflammatory conditions, however, endothelial DARC is involved in the transport of chemokines from the inflamed tissue to the luminal side of the endothelium [29], thereby facilitating leukocyte recruitment and aggravating inflammation [30]. Although elevated expression of DARC has been demonstrated on the endothelium of various inflamed tissues [31–33] and its expression has been linked to leukocyte migration in vitro [21], its contribution to the infiltration of PMNs into the lung is not known.
In the present study, we sought to identify mechanisms by which DARC regulates PMN trafficking in the lung. In a murine model of LPS-induced lung injury, we determined the role of DARC for the different migration steps in the lung. We further determined the effects of DARC for the spatial distribution of relevant CXC chemokines and characterized the contribution of endothelial versus erythrocyte DARC by creating bone marrow chimeras.
Results
DARC limits LPS-induced PMN recruitment into the BAL
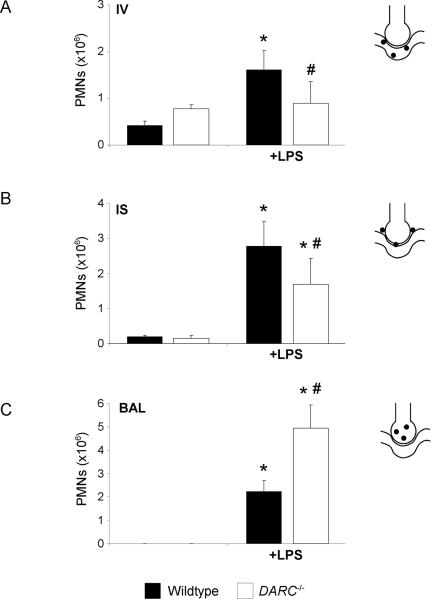
We used a flow cytometry-based method to determine LPS-induced PMN migration into the different compartments of the lung. Baseline blood PMN counts were similar in wildtype and Darc−/− mice (data not shown). After 24h, LPS inhalation induced significant PMN recruitment into all compartments of the lung of wildtype and Darc−/− mice (Figure 1). LPS-induced accumulation of PMNs in the intravascular compartment was significantly decreased in Darc−/− mice compared with wild-type mice (0.9 ± 0.5 ×106 vs. 1.6 ± 0.4×106, P < 0.05), whereas PMNs in the alveolar space were significantly increased (4.9 ± 1.0 ×106 vs. 2.2 ± 0.5}106, P < 0.05). Consistent with increased migration of PMNs into the bronchoalveolar space of Darc−/− mice, PMN accumulation in the interstitial space was relatively reduced in these mice (1.7 ± 0.7 ×106 vs. 2.8 ± 0.3×106, P < 0.05). These data suggest that DARC, although promoting PMN recruitment from the blood to the pulmonary microcirculation, limits transmigration into the alveolar space in vivo.
Figure 1.
LPS-induced migration of PMNs into the different lung compartments of wildtype (black bars) and Darc−/− (white bars) mice. Accumulation of PMNs in the vasculature (IV) (A), the lung interstitium (IS) (B), and the bronchoalveolar space (BAL) (C) were analyzed. Values are means ± SD of n = 4 animals. * P < 0.05 versus negative control without LPS, # P < 0.05 versus wildtype mice within the same treatment group.
Microvascular permeability
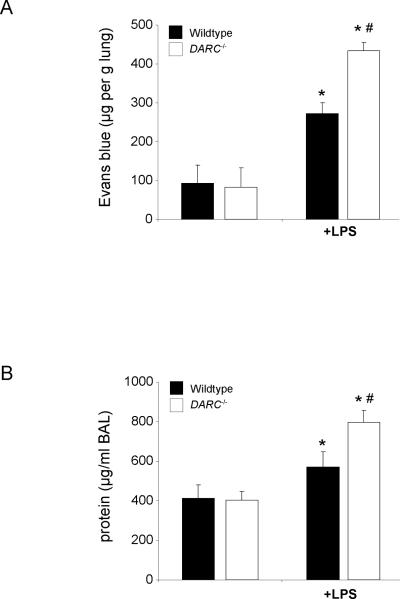
Disturbance of the endothelial barrier function and efflux of protein-rich fluid into the lung tissue is one of the critical events in ARDS that can accompany PMN infiltration. We therefore determined the role of DARC in LPS-induced microvascular permeability assessed by the extravasation of Evans blue (Figure 2A) and by total protein concentration in the BAL (Figure 2B). LPS induced a significant increase in microvascular permeability in wildtype (271 ± 28 vs. 92 ± 47 μg/g lung, P < 0.05) and Darc−/− mice (433± 21 vs. 83 ± 49 μg/g lung, P < 0.05). The LPS-induced increase in microvascular permeability was significantly higher in Darc−/− mice (433 ± 21 vs. 271 ± 28 μg/g lung, P < 0.05). Similarly, LPS-induced total protein Darc−/− mice in the BAL was significantly higher in Darc−/− mice compared to wildtype mice (797 ± 58 vs. 570 ± 76 μg/ml, P < 0.05).
Figure 2.
LPS-induced microvascular permeability in wildtype and Darc−/− mice, assessed by the extravasation of Evans blue into the lung (A) and total protein concentration in the BAL (B). LPS inhalation induced a significant increase in Evans blue extravasation in wildtype (black bars) and Darc−/− mice (white bars). Evans blue extravasation increase in Darc−/− mice was significantly higher than in wildtype mice (P < 0.05). Similarly, LPS-induced total protein concentration in the BAL was significantly higher in Darc−/− mice compared to wildtype mice. Data are mean ± SD from 4 animals, * P < 0.05 versus negative control within the same strain, # P < 0.05 versus wildtype mice within the same treatment group.
