Abstract
Autofluorescence bronchoscopy (AFB) is one of the newly developed diagnostic tools to detect the pre-cancerous lesions in the bronchial tissue. The utility of D-Light/AFB in the detection of pre-cancerous lesions was compared to the standard white light bronchoscopy (WLB). In 113 patients (male 106, female 7), who visited hospital for evaluation of lung cancer, WLB and AFB were done and 364 biopsy specimens were obtained from November 2001 to August 2002. The bronchoscopic findings on WLB and AFB were compared to the pathological findings. The pathologic diagnoses of the specimens were as follows: normal in 96; hyperplasia in 69; metaplasia in 32; mild dysplasia in 13, moderate dysplasia in 6, severe dysplasia in 4; carcinoma in situ in 6; invasive carcinoma in 57. The relative sensitivity of adjunctive AFB to WLB vs. WLB alone was 1.5 in moderate dysplasia or worse lesions, and 3.2 in intraepithelial neoplasia. The specificity of adjunctive AFB and WLB alone were 0.91 and 0.5, respectively. The adjunctive AFB to the standard WLB increased the detection rate of the localized pre-invasive lesions. However, there was high rate of false positive in AFB.
Keywords: Bronchoscopes, Fluorescence; Bronchoscopy; Lung Neoplasms
INTRODUCTION
It was reported that there were 8,284 new cases of lung cancer in Korea diagnosed in 2001 (1), and the incidence and the number of lung cancer deaths continue to increase. Since the overall 5-yr survival for patient with lung cancer is <15% (2), the future line in both the prevention of new disease by reducing the prevalence of tobacco use, and being able to accurately identify early stage disease will be amenable to curative therapy. Saccomanno et al. (3) described the cellular progression of bronchial tissue from atypical metaplasia to dysplasia to carcinoma in situ (CIS) and invasive carcinoma. It has also been shown that the identification and treatment of these pre-invasive lesions in other organs, such as the cervix and colon, can lead to significant improvement in cancer related mortality (4, 5).
Unfortunately, early detection has not been shown to improve survival in lung cancer patients, and physicians usually do not recommend screening for lung cancer, such as sputum cytology, chest radiology or bronchoscopy. Recent advances in screening include the use of ultra-fast CT, serum biomarkers, and sputum DNA, however their role remains to be defined (6, 7). Bronchoscopy for the detection of early lung cancer may be helpful for two reasons: First, carcinogenesis is slow process evolving over years (3). Secondly, about 50-60% of all lung cancer, especially squamous cell carcinoma, develops in the central airways. These cancers can be detected by bronchoscopy, but are generally roentgenographically occult (8). The main problem for conventional bronchoscopy is its low sensitivity and specificity for detection of early malignant cancer (9). Pre-invasive lesions usually are small and only some cell layer thick (10). Their endoscopic signs therefore are very subtle and can be missed even by experienced bronchoscopists. To improve the sensitivity and specificity of conventional endoscopy, diagnostic fluorescence procedures were developed (11).
Autofluorescence bronchoscopy (AFB) is an emerging endoscopic tool that can identify pre-cancerous lesions in the central tracheobronchial tree, predominantly squamous cell carcinoma. There are four available systems of AFB around the world, but the two most widely used systems are Lung Imaging Fluorescence Endoscopic device (LIFE) and D-light/AFB system. There was only one report (12) of using LIFE, but no report with D-light/AFB system in Korea. The aim of this study was to compare the utility of D-Light/AFB versus the standard white light bronchoscopy (WLB) in the detection of pre-cancerous lesions.
MATERIALS AND METHODS
The study was done during 15 November 2001 to 31 August 2002 in Kosin University Gospel Hospital, Busan, Korea. The criteria for enrollment were as follows: 1) patients with known or suspected bronchogenic cancer who scheduled for bronchoscopy as a part of standard diagnostic workup, 2) follow-up patients who were completely treated with surgery or radiotherapy, 3) positive cytological findings, and 4) smokers older than 40 yr and/or evidence of chronic obstractive pulmonary disease. The exclusion criteria were suspected lung infection, in state of cancer treatment, pregnancy, bleeding disorders, and known reaction to topical lidocaine.
