Abstract
Thromboembolic events are reported to occur with a high frequency in the setting of malignancy. However, reports on an association between cholangiocarcinoma and pulmonary thromboembolism, thus far, are almost lacking. We present here an unusual case of a 56-yr-old patient presenting cholangiocarcinoma and unexplained pulmonary thromboembolism. The patient had been quite healthy before the diagnosis. Coagulation tests showed elevated levels of fibrinogen, fibrinogen degradation product (FDP), D-dimer, and IgM anticardiolipin antibody (aCL Ab). The thromboemboli were resolved 3 weeks after anticoagulant therapy using low-molecular-weight-heparin. Then, follow-up coagulation tests showed a marked decrease to normal in aCL Ab titer as well as the normalization of FDP and D-dimer levels. In this case, we describe pulmonary thromboembolism caused by hypercoagulable state associated with cholangiocarcinoma and speculate that such a thrombotic phenomenon could be regressed by anticoagulant therapy.
Keywords: Cholangiocarcinoma; Pulmonary Embolism; Heparin; Heparin, Low-Molecular-Weight
INTRODUCTION
Cholangiocarcinoma is a highly devastating tumor arising from the epithelial cells of bile ducts. A recent epidemiologic survey reported that the incidence of cholangiocarcinoma is increasing in Western countries and the curative therapeutic approach against this tumor is not available at the present time (1). Hematologic abnormalities are often found in a variety of tumorous condition. In particular, thromboembolic disorders have been reported with an elevated frequency in cancer patients (2-5). However, few data on this subject are available in patients with cholangiocarcinoma (2-4).
We report a case of unexplained pulmonary thromboembolism associated with cholangiocarcinoma, in which coagulation tests showed elevated levels of fibrinogen, fibrinogen degradation product (FDP), D-dimer, and positive anticardiolipin antibody (aCL Ab).
CASE REPORT
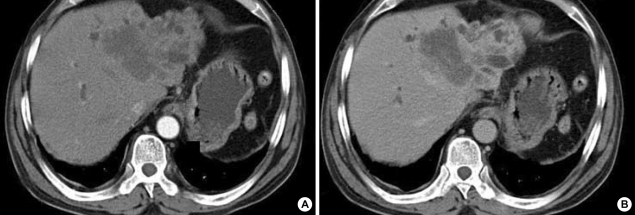
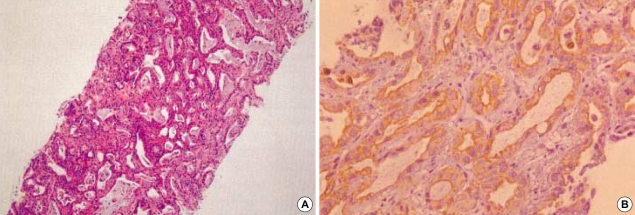
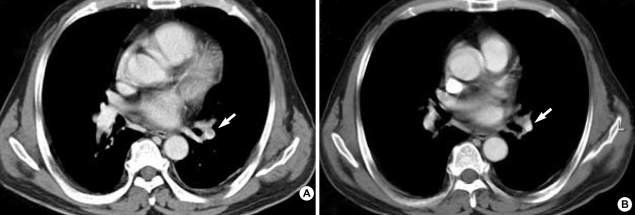
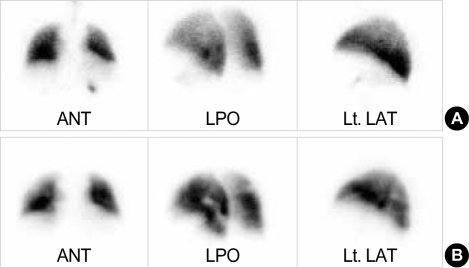
A 56-yr-old man was admitted to our hospital with a complaint of weight loss (6 kg/3 months), and mild shortness of breath at room air. He denied all past history of smoking, excessive alcohol drinking, or chronic diseases. On physical examination, both sclerae were grossly normal, breathing sound was clear, and no cardiac murmur was heard. Abdominal sound was normoactive, and organomegaly was not clear. Arterial blood gas analysis showed pH 7.45, PaO2 65 mmHg, PaCO2 36 mmHg, and O2 saturation 90%. There was no evidence of cardiomegaly, mass shadow, or pulmonary edema in both lung fields on chest radiograph. Laboratory findings showed alanine aminotransferase of 52 U/L, total bilirubin of 1.53 mg/dL, alkaline phosphatase of 597 U/L, and γ-GTP of 126 U/L. Hepatitis B virus surface antigen and antibody to hepatitis C virus were all negative. To evaluate biochemically abnormal findings, abdominal ultrasonography and dynamic CT scan were performed, which showed an ill-defined, poorly enhanced 6.5×7×7 cm-sized mass