Compartmental distribution of chemokines
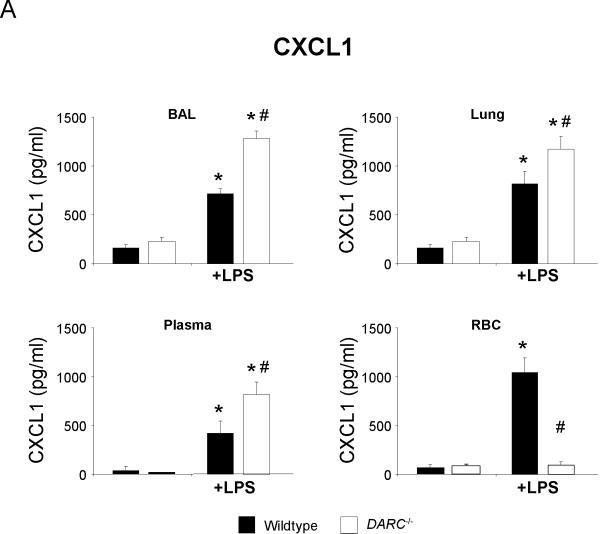
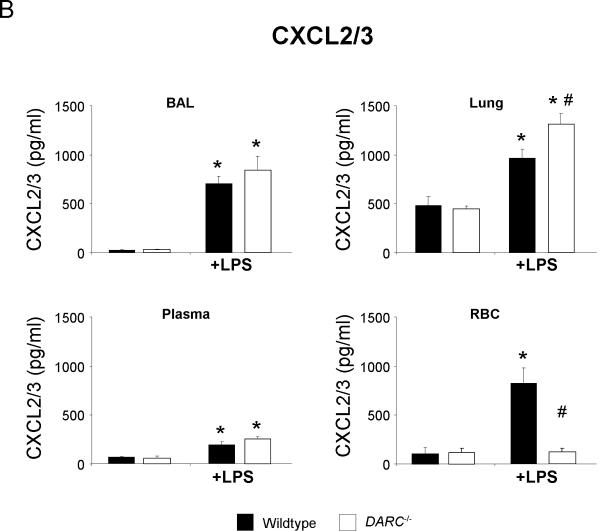
To determine whether DARC mediates its effects in acute lung injury by altering the spatial distribution of chemokines, we measured the concentration of the two essential CXCR2 ligands CXCL1 and CXCL2/3 in BAL, lung homogenate, plasma, and RBC lysate. Baseline concentrations of both chemokines did not differ between wildtype and Darc−/− mice (Figure 3). LPS significantly increased the release of CXCL1 into the BAL and lung tissue (Figure 3A). This increase was significantly higher in Darc−/− mice than in wildtype mice (P < 0.05 in either compartment). Similar results were obtained for CXCL2/3 (figure 3B).
Figure 3.
LPS-induced release of essential chemokines CXCL1 (A) and CXCL2/3 (B) into BAL, lung, plasma, and red blood cell lysate of wildtype (black bars) and Darc−/− mice (white bars). Values are means ± SD of n = 4 animals. * P < 0.05 versus negative control without LPS, # P < 0.05 versus wildtype mice within the same treatment group.
Upon LPS inhalation, CXCL1 and CXCL2/3 are released from alveolar macrophages into the alveolar airspace and transported to the systemic circulation where they can bind to DARC on red blood cells and on pulmonary endothelial cells [16]. Resident cells in the lung interstitium might be another source of chemokines. We reasoned that DARC deficiency would influence the spatial distribution of CXCL chemokines in response to LPS inhalation. Baseline plasma concentration of CXCL1 and CXCL2/3 was near the detection limit of the ELISA assay in wildtype and Darc−/− mice, but increased significantly upon LPS stimulation. The plasma CXCL1 increase was markedly enhanced in Darc−/− mice as compared to wildtype (P < 0.05). No such difference was observed with CXCL2/3. Consistent with the concept of DARC functioning as a chemokine sink, we found large amounts of CXCL1 and CXCL2/3 bound to red blood cells of wildtype but not of Darc−/− mice.
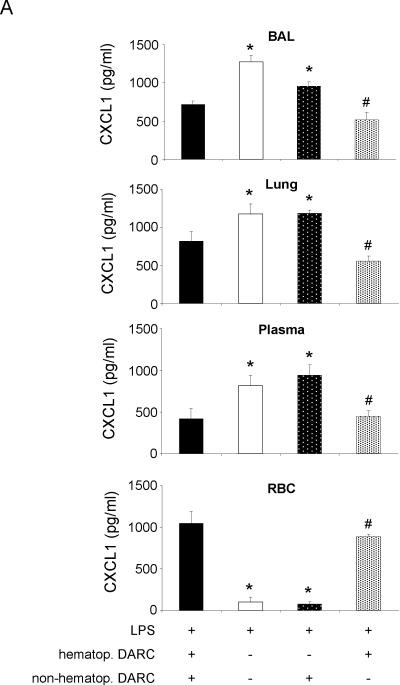
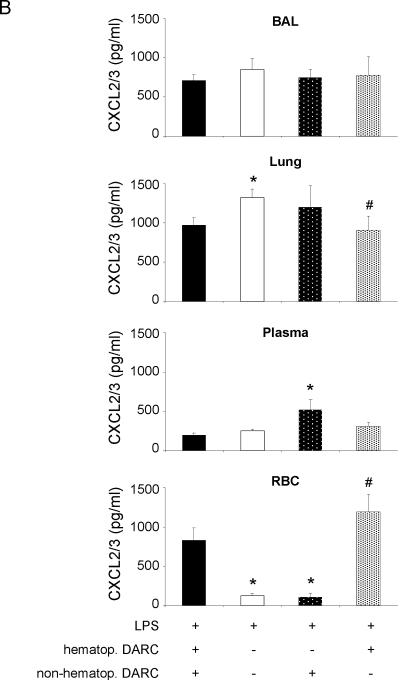
Compartmental distribution of CXCL1 and CXCL2/3 in chimeric mice that expressed DARC only on hematopoietic cells was similar to wildtype mice (Figure 4). Accordingly, chimeric mice that expressed DARC only on non-hematopoietic cells showed an LPS-induced chemokine distribution that was similar to Darc−/− mice, indicating that in our model, compartmentalization of chemokines was mainly regulated by DARC on hematopoietic cells. These studies also provide evidence that in chimeric mice, reconstitution with donor-derived red blood cells was almost complete as chemokines did not bind to red blood cells of chimeric mice that received DARC−/− bone marrow.
Figure 4.
LPS-induced release of essential chemokines CXCL1 (A) and CXCL2/3 (B) into BAL, lung, plasma, and red blood cell lysate of chimeric mice. Means ± SD of n = 4 animals. * P < 0.05 versus mice expressing DARC on all cells, # P < 0.05 versus mice that do not express DARC at all.