Bronchoscopy was performed with local anesthesia with or without sedation using D-Light/AFB (Karl Storz, Tuttlingen, Germany) and the standard white light bronchoscopy (WLB: BF-1T200, Olympus, Tokyo, Japan). D-Light/AFB system can be used without the application of a photosensitive drug such as ALA. The detection of autofluorescence was performed by flexible bronchoscopes. The color of the normal mucosa is dominated by a green fluorescence (Fig. 1), while malignant abnormal tissues appearing as red/brown areas against a normal gray/blue background. Fig. 2 shows the abnormal florescence and white light bronchoscopic findings of a 74 yr old male patient with CIS in left upper lobe bronchus. He was positive in sputum cytology, but he showed roentgenographically occult chest radiography finding. Fig. 3 shows the pathologic finding of that patient. Details of D-light/AFB systems have been reported elsewhere (13). The investigation was done first with white light mode, and then switching to blue light mode for the detection of the specific autofluorescence. Biopsies were taken from all abnormal areas detected by WLB and/or AFB. Additionally, in almost all patients random biopsies were taken from normally appearing bronchial tissue (2 point classification system used; normal and abnormal finding). Three more biopsy specimens were taken in each biopsy sites. The duty pathologist without knowledge of clinical findings evaluated biopsy slides first, and then one lung pathologist who was blinded to the previous results reviewed all. The pathologists classified the biopsy slides based on WHO classification (14). All biopsies were classified into one of eight groups as follows: normal, hyperplasia, metaplasia, mild atypia, moderate atypia, severe atypia, carcinoma in situ (CIS), and invasive carcinoma. Intraepithelial neoplasia was defined as lesions that were graded as moderate/severe dysplasia or CIS. Hyperplasia or worse was defined as including hyperplasia to invasive carcinoma. Moderate dysplasia or worse also has the similar meaning.
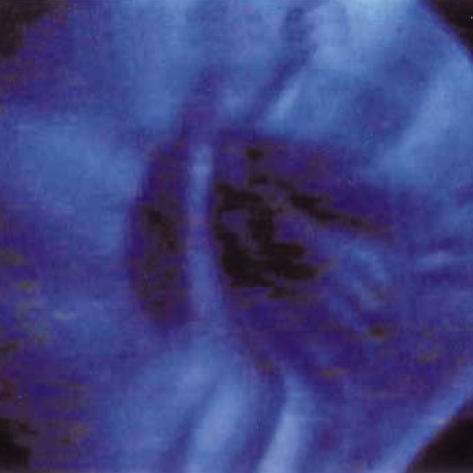
Fig. 1.
Normal fluorescence image.
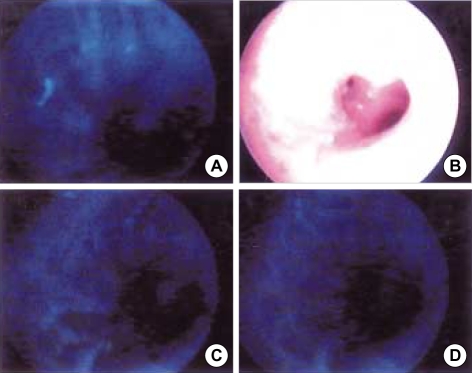
Fig. 2.
Abnormal fluorescence (A, C, and D) and white light bronchoscopic findings (B) of a 74 yr old male patient with carinoma in situ in left upper lobe bronchus, positive in sputum cytology, but roentgenographically occult lung cancer.
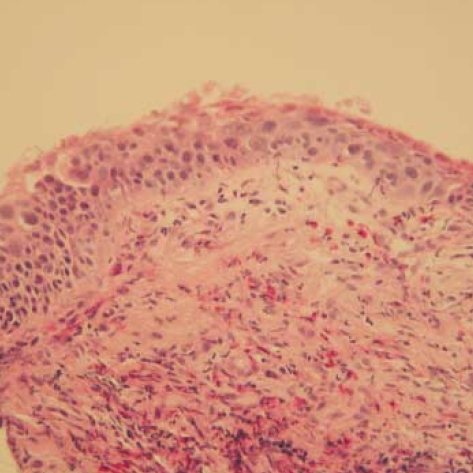
Fig. 3.
Carcinoma in situ: full thickness change with cells showing cytologic features of squamous carcinoma (H & E, ×100).
