Abstract
This study evaluated the sensitivities and false positive rates of the screening test using ultrasonographic measurement of thickness of nuchal translucency (NT) with different cut-offs for chromosomal aberration in a Korean population. We included 2,570 singleton pregnancies undergoing ultrasound between 11 weeks and 14 weeks of gestation in this study. We analyzed the sensitivities of NT alone for screening chromosomal aberration using three cut-offs -2.5 mm, 3.0 mm, and 95th percentile for each crown rump length (CRL). There were 31 chromosomal aberrations (1.2%) including 12 cases of trisomy 21. The numbers of chromosomal aberrations that were detected by NT with different cut-offs of 2.5 mm, 3.0 mm and the 95th percentile CRL were 22, 18 and 23, respectively. At a threshold of 2.5 mm, the sensitivity and the false positive rate for total chromosomal aberrations were 67.7% and 6.3%, respectively. At 3.0 mm, those were 54.8% and 3.5%, respectively. At the 95th percentile CRL, those were 70.9% and 5.8%, respectively. The use of CRL-dependent cut-offs for nuchal translucency improves the detection of chromosomal aberrations when compared to fixed cut-offs in a Korean population.
Keywords: Nuchal Translucency Measurement, Sensitivity and Specificity, Chromosome Aberrations
INTRODUCTION
The first screening method of screening for trisomy 21 was based on the maternal age. Since the late 1980s, a test was universalized for three fetoplacental products (alpha-fetoprotein, unconjugate estriol, and total hCG) in the maternal serum at the second trimester of gestation. In addition, various sonographic markers such as nuchal fold thickness and femur length in second trimester were introduced, and the nuchal translucency (NT) became important as the early screening method for chromosomal abnormality (1-3). The test, using maternal serum biochemical markers, has a relatively fixed cut-off of 1:270, and the sensitivity is known to be about 60%. Many studies have reported that the thickened NT could identify about 75% of affected fetuses for a false positive rate of 5%, but various cut-offs were used in those studies (4-7).
Although many studies have asserted that different cut-offs of NT according to the crown rump length (CRL) should be used for screening chromosomal aberration, there are few studies actually comparing the sensitivities and false positive rates of NT with different cut-offs within the same group. In this study, we report herein on those values of NT for chromosomal aberration with three different cut-offs, 2.5 mm, 3.0 mm and the 95th percentile for the CRL in a Korean population.
MATERIALS AND METHODS
This study was conducted with 2,570 pregnancies by searching our clinical database from January 2001 through December 2001. The inclusion criteria were singleton pregnancies who examined ultrasonographic fetal NT measurement between 11 weeks and 14 weeks of gestation at our institution and performed regular antenatal care during the pregnancy with known pregnancy outcome. Pregnancy outcomes were ascertained from the obstetric and neonatal medical records. This study was approved by the institutional review board.
The ultrasonographic scans of fetal NT measurement were carried out by 5 sonographers and 2 obstetricians experienced first-trimester scans more than 5 yr. Fetal NT thickness was measured using the transabdominal ultrasonography (HDI 3000, ATL, Bothell, WA, U.S.A.) in a good mid-sagittal section of the fetus with magnification so that the fetus occupied at least 75% of the image (8). In cases of visualization proved difficult or suspicious findings, a transvaginal ultrasonography was also used. The NT was defined as the black area between the inner skin on the outline echo and the outer border of the soft tissue overlying the cervical spine. The maximal thickness of the black area was measured with a caliper placed on the lines to 0.1 mm when the sagittal section of the fetuses was obtained. At same time, the fetal CRL was also measured. The range of fetal CRL was from 45 mm to 84 mm. Great care was also taken to clearly distinguish between the fetal skin and the amniotic membrane. In some cases this could only be achieved by asking the mother to cough or by tapping the maternal abdomen. In some cases, it was helpful to use the cineloop function (a function that permits the replay of ultrasound images taken in the past few seconds) to visualize the fetus in a satisfactory position. At least three measurements were taken during the scan and the largest of the three measurements was recorded.