with several daughter nodules in the left lobe of the liver (Fig. 1). Tests for tumor markers revealed alfa-fetoprotein of 6.19 ng/mL, CA 19-9 of 773.2 U/mL, and CEA of 615.5 ng/mL. Ultrasonography-guided needle biopsy for the liver mass was performed, and then, the histological findings were compatible with cholangiocarcinoma (Fig. 2). On chest CT scan for both of unexplained mild dyspnea and tumor staging, a low density due to filling defect in the left interlobar pulmonary artery was found without any evidence of other metastatic nodules (Fig. 3A). Pulmonary perfusion scan showed multiple perfusion defects in the left lower lung fields (Fig. 4). Echocardiographic examination revealed no evidence of vegetation on the cardiac valves or intracardiac thrombus. Based on the radiological and symptomatic findings of the patient, the diagnosis of pulmonary thromboembolism was made. At the time of diagnosis, coagulation tests showed elevated levels of blood clotting factors, such as D-dimer of 5,690 ng/mL, fibrinogen of 746 mg/dL, fibrinogen degradation product (FDP) of 8.02 µg/mL, and positive IgM anticardiolipin antibody (aCL Ab) of 73 PL (normal limit: <20 PL). The prothrombin time (PT) was 10.8 sec (international neutralization ratio [INR]=0.98), activated partial prothrombin time (aPTT) was 33.2 sec, and other coagulation factors including protein C and S activities, lupus anticoagulant and antithrombin III are all within the normal range. To treat the pulmonary thromboemboli, anticoagulation therapy using low-molecular-weight-heparin (LMWH) in therapeutic dose of 10 IU/kg every 12 hr was given subcutaneously. The patient's symptom was relieved with LMWH treatment over time and the follow up CT scan at 3 weeks after the diagnosis showed an almost complete resolution of the thromboemboli (Fig. 3B). Blood oxygenation was also increased to PaO2 of 83 mmHg and O2 saturation of 97%. Follow-up coagulation tests demonstrated the normalization of FDP, D-dimer, and IgM aCL Ab titer, but only a slight decrease in fibrinogen level (Fig. 5). Systemic chemotherapy for the cholangiocarcinoma was performed. The patient has been followed up without further thrombosis during the next 3 months.
Fig. 1.
Abdominal dynamic CT scan show about 6.5×7×7 cm-sized and ill-defined mass with several daughter nodules in the left lobe. The huge mass with a dilatation of intrahepatic bile ducts is not enhanced on the arterial phase (A), but shows delayed enhancement on the portal phase (B), indicating cholagiocarcinoma.
Fig. 2.
Photomicrograph of liver biopsy specimens. Moderately differentiated adenocarcinoma is shown in the hematoxylin-eosin stain (A; original magnification ×100). On the immunohistochemical staining by using cytokeratin 19 (CK 19), dark-brown staining patterns are observed on the epithelium of proliferating bile ducts (B; original magnification ×400).
Fig. 3.
Initial chest CT scan shows (A) a filling defect with lower density in the left interlobar pulmonary artery, indicating pulmonary thromboembolism. (B) Follow-up chest CT scan after anticoagulant therapy over 3 weeks revealed the interval regression of the thromboembolism.