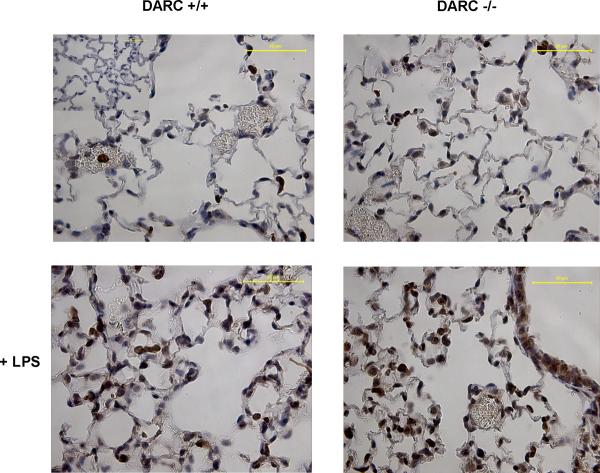
To determine where the increased CXCL1 was localized in the lung of Darc−/− mice, we stained lung paraffin sections with an anti-CXCL1 antibody (Figure 5). Three hours after LPS exposure, CXCL1 accumulation in the lung of Darc−/− mice was higher than in wildtype mice. Most CXCL1 was found on PMNs that were in or near lung capillaries.
Figure 5.
LPS-induced CXCL1 expression in the lungs of wildtype and Darc−/− mice shown by immunohistochemistry. Lung paraffin sections were stained with a CXCL1 antibody (R&D Systems). CXCL1 expression mostly represents PMNs that have been recruited to the lung (dark cells). Images are representative of n = 4 animals. Insert: isotype control.
PMN trafficking in chimeric mice
DARC is expressed on red blood cells and on non-hematopoietic cells such as endothelial and epithelial cells. To characterize the contribution of DARC on hematopoietic and non-hematopoietic cells, we created chimeric mice by transferring BM between wildtype and Darc−/− mice. Irradiated mice that received BM from mice of the same genotype were used as controls.
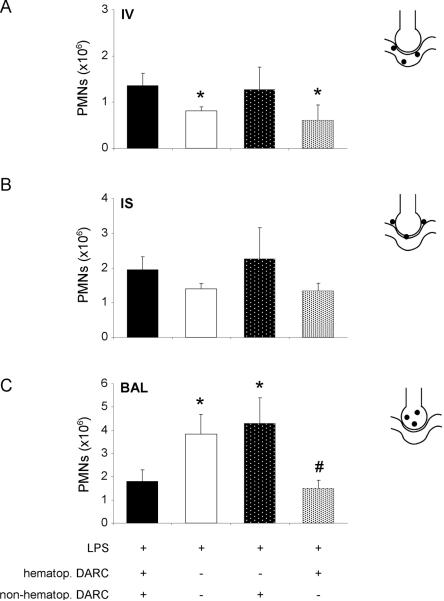
In control mice (BM transfer from wildtype to wildtype, figure 6), LPS-induced PMN accumulation in the different lung compartments was similar to wildtype mice (figure 1). Similarly, Darc−/− that had received Darc−/− bone marrow (figure 6) showed similar neutrophil recruitment as Darc−/− mice (figure 1), indicating that irradiation did not affect migratory activity.
Figure 6.
Contribution of DARC on hematopoietic and non-hematopoietic cells to LPS-induced migration of PMNs into the different compartments of the lung were analyzed by chimeric mice. Reconstitution of blood neutrophils was >90% complete (data not shown). Accumulation of PMNs in the vasculature (IV) (A), the lung interstitium (IS) (B), and the bronchoalveolar space (BAL) (C) are displayed. Means ± SD of n = 4 animals. * P < 0.05 versus mice expressing DARC on all cells, # P < 0.05 versus mice that do not express DARC at all.
When wildtype mice were reconstituted with bone marrow from Darc−/− mice, PMN migration into the alveolar space was enhanced significantly (figure 6C). In these mice, PMN accumulation in all lung compartments was not different from completely DARC null mice. When DARC was expressed on hematopoietic cells only, intravascular and interstitial PMN accumulation were similar to mice that did not express DARC at all. In contrast, PMNs counts in the BAL were significantly reduced when compared to Darc−/− mice (1.4 ± 0.3 ×106 vs. 3.8 ± 0.9×106, P < 0.05). These findings indicate that DARC on bone marrow-derived cells, i.e. mostly red blood cells, prevents excessive transmigration of PMNs into the alveolar space. In contrast, DARC on non-hematopoietic appeared to have only minor effects in our model.
Discussion
In the present study, we sought to characterize pathways that involve DARC in the pathophysiology of acute lung injury. In an experimental model induced by LPS inhalation, DARC on red blood cells prevented excessive infiltration of PMNs into the alveolar space. Increased PMN accumulation in Darc−/− mice was associated with elevated amounts of two essential CXC chemokines, CXCL1 and CXCL2/3, in the BAL. Both chemokines also appeared in the blood of wildtype and Darc−/− mice. In wildtype mice that received LPS, CXCL1 was mostly bound to red blood cells. In Darc−/− mice, LPS inhalation increased plasma levels of CXCL1. Our data is consistent with a dual role of erythrocyte DARC acting as a chemokine sink and endothelial DARC promoting adhesion to and transmigration through the pulmonary endothelium.
The majority of African Americans and West Africans lack DARC on their erythrocytes [34]. In addition to providing resistance to malaria, Darc deficiency has been related to increased incidence and mortality of various malignant diseases, most likely due to the failure of these subjects to eliminate angiogenic chemokines from the blood [35;36]. Whether Darc deficiency has an impact on the course of inflammatory diseases in humans remains unclear. Racial differences in the course of sepsis have become evident with significantly higher incidence and poorer outcome in nonwhite individuals [37]. Although clinically relevant differences in the cytokine profile between African and White Americans have been identified [38;39], the role of DARC has not been addressed. In an experimental study, intravenous LPS-injection resulted in higher plasma levels of monocyte chemoattractant protein-1 (CCL2) and GRO-α (CXCL1) in DARC-positive compared to DARC-negative subjects [40]. However, these differences did not translate into altered leukocyte counts or any organ dysfunction, and the authors of that study concluded that DARC has no protective effect in their model.