We underwent fetal karyotyping in one hundred twenty five women with increased fetal NT (≥2.5 mm) by chorionic villus sampling or amniocentesis. Fetal karyotyping was also offered to women who were maternal age of 35 yr old or more at the time of delivery (n=158), had a positive result on the triple test (n=92), had the history of chromosomal abnormality in any previous pregnancy (n=8), had major fetal structural anomalies on ultrasound examination (n=12), and others (n=24). The karyotyping was also performed in Intrauterine fetal death (IUFD) (n=4) cases. Among the cases who had not been offered fetal karyotyping, we examined the neonates or stillborn for phenotypic abnormality indicating chromosomal aberrations. We excluded the cases (n=8) who had terminated their pregnancies because of major structural anomaly on antenatal ultrasound without fetal karyotyping.
The frequencies of chromosomal aberrations were analyzed and the sensitivities and false positive rates were calculated according to three different cut-offs, namely 2.5 mm, 3.0 mm and the 95th percentile for each fetal CRL. We used the data about the 95th percentile of NT for each CRL of our institution (9).
RESULTS
A total of 2,570 single pregnancies were included in our analysis. The mean maternal age was 29.9±3.3 yr old. The mean CRL was 60.1±9.1 mm. The frequencies of fetuses with increased NT according to cut-offs of 2.5 mm, 3.0 mm, and the 95th percentile of each CRL were 7.1%, 4.1% and 6.5% respectively.
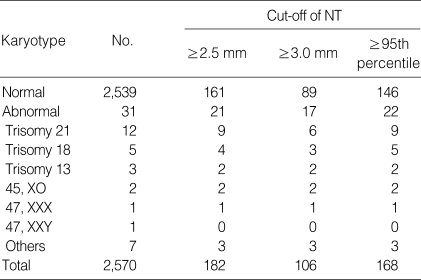
Table 1 shows the types and frequencies of chromosomal aberrations according to the various cut-offs of NT. There were a total of 31 cases (1.2%) of chromosomal aberrations in this study. Among these cases, trisomy 21 was most frequent chromosomal aberration (38.7% [12/31]). The frequencies of chromosomal aberrations that ultrasonographic NT measurement was thickened than that of 2.5 mm, 3.0 mm, and the 95th percentile of NT measurement at each CRL were 11.5% (21/161), 15.9% (17/89), and 13.2% (22/146), respectively. We could detect one more case of trisomy 18 when we used the 95th percentile as the cut-off than the fixed cut-off of 2.5 mm.
Table 1.
Types and frequencies of chromosomal aberrations according to different cut-offs of NT in 2,570 cases
Others: 47,XX+21/46,XX, de novo 45,XX t(13;14) (q10;q10), 47,XY+MAR/46,XY, 45,X/46,X+MAR, 46,XX/46,XY.
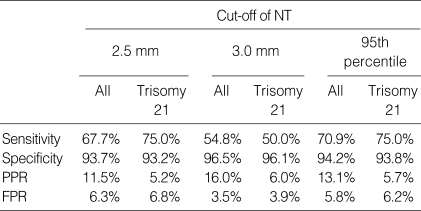
Table 2 shows the sensitivities and false positive rates of NT with each cut-off for screening all the chromosomal aberrations and trisomy 21. The sensitivity for detection of chromosomal aberrations was high (70.9%) in the 95th percentile as the cut-off compared with the fixed cut-offs. The false positive rate was 3.5% when using 3.0 mm, but the sensitivity was 54.8%. When using the 95th percentile of NT, the sensitivity was higher and the false positive rate was lower than those of using 2.5 mm for detecting chromosomal aberrations. The sensitivities of the NT measurement to detect trisomy 21 with two cut-offs, 2.5 mm and the 95th percentile were same (75%), and the false positive rate of the 95th percentile (6.2%) was lower than that of 2.5 mm (6.8%).
Table 2.
Sensitivity, specificity, positive predictive rate, false positive rate of each cut-off of NT for screening all chromosomal aberrations and trisomy 21
PPR, Positive predictive rate; FPR, False positive rate.
DISCUSSION
The various screening methods for detecting chromosomal aberration have been investigated and many studies are ongoing to search for a more efficient screening method. Among the various methods, we evaluated the sensitivities and false positive rates of the screening test of NT measurement alone (not including maternal age) in the first trimester for chromosomal aberrations by using three different cut-offs in a Korean population.