Fig. 4.
Ventilation-perfusion scan reveals that no definite ventilatory abnormality in both lungs is shown (A), but multiple perfusion defects are found on the left lower lobe (B).
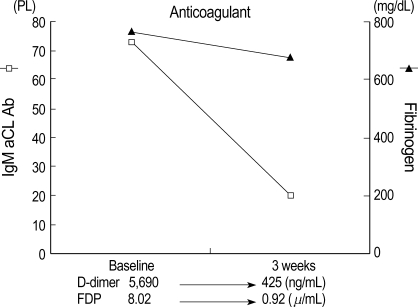
Fig. 5.
Changes in FDP, D-dimer, fibrinogen, and aCL Ab levels during anticoagulant therapy. The levels of FDP, D-dimer, and aCL Ab were normalized, but fibrinogen level was slightly decreased.
DISCUSSION
In 1865, Trousseau first described the relationship between neoplastic and thromboembolic disease (6). Since then, a variety of thromboembolic events including spontaneous, recurrent, or migratory vascular thrombosis, microangiopathy, acute bleeding diathesis, and disseminated intravascular coagulation in cancer patients have been termed Trousseau's syndrome (5, 7). It also presents as non-bacterial thrombotic endocarditis (NBTE), deep vein thrombosis, pulmonary/mesenteric thrombosis, and stroke (2-9). The prevalence of the syndrome was reported to be from 1% to 11% (9, 10). Under the conditions, clinical manifestation of blood coagulopathies ranges from subclinical status to full-blown massive thromboembolisms causing a fatality (11). This syndrome often precedes the initial manifestation of malignancy (8), so that unexplained thromboembolic events occasionally provide a clue to diagnosis of occult malignancy (9).
Our case has a pulmonary thromboembolism associated with cholangiocarcinoma. To our knowledge, there has been only one case reported on the association of cholangiocarcinoma with pulmonary thromboembolism (2). There was no evidence of immobilization, surgery, or trauma, which are known as risk factors for pulmonary thromboembolism or deep vein thrombosis. Concerning the pulmonary emboli in this case, the question of whether the emboli came from the malignant cells or were induced via blood hypercoagulability could be raised. However, the follow-up chest CT showed an almost complete resolution of the thromboemboli by anticoagulant using LMWH. This finding, thus, suggests that the pulmonary emboli was not caused by malignant cell cluster but caused by blood hypercoagulability.
NBTE, as a manifestation of Trousseau's syndrome, has been reported in up to 1.3% of patients dying of cancer (12). Cerebral arteries are most commonly involved sites (4). In our case, no evidence of vegetations was seen on echocardiography, and the patient had no neurologic abnormalities. However, the possibility of NBTE should be considered in all cancer patients presenting with acute stroke syndrome, since it is responsible for around one-third of cerebral infarctions and the diagnosis can be often difficult to establish antemortem (4). Apart from NBTE, the possibility of antiphospholipid syndrome (APS) may also be raised, given the positive test for aCL Ab in our case. APS is suspected by the occurrence of one or more thromboembolic events, pregnancy loss, or premature births in the presence of antiphospholipid antibodies (aPL Ab). Of note, the syndrome is excluded if there is associated infection, cancer, and the use of drug known to induce aPL Ab (13). Thereby, the sole diagnosis of APS is less likely in the present case.
The full mechanism of cancer-induced coagulopathy has not yet been clarified. Recently, possible hypotheses explaining this phenomenon have been proposed (11, 14). Growing tumor leads to tissue hypoxia, and then, tumor cells in turn produce procoagulant and angiogenic factors. Tumor cells can also interact with host cells and vascular endothelium, causing endothelial cell injury. In addition, host blood cells and smooth muscle cells secrete procoagulant factors. Moreover, the coagulation and fibrinolytic activities are dysregulated as well. These tumor cell- and host cell-induced, dysregulated coagulation and fibrinolysis are considered to provide a synergistic means of inducing a cancer-related hypercoagulable state (11, 14).