The concept of erythrocyte DARC acting as a sink for circulating chemokines derives from early in vitro studies demonstrating that in the presence of red blood cells, interleukin 8 (IL-8) rapidly disappears from the plasma [41]. Later, the IL-8 binding site on erythrocytes was identified as DARC [24], which suggested that DARC might be a negative regulator of inflammation. This hypothesis was supported by the finding that IL-8 lost its ability to activate PMNs when bound to red cells [41]. Consistent with this concept, Darc-deficient mice exhibited increased numbers of PMNs in lungs and liver after an intraperitoneal LPS challenge [25]. However, this concept did not remain unchallenged as others found less PMN infiltration into the lungs or kidneys in response to i.p. LPS [26;42]. Our data shed some light on these apparent discrepancies, because in the previous studies, neutrophil recruitment was not measured separately in the lung compartments. Using a recently developed technique that allows detection of PMNs in all three lung compartments revealed that LPS-induced transepithelial migration of PMNs into the alveolar airspace was significantly elevated in Darc−/− mice whereas PMN accumulation in the pulmonary vasculature and in the lung interstitium was lower than in wildtype mice, suggesting that DARC is not required for PMN adhesion to and migration through the pulmonary endothelium. However, PMN entry into the BAL appears to be DARC-dependent.
Elevated plasma levels of chemokines in Darc deficient subjects have been found by some [43], but not by others [40], suggesting that DARC not only contributes to the clearance of chemokines but also to their release, thus maintaining steady chemokine levels in the blood [44]. In our study, LPS inhalation resulted in increased levels of CXCL1 in BAL and plasma of Darc−/− mice. The same trend was found for CXCL2/3, although less prominent. The murine chemokines CXCL1 and CXCL2/3 are the major chemoattractants responsible for recruiting neutrophils in various inflammatory models. In the lung, both CXC chemokines are mainly produced by type II pneumocytes and alveolar macrophages. We measured chemokines when chemotactic activity of the BAL was highest (data not shown). There is conflicting evidence as to the individual importance of CXCL1 and CXCL2/3 in neutrophil recruitment but it is possible that three hours after LPS exposure, different amounts of both chemokines are found. Synthesis of both chemokines requires TLR-dependent pathways, however, downstream pathways involve distinct adaptor proteins [45], and this may explain differences in the concentrations of both chemokines in our study. In addition, distinct amount of both chemokines are released from circulating neutrophils and may contribute to the different concentrations in the plasma [46;47].
Elevated chemokine levels in the BAL were associated with increased PMN transmigration into the alveolar space of Darc−/− mice. Our data support the concept of erythroid DARC being a chemokine sink that rapidly removes chemokines from the circulation, thereby also diminishing the concentration of alveolar chemokines as they will follow a concentration gradient between lung and blood. Chemokine levels in the BAL are higher in Darc−/− mice, but elevated plasma chemokine levels result in a similar or even reduced chemotactic concentration gradient between plasma and BAL. At the same time, unbound chemokines in the blood of Darc−/− mice retain their ability to activate circulating PMNs, which might lead to mechanical alterations that are sufficient to promote sequestration of PMNs in the pulmonary microcirculation [17]. An intrapulmonary stimulus (LPS inhalation) causes chemokines to be released primarily into the alveolar space before they are transported into the systemic circulation [16].
Chemokine-producing cells in the alveolar space include alveolar macrophages and Type II pneumocytes [12]. In addition, recruited and migrated PMNs contribute to the release of chemokines into the BAL. The present findings are likely specific to the origin of the inflammatory stimulus in the alveolar space. Although our current understanding of endothelial DARC is limited, transport of chemokines from the inflamed tissue to the luminal surface of the vascular endothelium has emerged as one of its roles [21;29;30]. Chemokines that are transported to the apical side of the endothelium are, at least in part, immobilized and presented to circulating leukocytes, thereby promoting leukocyte adhesion and transmigration to the site of inflammation [48]. Therefore, endothelial DARC has been considered a critical regulator of leukocyte transmigration. This concept is supported by the fact that DARC is selectively expressed on subsets of vascular endothelial cells, such as postcapillary venules, that are the preferred sites of leukocyte extravasation. Accordingly, CXCL1-induced migration of PMNs across an endothelial monolayer was reduced in the presence of a functional blocking antibody to DARC [30]. In the lung, intratracheal LPS instillation led to elevated concentrations of CXCL1 and CXCL2/3 in the BAL when endothelial DARC was selectively removed [43], which is consistent with a role of DARC in transporting these chemokines across the endothelial membrane. From the present study (figures 3 and 5), the lung interstitium appears to be another reservoir of CXC chemokines. It remains speculative whether chemokines were produced within the interstitial space or originated from the alveolar space. From our migration studies (figure 1), it becomes evident that PMNs accumulate in large numbers in the interstitium and therefore, chemokine release into this compartment will likely occur, and this is consistent with the chemokine distribution demonstrated in figure 5. Alternatively, chemokines may follow a hydrostatic gradient that is associated with LPS-induced increase in microvascular permeability (figure 2). A recent study demonstrated DARC-dependent transport of chemokines from the abluminal to the luminal side of endothelial cells [21]. However, these experiments did not consider hydrostatic gradients that may contribute to the chemokine transport in our model.
In our study, DARC on hematopoietic cells mainly contributed to the effects of leukocyte trafficking. When wildtype mice were reconstituted with bone marrow from Darc−/− mice, PMN migration into the alveolar space was enhanced significantly. In contrast, leukocyte trafficking in mice that expressed DARC only on non-hematopoietic cells was similar to Darc−/− mice, suggesting that in our model, DARC on non-hematopoietic appeared to have only minor effects.
Despite recent evidence of the biological relevance of endothelial DARC [21], these findings are consistent with previous data reporting a minor significance of endothelial DARC in a model of LPS-induced lung injury [43]. In that study [43], however, infiltration of PMNs into the BAL was lower when erythrocyte DARC was absent and plasma chemokine levels were lower in Darc deficient mice. This is in strong contrast to our findings. One important difference between the two studies is that Lee at al. (51) studied a mild injury with lower PMN counts in the BAL measured at an early time point. This limited PMN recruitment was not associated with increased microvascular permeability and resulted in low CXCL chemokine levels. DARC appears to be more significant in severe inflammatory disorders [25], presumably because the red blood cell sink for chemokines begins to play a role only when large amounts of chemokines are produced. Mild lung injury is likely dominated by the effects of DARC on transendothelial chemokine transport and presentation. Our data help to resolve the apparent discrepancy between the previous studies, because we show the distinct impact of both roles of DARC by bone marrow chimerism and PMN counting in all three compartments.