The sensitivity of the NT measurement with 3.0 mm as a cut-off was 54.8% in this study. The early studies about NT measurement mainly used 3.0 mm as the cut-off and the reported various sensitivities were from 39% through 93% (10-12). This wide range of results may be due to the different study populations and/or to an inadequate gestational age at the time of NT measurement. Most of these studies were mainly targeted to a high risk population that was scheduled for fetal karyotyping (11, 12), so the sensitivity of detecting an affected fetus with an increased NT would be higher than that used in the general population. On the other hand, some early studies reported low sensitivity in their results (13, 14). In those studies, the adequate gestational age (in weeks) of the NT measurement was not established, so cases that measured earlier than 11 weeks of gestation were also included as a large proportion of the subjects. All the cases in which the translucency was not visible or measurable were included in the classification of less than 1.0 mm. The cases under 10 weeks of gestational age had the probability of having an immeasurable NT, and the studies that included these subjects would have results with low sensitivity. Currently, the adequate period of the NT measurement is known to be the time between 11 and 14 weeks of gestation (8). In this study, we included pregnant woman in this period.
As extensive data is accumulated regarding the first-trimester NT screening of populations at high risk, attention has been drawn to those populations at low risk and general population (15-17). This data is of major importance because these populations represent the women for whom the vast majority of pregnancies with fetuses having abnormal chromosomes will occur.
The results of the present study are similar to those results of Hafner et al. (16). They assessed what percentage of chromosomal anomalies could be detected by a NT measured in 4,233 unselected women. They diagnosed one additional Down syndrome case by using a sliding scale for the cut-off value with two standard deviations instead of the fixed value of 2.5 mm. So, they suggested that detection rate could probably be improved by raising the values with increasing gestational age. We used the 95th percentile of each CRL as the cut-off value in this study, and we could detect one additional case of trisomy 18.
Recent studies have reported the sensitivities of NT according to various gestational age-dependent cut-offs for detecting chromosomal abnormality (15, 17, 18). One of the largest multicenter study (18) showed that the sensitivity of NT with gestational age-dependent cut-offs for trisomy 21 was 77% for a false positive rate of 5%.
The frequency of trisomy in our study is higher than that of other studies. We presume the reason for high occurrence of trisomy in this study is that we included two cases of trisomy referred from private clinics with abnormal ultrasound findings in first trimester and another two cases of trisomy with IUFD before amniocentesis (The NT thickness of those cases were all increased over 3 mm). Although the sensitivities and false positive rates of NT for detecting of chromosomal aberration were changed after excluding those four cases (62.9%, 48.1%, 66.6% and 6.4%, 3.6%, 5.8% according to cut-offs 2.5 mm, 3.0 mm, 95th percentile), the superiority of 95th percentile NT value as the cut-off for screening test was not changed.
This study is the first study to compare the sensitivities of nuchal translucency alone as a screening test for chromosomal aberration with different cut-offs in a Korean population. When using the 95th percentile of NT, we identified that the sensitivity was higher than that of fixed cut-off for detecting chromosomal aberrations. So, we can suggest that this cut-off should be used for the nuchal translucency measurement screening method in Korean population.
References
- 1.Benacerraf BR, Neuberg D, Bromley B, Frigoletto FD., Jr Sonographic scoring index for prenatal detection of chromosomal abnormalities. J Ultrasound Med. 1992;11:449–458. doi: 10.7863/jum.1992.11.9.449. [DOI] [PubMed] [Google Scholar]
- 2.Benacerraf BR, Nadel A, Bromley B. Identification of second trimester fetuses with autosomal trisomy by use of a sonographic scoring index. Radiology. 1994;193:135–140. doi: 10.1148/radiology.193.1.8090881. [DOI] [PubMed] [Google Scholar]