D-dimer and FDP levels are significantly elevated in patients with deep venous thrombosis, pulmonary embolism, and disseminated intravascular coagulation (15, 16). Serial monitoring of those levels is reported to be of value in evaluating the response to treatment, and persistently positive levels suggest a higher risk of recurrent episodes of thromboembolism (15, 16). In our patient, highly elevated levels of D-dimer and FDP at baseline were improved and significantly reduced following anticoagulant therapy, suggesting that the patient achieved a good response to anticoagulant therapy. Interestingly, aCL Ab titer was also normalized together with a resolution of the pulmonary thromboembolism following anticoagulant therapy. Although an association between the presence of aCL Ab and thromboembolic events in healthy populations is still debated (17), previous reports consistently demonstrated an positive correlation between the two in the setting of malignancy (18-20). Among the coagulation factors examined, aCL Ab was more likely to be clinically correlated with thrombotic process in our patient, suggesting that it might play a role in the development of the thromboemboli. Taken together, under the cancer-induced hypercoagulable state as reflected in the elevated levels of fibrinogen, FDP, and D-dimer, aCL Ab might have triggered or enhanced, at least in part, the thrombosis in our case.
For patients with Trousseau's syndrome, therapy targeted for the underlying malignancy may be reasonable. It is because as long as tumor persists, thrombotic process will be ongoing (9). For this complication, anticoagulant is needed as a prophylaxis and treatment. A recent experimental study demonstrated the advantage of LMWH over unfractionated heparin for cancer-related thromboembolism (21). Consistently, LMWH is also reported to have a therapeutic benefit in clinical applications (9, 22). We chose LMWH as the firstline anticoagulant for the thromboemboli and the present case supported the efficacy of LMWH. Despite the relatively short-term follow-up interval of 3 weeks, the pulmonary thromboembolism was successfully treated by LMWH and never recurred during the next 3 months.
In conclusion, we herein report an unusual case of Trousseau's syndrome associated with cholangiocarcinoma and pulmonary thromboembolism. This is a case of Trousseau's syndrome with cholangiocarcinoma supported by clinical evidence of abnormally increased blood clotting factors, such as fibrinogen, FDP, D-dimer, and aCL Ab. Although the precise mechanisms have not yet been confirmed, this case highlights the implication that procoagulant factors or aPL Ab may play an important role in the thrombotic process in cancer patients, and anticoagulant therapy with LMWH may be helpful to relieve thrombotic symptoms.
References
- 1.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–969. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez JL, Riancho JA, Gonzalez-Macias J. Cholangiocarcinoma presenting as Trousseau's syndrome. Am J Gastroenterol. 1998;93:847–848. doi: 10.1111/j.1572-0241.1998.847a_a.x. [DOI] [PubMed] [Google Scholar]
- 3.Ching CK. Trousseau's syndrome in a patient with cholangiocarcinoma. Am J Gastroenterol. 1991;86:928–929. [PubMed] [Google Scholar]
- 4.Tasi SH, Juan CJ, Dai MS, Kao WY. Trousseau's syndrome related to adenocarcinoma of the colon and cholangiocarcinoma. Eur J Neurol. 2004;11:493–496. doi: 10.1111/j.1468-1331.2004.00814.x. [DOI] [PubMed] [Google Scholar]
- 5.Sack GH, Jr, Levin J, Bell WR. Trousseau's syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore) 1977;56:1–37. [PubMed] [Google Scholar]