Various experimental models of ALI have been established. We chose a model of LPS inhalation as this method leads to a consistent migration of PMNs into all compartments of the lung. As a downside of this model, LPS inhalation results in a transient pulmonary inflammation that is self-limiting and therefore not suitable for survival studies. It will be very interesting to study the phenotype of the DARC knockout mice in other, more aggressive models of ALI, including models of VILI [49] or live bacteria as both models involve chemokine-dependent PMN trafficking. We speculate that DARC knockout mice would be much more susceptible to VILI and therefore exhibit increased mortality.
Other pathways regulating chemokine-dependent pulmonary leukocyte trafficking have been discussed. Extracellular adenosine, for instance, plays a major role in various models of pulmonary inflammation [50;51] and is directly linked to the release of chemotactic chemokines [52]. Whether DARC is involved in adenosine-dependent inflammatory pathways remains speculative but it appears attractive to confirm the protective effects of various adenosine receptors in DARC deficient individuals.
Our study confirms and extends previous reports that DARC is a critical regulator of the compartmentalization of relevant chemokines and has therefore direct effects on the directed leukocyte trafficking to inflamed tissue. We show that erythrocyte DARC limits excessive PMN infiltration into the lung, whereas endothelial DARC has pro-inflammatory properties by presenting chemokines on the luminal surface of the inflamed vascular endothelium, thereby causing intravascular neutrophil accumulation.
Materials and methods
Mice
Wild type male C57Bl/6 mice were obtained from Jackson Labs (Bar Harbor, ME). Mice with a null mutation of the Darc gene (Darc−/− mice) were obtained from Nobuyo Maeda, University of North Carolina [25]. All animal experiments were approved by the Animal Care and Use Committee of the University of Virginia. Mice were eight to twelve weeks of age.
Generation of chimeric mice
Chimeric mice were generated by transferring bone marrow (BM) between Darc+/+ (wildtype) and Darc−/− mice as described earlier [53]. Briefly, recipient mice were lethally irradiated in two doses of 600 rad each (separated by 4 hours). This regimen results in >90% donor-derived neutrophils at 6 weeks of reconstitution. Bone marrow from donor mice was harvested from both femora and tibiae, and ~5 million cells were injected intravenously into recipient mice. Bone marrow transplantation (BMT) was performed in four groups of mice: 1) BM from Darc−/− into Darc+/+ (chimeric mice express DARC on non-hematopoietic cells only), 2) BM from Darc+/+ into Darc−/− (chimeric mice express DARC on hematopoietic cells only), 3) BM from Darc−/− into Darc−/−, and 4) BM from Darc+/+ into Darc+/+. Mice in groups 3 and 4 served as negative and positive controls for possible radiation effects. Chimeric mice were used for experiments six weeks after BMT.
Murine model of acute lung injury
Up to four mice were exposed to aerosolized LPS in a custom-built cylindrical chamber (20 × 9 cm) connected to an air nebulizer (MicroAir, Omron Healthcare, Vernon Hills, IL). LPS from Salmonella enteritidis (Sigma Co., St. Louis, MO) was dissolved in 0.9% saline (500μg/ml) and mice inhaled LPS for 30 minutes. As previously shown, this mimics several aspects of acute lung injury including PMN recruitment into all compartments of the lung, increase in vascular permeability [54], release of chemokines and disruption of the pulmonary architecture [55]. Control mice were exposed to saline aerosol.
PMN trafficking in the lung
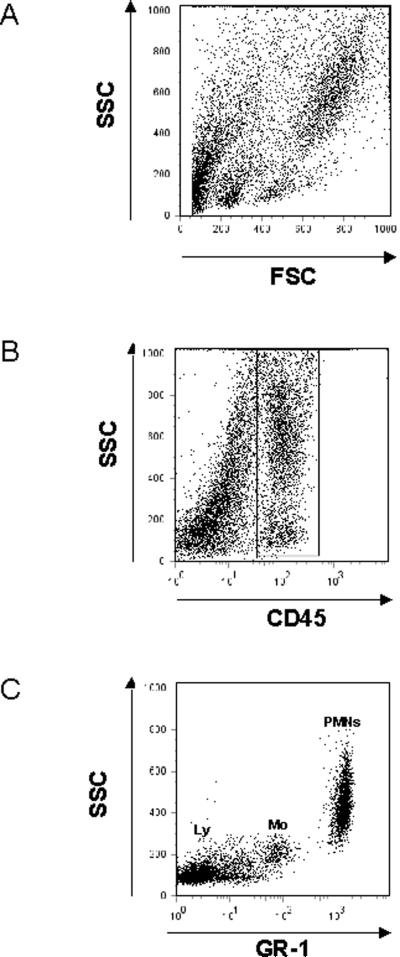
PMN recruitment into the different compartments of the lung (pulmonary vasculature, interstitium, alveolar airspace) was assessed as described [54]. Briefly, 12 hours after LPS exposure, intravascular PMNs were labeled by an intravenous injection of Alexa 633-labeled GR-1. After 5 minutes, mice were euthanized and non-adherent PMN were removed from the pulmonary vasculature by flushing 10ml of PBS at 25 cmH2O through the spontaneously beating right ventricle. BAL was withdrawn and lungs were removed, minced and digested in the presence of excess unlabeled anti-GR-1 to prevent possible binding of the injected antibody to extravascular PMN. A cell suspension was prepared by passing the digested lungs through a 70 μm cell strainer (BD Falcon, Bedford, MA). Total cells in BAL and lung were counted and the percentage of PMNs determined by flow cytometry. In the BAL, PMNs were identified by their typical appearance in the forward/sideward scatter and their expression of CD45 (clone 30-F11), 7/4 (clone 7/4), and GR-1 (clone RB6-8C5). In the lung, the expression of GR-1 was used to distinguish intravascular (CD45+7/4+GR-1+) from interstitial (CD45+7/4+GR-1−) PMNs, which were not reached by the injected antibody [54]. The GR-1 antibody recognizes both Ly6C on monocytes and Ly6G on neutrophils. However, expression of Ly6C on monocytes is much lower than expression of Ly6G on neutrophils, so PMNs can be safely distinguished by flow cytometry (Figure 7).
Figure 7.