- 3.Nicolaides KH, Azar G, Byrne D, Mansur C, Marks K. Fetal nuchal translucency: Ultrasound screening for chromosomal defects in first trimester of pregnancy. BMJ. 1992;304:867–869. doi: 10.1136/bmj.304.6831.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandya PP, Snijders RJ, Johnson SP, De Lourdes Brizot M, Nicolaides KH. Screening for fetal trisomies by maternal age and fetal nuchal translucency thickness at 10 to 14 weeks of gestation. Br J Obstet Gynaecol. 1995;102:957–962. doi: 10.1111/j.1471-0528.1995.tb10902.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee JY, Choi KH, Park CW, Yun TS, Park CJ, Jang PR, Park YS. Fetal nuchal translucency measurement for detection of chromosomal abnormalities in the first trimester of high risk pregnancy. Korean J Obstet Gynecol. 1998;41:2739–2742. [Google Scholar]
- 6.Schwarzler P, Carvalho JS, Senat MV, Masroor T, Campbell S, Ville Y. Screening for fetal aneuploidies and fetal cardiac abnormalities by nuchal translucency thickness measurement at 10-14 weeks of gestation as part of routine antenatal care in an unselected population. Br J Obstet Gynaecol. 1999;106:1029–1034. doi: 10.1111/j.1471-0528.1999.tb08109.x. [DOI] [PubMed] [Google Scholar]
- 7.Zoppi MA, Ibba RM, Floris M, Monni G. Fetal nuchal translucency screening in 12,495 pregnancies in Sardinia. Ultrasound Obstet Gynecol. 2001;18:649–651. doi: 10.1046/j.0960-7692.2001.00583.x. [DOI] [PubMed] [Google Scholar]
- 8.Nicolaides KH, Sebire NJ, Snijders RJ. The 11-14 weeks scan: The diagnosis of fetal abnormalities. In: Nicolaides KH, editor. Nuchal translucency and chromosomal defects. London, UK: Parthenon Publishing; 1999. pp. 3–65. [Google Scholar]
- 9.Chung JH, Yang JH, Song MJ, Cho JY, Lee YH, Park SY, Moon MJ, Lim HJ, Choi JS, Kim JO, Shin JS, Ahn HK, Han JY, Kim MY, Choi KH, Ryu HM. The distribution of fetal nuchal translucency thickness in normal Korean fetuses. J Korean Med Sci. 2004;19:32–36. doi: 10.3346/jkms.2004.19.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolaides KH, Brizot ML, Snijders RJ. Fetal nuchal translucency: ultrasound screening for fetal trisomy in the first trimester of pregnancy. Br J Obstet Gynaecol. 1994;101:782–786. doi: 10.1111/j.1471-0528.1994.tb11946.x. [DOI] [PubMed] [Google Scholar]
- 11.Szabo J, Gellen J, Szemere G. First-trimester ultrasound screening for fetal aneuploidies in women over 35 and under 35 years of age. Ultrasound Obstet Gynecol. 1995;5:161–163. doi: 10.1046/j.1469-0705.1995.05030161.x. [DOI] [PubMed] [Google Scholar]
- 12.Brambati B, Cislaghi C, Tului L, Alberti E, Amidani M, Colombo U, Juliani G. First-trimester Down's syndrome screening using nuchal translucency: a prospective study in patients undergoing chorionic villus sampling. Ultrasound Obstet Gynecol. 1995;5:9–14. doi: 10.1046/j.1469-0705.1995.05010009.x. [DOI] [PubMed] [Google Scholar]
- 13.Bewley S, Roberts LJ, Makinson AM, Rodeck CH. First trimester fetal nuchal translucency: problems with screening the general population 2. Br J Obstet Gynaecol. 1995;102:386–388. doi: 10.1111/j.1471-0528.1995.tb11290.x. [DOI] [PubMed] [Google Scholar]
- 14.Scott F, Boogert A, Sinosich M, Anderson J. Establishment and application of a normal range for nuchal translucency across the first trimester. Prenat Diagn. 1996;16:629–634. doi: 10.1002/(SICI)1097-0223(199607)16:7<629::AID-PD922>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 15.Schuchter K, Wald N, Hackshaw AK, Hafner E, Liebhart E. The distribution of nuchal translucency at 10-13 weeks of pregnancy. Prenat Diagn. 1998;18:281–286. [PubMed] [Google Scholar]
- 16.Hafner E, Schuchter K, Liebhart E, Philipp K. Results of routine fetal nuchal translucency measurement at weeks 10-13 in 4,233 unselected pregnant women. Prenat Diagn. 1998;18:29–34. [PubMed] [Google Scholar]
- 17.Theodoropoulos P, Lolis D, Papageorgiou C, Papaioannou S, Plachouras N, Makrydimas G. Evaluation of first trimester screening by fetal nuchal translucency and maternal age. Prenat Diagn. 1998;18:133–137. [PubMed] [Google Scholar]
- 18.Snijders RJ, Noble P, Sebire N, Souka A, Nicolaides KH. UK multicenter project on assessment of risk of trisomy 21 by maternal age and fetal nuchal translucency thickness at 10-14 weeks of gestation. Lancet. 1998;352:343–346. doi: 10.1016/s0140-6736(97)11280-6. [DOI] [PubMed] [Google Scholar]