The sensitivity and specificity of both bronchoscopy procedures were calculated in the standard manner. Thus the sensitivity was defined as the percentage (visually suspicious and histologically positive/total number of histologically positive), and specificity also by the similar way. Positive predictive values and negative predictive values were also calculated for both WLB and AFB in a similar manner. The relative sensitivity, or the ratio of sensitivity of WLB+AFB as compared with WLB alone was calculated to evaluate the contribution of the fluorescence examination to detect neoplastic lesions. A relative sensitivity test >1 would indicate a significant improvement. The data were analyzed on a per lesion basis. For the per lesion analysis, each biopsy specimen was evaluated and considered positive if the final diagnosis was positive.
RESULTS
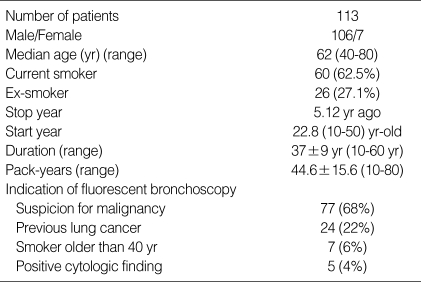
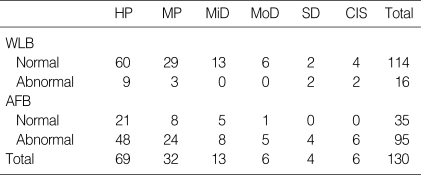
There were 113 patients (male 106, female 7) with median age of 62 yr (range 40-80). Almost all patients (86.9%) were current or ex-smokers with mean pack-years of 44.6 (Table 1). A total of 364 biopsy specimens were obtained. Of these, 81 were not valuable; inflammation in 63 and inadequate specimens in 18. The final pathologic diagnoses of the specimens were as follows: Normal in 96; hyperplasia in 69; metaplasia in 32; mild dysplasia in 13, moderate dysplasia in 6, severe dysplasia in 4; CIS in 6; invasive carcinoma in 57 (Table 2). Thus, 73 biopsy specimens (25.7%) were graded as moderate dysplasia or worse; this was then used as the standard to determine the relative sensitivity in intraepithelial neoplasia of the bronchoscopic procedure.
Table 1.
Characteristics of patients
Table 2.
Histology of biopsy specimen
CIS, Carcinoma in situ.
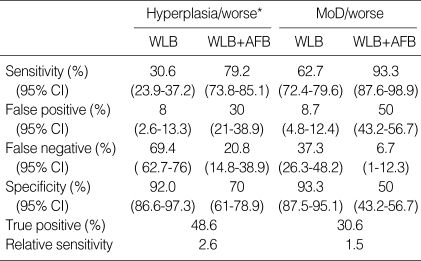
On the lesion-by-lesion analysis, WLB alone detected severe dysplasia 2 among 4, CIS 2 among 6, but moderate dysplasia could not be found. In addition of AFB, we missed only 1 moderate dysplasia (Table 3). The sensitivity of WLB was 30.6%, adjunctive AFB to WLB 79.2%, the specificity of WLB 92%, adjunctive AFB to WLB 70% in hyperplasia or worse lesions. The sensitivity of WLB was 30.6%, WLB+AFB 79.2%, the specificity of WLB 92%, WLB+AFB 70% in moderate dysplasia or worse lesions. The relative sensitivity of adjunctive AFB to WLB vs. WLB alone was 2.6 in hyperplasia or worse and 1.5 in moderate dysplasia or worse lesions (Table 4).
Table 3.
Results of fluorescence bronchoscopy according to histologic classification
*Hyperplasia/worse, intraepithelial neoplasia and invasive carcinoma.
MoD, moderate dysplasia; WLB, white light bronchosocpy; AFB, autofluorescence bronchoscopy.
Table 4.
Results of WLB and FAB according to pathologic finding
HP, hyperplasia; MP, metaplasia; MiD, mild dysplasia; MoD, moderate dysplasia; SD, severe dysplasia; CIS, carcinoma in situ; WLB, white light bronchoscopy; AFB, autofluorescence bronchscopy.
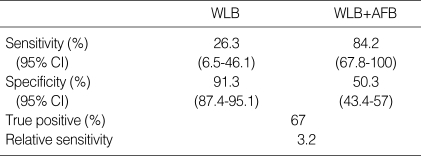
Of the 16 intraepithelial lesions, only 4 were by WLB alone. The addition of the AFB identified 15 sites. Therefore, the relative sensitivity of WLB+AFB vs. WLB alone for intraepithelial lesions was 3.2. The specificity of adjunctive AFB and WLB alone were 0.5 and 0.91, respectively (Table 5). Fifty-seven lesions were classified as invasive carcinoma.