Identification of PMNs by flow cytometry. Leukocytes were identified by their typical appearance in the forward/sidescatter (A) and their expression of CD45 (B). Among all leukocytes, PMNs were identified by their expression of GR-1 (C) and 7/4 (not shown). Please note that monocytes (Mo) also express low levels of Ly6C that is recognized by the GR-1 antibody. Mean fluorescent intensity, however, is lower than in PMNs. Lymphocytes (Ly) are not recognized by the GR-1 antibody.
Pulmonary microvascular permeability
Pulmonary microvascular permeability in wildtype and Darc−/− mice was determined by measuring extravasation of Evans blue dye [56]. Evans blue (20mg/kg; Sigma-Aldrich) was injected intravenously 30 minutes prior to euthanasia. Lungs were perfused through the spontaneously beating right ventricle to remove intravascular dye. Lungs were removed and Evans blue was extracted as described [57]. The absorption of Evans blue was measured at 620 nm and corrected for the presence of heme pigments: A620 (corrected) = A620 − (1.426 × A740 + 0.030) [58]. Extravasated Evans blue was determined in the different animal groups 6 hours after LPS or saline inhalation and calculated against a standard curve (micrograms Evans blue dye per gram lung).
BAL total protein concentration
BAL fluid total protein levels were determined using a bicinchoninic acid (BCA) protein assay (Pierce, Omnilabo International, The Netherlands) according to manufacturers' instructions with BSA as standard [59].
Compartmental distribution of chemokines
CXCL1 and CXCL2/3 are released into the alveolar space by alveolar macrophages and Type II pneumocytes, thereby generating a chemotactic gradient that attracts CXCR2-expressing leukocytes from the peripheral blood to the pulmonary microcirculation. To determine whether DARC is involved in the spatial distribution of CXCL1 and CXCL2/3, LPS-induced release of CXCL1 and CXCL2/3 was measured in BAL, lung homogenate (BAL removed), plasma, and red blood cell (RBC) lysates of wild-type, Darc−/−, and chimeric mice using enzyme-linked immunosorbent assay kits (detection limit 15pg/ml, R&D Systems, Minneapolis, MN) [51]. To isolate RBCs, heparinized blood was centrifuged (1000 rpm for 15 minutes at room temperature), the pellet was transferred to a borosilicate glass tube and re-centrifuged in 1ml 2% methyl cellulose. The upper phase was removed, and 200μl RBCs from the bottom were washed with PBS. 50μl RBCs were lysed, washed again, and the supernatant was stored at −80°C.
Immunohistochemistry
Wildtype and Darc−/− mice were euthanized 3 hours after exposure to LPS. Control mice did not receive LPS. The pulmonary circulation was perfused free of blood, the trachea was cannulated, and the lung was inflated with 4% paraformaldehyde (PFA) for ten minutes at 25cmH2O. The lungs were subsequently removed and fixed in PFA for 24 hours. Paraffin-embedded sections (5 μm) were stained for PMNs utilizing the avidin-biotin technique (Vector Laboratories) as previously described [60]. Briefly, deparaffinized and rehydrated sections were incubated with avidin, 10% goat serum, and 0.5% fish skin gelatin oil (FSGO) for one hour to block nonspecific binding. After washing with PBS, a polyclonal antibody against mouse CXCL1 (R&D Systems) was added (1 μg/ml) and incubated overnight. Sections were then washed and incubated with 5 μg/ml biotinylated rabbit anti-goat IgG (Vector Laboratories) for one hour, followed by avidin-biotin-peroxidase complexes (Vectastain Elite ABC kit, Vector Laboratories), washed with PBS, incubated with diaminobenzidine (DAB kit, Vector Laboratories) and counterstained with hematoxylin.
Statistical analysis
Statistical analysis was performed with JMP Statistical Software (version 7.0, SAS Institute Inc., Cary, NC). Differences between the groups were evaluated by one way analysis of variance (ANOVA) followed by a post hoc Tukey test. Data were presented as mean ± SD and P < 0.05 was considered statistically significant.
Acknowledgement
This study was supported by the German Research Foundation (grant RE 1683/3-1 to J. Reutershan), by NIH grant HL73361 to K. Ley.
List of abbreviations
- DARC
Duffy antigen receptor for chemokines
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- PMNs
polymorphonuclear leukocytes
- BAL
bronchoalveolar fluid
- RBC
red blood cells
- BM
bone marrow
- BMT
bone marrow transplantation
- LPS
lipopolysaccharide
Footnotes
Conflict of interest None
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N.Engl.J.Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Azoulay E, Darmon M, Delclaux C, Fieux F, Bornstain C, Moreau D, Attalah H, Le Gall JR, Schlemmer B. Deterioration of previous acute lung injury during neutropenia recovery. Crit Care Med. 2002;30:781–786. doi: 10.1097/00003246-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, Gunther KL, Rashkin MC, Keeton DA, Pattishall EN. Changes in the inflammatory response of the lung during acute respiratory distress syndrome: prognostic indicators. Am.J.Respir.Crit Care Med. 1996;154:76–81. doi: 10.1164/ajrccm.154.1.8680703. [DOI] [PubMed] [Google Scholar]
- 4.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am.J.Physiol Lung Cell Mol.Physiol. 2000;279:L1137–L1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 5.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Rot A, Von Andrian UH. Chemokines in Innate and Adaptive Host Defense: Basic Chemokinese Grammar for Immune Cells. Annu.Rev.Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 7.Neptune ER, Bourne HR. Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs. Proc.Natl.Acad.Sci.U.S.A. 1997;94:14489–1449. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu D, LaRosa GJ, Simon MI. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261:101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- 9.Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J.Clin.Invest. 2002;110:1703–1716. doi: 10.1172/JCI15849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lomas-Neira JL, Chung CS, Wesche DE, Perl M, Ayala A. In vivo gene silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage-induced, neutrophil-mediated septic acute lung injury. J.Leukoc.Biol. 2005;77:846–853. doi: 10.1189/jlb.1004617. [DOI] [PubMed] [Google Scholar]