- 6.Trousseau A. Phlegmasia Alba Dolens. Clinique medicale de l'Hotel-Dieu de Paris, London: New Syndeham Society. 1865;3:94. [Google Scholar]
- 7.Song MK, Kim YS, Lee KM, Kim SK, Chang J, Kim SK, Lee WY. A case of Trousseau's syndrome associated with lung cancer. Tuberc Respir Dis. 1995;42:941–946. [Google Scholar]
- 8.Nakayama M, Iha T, Kanazawa K. Unusual Trousseau' syndrome in ovarian carcinosarcoma: multiple systemic thromboembolic events. J Obstet Gynaecol. 2002;22:699–700. doi: 10.1080/014436102762062475. [DOI] [PubMed] [Google Scholar]
- 9.Walsh-McMonagle D, Green D. Low-molecular-weight heparin in the management of Trousseau's syndrome. Cancer. 1997;80:649–655. [PubMed] [Google Scholar]
- 10.Minna JD, Bunn PA., Jr . Paraneoplastic syndromes. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. 3rd edition. Philadelphia: JB Lippincott; 1989. pp. 1920–1940. [Google Scholar]
- 11.Denko NC, Giaccia AJ. Tumor hypoxia, the physiological link between Trousseau's syndrome (carcinoma-induced coagulopathy) and metastasis. Cancer Res. 2001;61:795–798. [PubMed] [Google Scholar]
- 12.Rogers LR, Cho ES, Kempin S, Posner JB. Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am J Med. 1987;83:746–756. doi: 10.1016/0002-9343(87)90908-9. [DOI] [PubMed] [Google Scholar]
- 13.Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 14.von Tempelhoff GF, Heilmann L, Hommel G. Tumor hypoxia, the physiological link between Trousseau's syndrome (carcinoma-induced coagulopathy) and metastasis [letter] Cancer Res. 2001;61:7697–7698. [PubMed] [Google Scholar]
- 15.Horan JT, Francis CW. Fibrin degradation products, fibrin monomer and soluble fibrin in disseminated intravascular coagulation. Semin Thromb Hemost. 2001;27:657–666. doi: 10.1055/s-2001-18870. [DOI] [PubMed] [Google Scholar]
- 16.Kuruvilla J, Wells PS, Morrow B, MacKinnon K, Keeney M, Kovacs MJ. Prospective assessment of the natural history of positive D-dimer results in persons with acute venous thromboembolism (DVT or PE) Thromb Haemost. 2003;89:284–287. [PubMed] [Google Scholar]
- 17.Runchey SS, Folsom AR, Tsai MY, Cushman M, McGovern PD. Anticardiolipin antibodies as a risk factor for venous thromboembolism in a population-based prospective study. Br J Haematol. 2002;119:1005–1010. doi: 10.1046/j.1365-2141.2002.03949.x. [DOI] [PubMed] [Google Scholar]
- 18.Proven A, Bartlett RP, Moder KG, Chang-Miller A, Cardel LK, Heit JA, Homburger HA, Petterson TM, Christianson TJ, Nichols WL. Clinical importance of positive test results for lupus anticoagulant and anticardiolipin antibodies. Mayo Clin Proc. 2004;79:467–475. doi: 10.4065/79.4.467. [DOI] [PubMed] [Google Scholar]
- 19.Caprini JA, Glase CJ, Anderson CB, Hathaway K. Laboratory markers in the diagnosis of venous thromboembolism. Circulation. 2004;109(Suppl 1):I4–I8. doi: 10.1161/01.CIR.0000122869.59485.36. [DOI] [PubMed] [Google Scholar]
- 20.Zuckerman E, Toubi E, Golan TD, Rosenvald-Zuckerman T, Sabo E, Shmuel Z, Yeshurun D. Increased thromboembolic incidence in anti-cardiolipin-positive patients with malignancy. Br J Cancer. 1995;72:447–451. doi: 10.1038/bjc.1995.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita S, Gebska MA, Kakkar AK, Scully MF. High affinity binding of heparin by necrotic tumour cells neutralises anticoagulant activity--implications for cancer related thromboembolism and heparin therapy. Thromb Haemost. 2001;86:616–622. [PubMed] [Google Scholar]
- 22.Zuger M, Demarmels Biasiutti F, Wuillemin WA, Furlan M, Lammle B. Subcutaneous low-molecular-weight heparin for treatment of Trousseau's syndrome. Ann Hematol. 1997;75:165–167. doi: 10.1007/s002770050336. [DOI] [PubMed] [Google Scholar]