Table 5.
Sensitivity, specificity, true positive, and relative sensitivity in intraepithelial neoplasia of WLB and AFB
Intraepithelial neoplasia, moderate dysplasia; severe dysplasia, and carcinoma in situ. WLB, white light bronchosocpy; AFB, autofluorescent bronchoscopy.
DISCUSSION
The fluorescent system is based on the different fluorescence emissions of normal tissue and tumor tissue, either spontaneously as autofluorescence (AF) light or as drug induced fluorescence by selective accumulation of exogenous applied photosensitizer in the tumor tissue. When a surface is illuminated by light, the light can be reflected, back-scattered, or absorbed. Additionally, light also causes the tissue to fluoresce, however this AF can not be seen during conventional WLB as the intensity of fluorescence is very low, and is overwhelmed by the reflected and back-scattered light. Tissue AF revealed the underlying biochemical changes occurring in the cells, specifically the electronic structure of absorption chromophores. The normal airway fluoresces green when exposed to light in the violet-blue spectrum (400-450 nm), and as sub-mucosal disease progresses from normal, to metaplasia, to dysplasia, to CIS, there is a progressive loss of the green AF, causing a red-brown appearance of the airway wall. This results from reductions in certain chromophores, increasing thickness of the epithelium, as well as an increase in angiogenesis that develops with more advanced disease (15). As intraepithelial neoplastic lesions are only a few cell layers thick, the surface mucosa typically appears relatively normal during WLB. In fact, Woolner et al. (16) found that only 29% of CIS was visible to the experienced bronchoscopist.
Lam et al. (17) introduced fluorescence bronchoscopy that is effective without the use of protoporphyrins for the purpose of localizing endobronchial lesions of moderate dysplasia or more malignant tissue. They initially used a 100 mW helium-cadmium laser to illuminate the airways with blue light (442 nm). Red and green fluorescence intensities were then measured, and expressed as a ratio. Lam's group found that the red/green ratios from areas with moderate dysplasia, severe dysplasia and CIS were significantly higher than those from normal areas (18). Their group went on to develop the LIFE system. The system of Karl-Storz system is based on a modified xenon light source. On a filter in the ocular of the bronchoscope and an optional integrating camera, this can be attached easily. There has been one study (19) comparing LIFE and D-light system. Both systems yielded comparable results. The examination time was significantly shorter with the D-light system, which may be explained by the more comfortable handling and the direct switch between white light and AF imaging.
When compared to the other studies, the relative sensitivity of 3.2 finding rate in the intraepithelial lesion is similar. False positive rate for AFB was 41.1%, compared with 8.7% for WLB. We gained 3.2-folds sensitivity in detecting pre-cancerous lesions by addition of AFB, but lost 0.55-folds decrease in specificity. The relative sensitivity of 1.5 in moderate dysplasia or worse lesions was also similar to the other report (20). That study was a large study with 1,173 patients (916 men) of mean age 58.7 yr using D-light system. Overall pre-invasive lesions (moderate to severe dysplasia, and CIS) were detected in 3.9% of the patients. The biopsy based sensitivity of WLB alone and WLB+AFB for detecting intraepithelial lesion was 57.9% compared with 82.3% (1.42-fold increase). The corresponding specificity was 62.1% compared with 58.4% (0.94-fold decrease). In an initial study of AFB, Lam et al. found a sensitivity of 48% for WLB, and sensitivity of 73% for AFB, a 50% improvement (17). Published data in more than 1,400 patients suggest that WLB alone detects on average only 40% of high-grade dysplasia and CIS, whereas AFB increased the detection rate up to 88% (21). The variation in results of both WLB and AFB can be explained by the quality of conventional WLB as well as the reproducibility of the bronchoscopic and pathologic interpretation (22). The latter problem will hopefully be addressed by utilizing a standardized classification of pre-invasive lung cancer developed by the WHO (14).
Kurie et al. (23) published a negative study. They investigated the additional yield AFB had on WLB in 39 current or former smokers, and found no significant increase in the detection of squamous cell metaplasia or dysplasia. A possible explanation for this is the fact that the prevalence of advanced disease was low in the population studied. The majority of patients were former smokers who had stopped smoking for median of 8 yr prior to the study. This contrasts to the studies where AFB has shown to be beneficial, in that enrolled patients had known or suspected lung cancer, and therefore a higher prevalence of advanced dysplasia/CIS (18).