- 11.Sue RD, Belperio JA, Burdick MD, Murray LA, Xue YY, Dy MC, Kwon JJ, Keane MP, Strieter RM. CXCR2 is critical to hyperoxia-induced lung injury. J.Immunol. 2004;172:3860–3868. doi: 10.4049/jimmunol.172.6.3860. [DOI] [PubMed] [Google Scholar]
- 12.Vanderbilt JN, Mager EM, Allen L, Sawa T, Wiener-Kronish J, Gonzalez R, Dobbs LG. CXC chemokines and their receptors are expressed in type II cells and upregulated following lung injury. Am.J.Respir.Cell Mol.Biol. 2003;29:661–668. doi: 10.1165/rcmb.2002-0227OC. [DOI] [PubMed] [Google Scholar]
- 13.Gazoni LM, Tribble CG, Zhao MQ, Unger EB, Farrar RA, Ellman PI, Fernandez LG, Laubach VE, Kron IL. Pulmonary macrophage inhibition and inhaled nitric oxide attenuate lung ischemiareperfusion injury. Ann.Thorac.Surg. 2007;84:247–253. doi: 10.1016/j.athoracsur.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 14.Maus UA, Koay MA, Delbeck T, Mack M, Ermert M, Ermert L, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J. Role of resident alveolar macrophages in leukocyte traffic into the alveolar air space of intact mice. Am.J.Physiol Lung Cell Mol.Physiol. 2002;282:L1245–L1252. doi: 10.1152/ajplung.00453.2001. [DOI] [PubMed] [Google Scholar]
- 15.Mehrad B, Wiekowski M, Morrison BE, Chen SC, Coronel EC, Manfra DJ, Lira SA. Transient lung-specific expression of the chemokine KC improves outcome in invasive aspergillosis. Am.J.Respir.Crit Care Med. 2002;166:1263–1268. doi: 10.1164/rccm.200204-367OC. [DOI] [PubMed] [Google Scholar]
- 16.Quinton LJ, Nelson S, Zhang P, Boe DM, Happel KI, Pan WH, Bagby GJ. Selective transport of cytokine-induced neutrophil chemoattractant from the lung to the blood facilitates pulmonary neutrophil recruitment. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2004;286:L465–L472. doi: 10.1152/ajplung.00153.2003. [DOI] [PubMed] [Google Scholar]
- 17.Mueller GA, Quinlan WM, Doyle NA, Doerschuk CM. The role of cytoskeletal proteins in neutrophil emigration during pneumonia in rabbits. Am.J.Respir.Crit Care Med. 1994;150:455–461. doi: 10.1164/ajrccm.150.2.8049829. [DOI] [PubMed] [Google Scholar]
- 18.Pallister I, Dent C, Topley N. Increased neutrophil migratory activity after major trauma: a factor in the etiology of acute respiratory distress syndrome? Crit Care Med. 2002;30:1717–1721. doi: 10.1097/00003246-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, Ley K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J.Clin.Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimberg BT, Udomsangpetch R, Xainli J, McHenry A, Panichakul T, Sattabongkot J, Cui L, Bockarie M, Chitnis C, Adams J, Zimmerman PA, King CL. Plasmodium vivax invasion of human erythrocytes inhibited by antibodies directed against the Duffy binding protein. PLoS.Med. 2007;4:e337. doi: 10.1371/journal.pmed.0040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pruenster M, Mudde L, Bombosi P, Dimitrova S, Zsak M, Middleton J, Richmond A, Graham GJ, Segerer S, Nibbs RJ, Rot A. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat.Immunol. 2009;10:101–108. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pruenster M, Rot A. Throwing light on DARC. Biochem.Soc.Trans. 2006;34:1005–1008. doi: 10.1042/BST0341005. [DOI] [PubMed] [Google Scholar]
- 23.He W, Neil S, Kulkarni H, Wright E, Agan BK, Marconi VC, Dolan MJ, Weiss RA, Ahuja SK. Duffy antigen receptor for chemokines mediates trans-infection of HIV-1 from red blood cells to target cells and affects HIV-AIDS susceptibility. Cell Host.Microbe. 2008;4:52–62. doi: 10.1016/j.chom.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horuk R, Darbonne WC, Hadley TJ, Miller LH. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993;261:1182–1184. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- 25.Dawson TC, Lentsch AB, Wang Z, Cowhig JE, Rot A, Maeda N, Peiper SC. Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC) Blood. 2000;96:1681–1684. [PubMed] [Google Scholar]
- 26.Luo H, Chaudhuri A, Zbrzezna V, He Y, Pogo AO. Deletion of the murine Duffy gene (Dfy) reveals that the Duffy receptor is functionally redundant. Mol.Cell Biol. 2000;20:3097–3101. doi: 10.1128/mcb.20.9.3097-3101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du J, Luan J, Liu H, Daniel TO, Peiper S, Chen TS, Yu Y, Horton LW, Nanney LB, Strieter RM, Richmond A. Potential role for Duffy antigen chemokine-binding protein in angiogenesis and maintenance of homeostasis in response to stress. J.Leukoc.Biol. 2002;71:141–153. [PMC free article] [PubMed] [Google Scholar]
- 28.Xu L, Ashkenazi A, Chaudhuri A. Duffy antigen/receptor for chemokines (DARC) attenuates angiogenesis by causing senescence in endothelial cells. Angiogenesis. 2007;10:307–318. doi: 10.1007/s10456-007-9084-y. [DOI] [PubMed] [Google Scholar]
- 29.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 30.Lee JS, Frevert CW, Wurfel MM, Peiper SC, Wong VA, Ballman KK, Ruzinski JT, Rhim JS, Martin TR, Goodman RB. Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo. J.Immunol. 2003;170:5244–5251. doi: 10.4049/jimmunol.170.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruhl H, Vielhauer V, Weiss M, Mack M, Schlondorff D, Segerer S. Expression of DARC, CXCR3 and CCR5 in giant cell arteritis. Rheumatology.(Oxford) 2005;44:309–313. doi: 10.1093/rheumatology/keh485. [DOI] [PubMed] [Google Scholar]
- 32.Patterson AM, Siddall H, Chamberlain G, Gardner L, Middleton J. Expression of the duffy antigen/receptor for chemokines (DARC) by the inflamed synovial endothelium. J.Pathol. 2002;197:108–116. doi: 10.1002/path.1100. [DOI] [PubMed] [Google Scholar]
- 33.Segerer S, Regele H, Mack M, Kain R, Cartron JP, Colin Y, Kerjaschki D, Schlondorff D. The Duffy antigen receptor for chemokines is up-regulated during acute renal transplant rejection and crescentic glomerulonephritis. Kidney Int. 2000;58:1546–1556. doi: 10.1046/j.1523-1755.2000.00316.x. [DOI] [PubMed] [Google Scholar]
- 34.Hadley TJ, Peiper SC. From malaria to chemokine receptor: the emerging physiologic role of the Duffy blood group antigen. Blood. 1997;89:3077–3091. [PubMed] [Google Scholar]
- 35.Shen H, Schuster R, Stringer KF, Waltz SE, Lentsch AB. The Duffy antigen/receptor for chemokines (DARC) regulates prostate tumor growth. FASEB J. 2006;20:59–64. doi: 10.1096/fj.05-4764com. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Ou ZL, Hou YF, Luo JM, Shen ZZ, Ding J, Shao ZM. Enhanced expression of Duffy antigen receptor for chemokines by breast cancer cells attenuates growth and metastasis potential. Oncogene. 2006;25:7201–7211. doi: 10.1038/sj.onc.1209703. [DOI] [PubMed] [Google Scholar]
- 37.Martin GS. The epidemiology of sepsis in the United States from 1979 through. 2000. [DOI] [PubMed]
- 38.Ness RB, Haggerty CL, Harger G, Ferrell R. Differential distribution of allelic variants in cytokine genes among African Americans and White Americans. Am.J.Epidemiol. 2004;160:1033–1038. doi: 10.1093/aje/kwh325. [DOI] [PubMed] [Google Scholar]
- 39.Reid CL, Perrey C, Pravica V, Hutchinson IV, Campbell IT. Genetic variation in proinflammatory and anti-inflammatory cytokine production in multiple organ dysfunction syndrome. Crit Care Med. 2002;30:2216–2221. doi: 10.1097/00003246-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Mayr FB, Spiel AO, Leitner JM, Firbas C, Kliegel T, Jilma-Stohlawetz P, Derendorf H, Jilma B. Duffy antigen modifies the chemokine response in human endotoxemia. Crit Care Med. 2008;36:159–165. doi: 10.1097/01.CCM.0000297875.55969.DB. [DOI] [PubMed] [Google Scholar]
- 41.Darbonne WC, Rice GC, Mohler MA, Apple T, Hebert CA, Valente AJ, Baker JB. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J.Clin.Invest. 1991;88:1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarbock A, Schmolke M, Bockhorn SG, Scharte M, Buschmann K, Ley K, Singbartl K. The Duffy antigen receptor for chemokines in acute renal failure: A facilitator of renal chemokine presentation. Crit Care Med. 2007;35:2156–2163. doi: 10.1097/01.ccm.0000280570.82885.32. [DOI] [PubMed] [Google Scholar]
- 43.Lee JS, Wurfel MM, Matute-Bello G, Frevert CW, Rosengart MR, Ranganathan M, Wong VW, Holden T, Sutlief S, Richmond A, Peiper S, Martin TR. The Duffy antigen modifies systemic and local tissue chemokine responses following lipopolysaccharide stimulation. J.Immunol. 2006;177:8086–8094. doi: 10.4049/jimmunol.177.11.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukuma N, Akimitsu N, Hamamoto H, Kusuhara H, Sugiyama Y, Sekimizu K. A role of the Duffy antigen for the maintenance of plasma chemokine concentrations. Biochem.Biophys.Res.Commun. 2003;303:137–139. doi: 10.1016/s0006-291x(03)00293-6. [DOI] [PubMed] [Google Scholar]
- 45.De FK, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J.Immunol. 2008;180:4308–4315. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- 46.Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr.Drug Targets.Inflamm.Allergy. 2005;4:273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 47.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol.Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 48.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 49.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J.Clin.Invest. 2008 doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC, Eltzschig HK. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2008 doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 51.Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor a2a stimulation in lipopolysaccharide-induced lung injury. J.Immunol. 2007;179:1254–1263. doi: 10.4049/jimmunol.179.2.1254. [DOI] [PubMed] [Google Scholar]
- 52.Murphree LJ, Sullivan GW, Marshall MA, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-kappaB in A(2A) adenosine receptor induction. Biochem.J. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forlow SB, Schurr JR, Kolls JK, Bagby GJ, Schwarzenberger PO, Ley K. Increased granulopoiesis through interleukin-17 and granulocyte colony-stimulating factor in leukocyte adhesion molecule-deficient mice. Blood. 2001;98:3309–3314. doi: 10.1182/blood.v98.12.3309. [DOI] [PubMed] [Google Scholar]
- 54.Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am.J.Physiol Lung Cell Mol.Physiol. 2005;289:L807–L815. doi: 10.1152/ajplung.00477.2004. [DOI] [PubMed] [Google Scholar]
- 55.Reutershan J, Chang D, Hayes JK, Ley K. Protective Effects of Isoflurane Pretreatment in Endotoxin-induced Lung Injury. Anesthesiology. 2006;104:511–517. doi: 10.1097/00000542-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 56.Green TP, Johnson DE, Marchessault RP, Gatto CW. Transvascular flux and tissue accrual of Evans blue: effects of endotoxin and histamine. J Lab Clin.Med. 1988;111:173–183. [PubMed] [Google Scholar]
- 57.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JGN. Protective Effects of Sphingosine 1-Phosphate in Murine Endotoxin-induced Inflammatory Lung Injury. Am.J.Respir.Crit.Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 58.Wang LF, Patel M, Razavi HM, Weicker S, Joseph MG, McCormack DG, Mehta S. Role of Inducible Nitric Oxide Synthase in Pulmonary Microvascular Protein Leak in Murine Sepsis. Am.J.Respir.Crit.Care Med. 2002;165:1634–1639. doi: 10.1164/rccm.2110017. [DOI] [PubMed] [Google Scholar]
- 59.Kolosova IA, Mirzapoiazova T, Moreno-Vinasco L, Sammani S, Garcia JG, Verin AD. Protective effect of purinergic agonist ATPgammaS against acute lung injury. Am.J.Physiol Lung Cell Mol.Physiol. 2008;294:L319–L324. doi: 10.1152/ajplung.00283.2007. [DOI] [PubMed] [Google Scholar]
- 60.Olson TS, Singbartl K, Ley K. L-selectin is required for fMLP- but not C5a-induced margination of neutrophils in pulmonary circulation. Am.J.Physiol Regul.Integr.Comp Physiol. 2002;282:R1245–R1252. doi: 10.1152/ajpregu.00540.2001. [DOI] [PubMed] [Google Scholar]