It appears that the increased sensitivity is associated with decreased specificity, resulting in many false-positive biopsies. Aside from the cost of the autofluorescence unit, the major downside to AFB is the lack of specificity. The specificity of AFB has been found to be low, with up to one third of area of abnormal fluorescence being found to be false positives (24). This results in a greater number of abnormal lesions being identified at the time of bronchoscopy, prolonging the procedure in order to biopsy the negative lesions, and added time and expense for the pathologist, who is required to examine all of the obtained tissue (25). There are many trials to increase the specificity. Quantitative fluorescence imaging or combined fluorescence-reflectance imaging may also be helpful in future bronchoscopic devices to address specificity. However it has been suggested that up to 50% of biopsies obtained from these areas carry molecular genetic lesions associated with malignancy, despite their normal histological appearance (26).
In conclusion, the adjunctive AFB to the standard WLB increased the detection rate of the localized pre-invasive lesions. However, there was high rate of false positive in AFB. Our findings do not support the general use of AFB as a screening tool for lung cancer, but suggest that it may be of use in certain groups.
Footnotes
This work was supported by research funds of Kosin University College of Medicine, Busan, Korea.
References
- 1.Shin HR, Ahn YO, Bae JM, Shin MH, Lee DH, Lee CW, Ohrr HC, Ahn DH, Ferlay J, Parkin DM, Oh DK, Park JG. Cancer incidence in Korea. Cancer Res Treatm. 2002;34:405–408. doi: 10.4143/crt.2002.34.6.405. [DOI] [PubMed] [Google Scholar]
- 2.Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975-2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–1299. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 3.Saccomanno G, Archer VE, Auerbach O, Saunders RP, Brennan LM. Development of carcinoma of the lung as reflected in exfoliated cells. Cancer. 1974;33:256–270. doi: 10.1002/1097-0142(197401)33:1<256::aid-cncr2820330139>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Anderson GH, Boyes DA, Benedet JL, Le Riche JC, Matisic JP, Suen KC, Worth AJ, Millner A, Bennett OM. Organization and results of the cervical cytology screening programme in British Columbia, 1955-1985. Br Med J. 1988;296:975–978. doi: 10.1136/bmj.296.6627.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winawer SJ, Zauber AG, O'Brien MJ, Ho MN, Gottlieb L, Sternberg SS, Waye JD, Bond J, Schapiro M, Panish JF, Ackroyd F, Kurtz RC, Shike M The National Polyp Study Workgroup. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Engl J Med. 1993;328:901–906. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 6.Thunnissen FB. Sputum examination for early detection of lung cancer. J Clin Pathol. 2003;56:805–810. doi: 10.1136/jcp.56.11.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunn PJ., Jr Early detection of lung cancer using serum RNA or DNA markers: ready for "prime time" or for validation? J Clin Oncol. 2003;21:3891–3893. doi: 10.1200/JCO.2003.07.976. [DOI] [PubMed] [Google Scholar]
- 8.Cortese DA, Pairolero PC, Bergstralh EJ, Woolner LB, Uhlenhopp MA, Piehler JM, Sanderson DR, Bernatz PE, Williams DE, Tayne WS, Fontana RS. Roentgenographically occult lung cancer: A ten-year experience. J Thorac Cardiovasc Surg. 1983;86:373–380. [PubMed] [Google Scholar]
- 9.Lam S, MacAuley C, LeRiche JC, Ikeda N, Palcic B. Early localization of bronchogenic carcinoma. Diagn Therap Endosc. 1994;1:75–78. doi: 10.1155/DTE.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasiell M. Metaplasia and atypical metaplasia in the bronchial epithelium: A histopathologic study. Acta Cytol. 1966;10:421–424. [Google Scholar]
- 11.Hayata Y, Kato H, Ono J, Matsushima Y, Hayashi N, Saito T, Kawate N. Fluorescence fiberoptic bronchoscopy in the diagnosis of early stage lung cancer. Recent Results. Cancer Res. 1982;82:121–130. doi: 10.1007/978-3-642-81768-7_11. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Shim JJ, Lee SR, Lee SY, Suh JK, Cho JY, Kim HG, In KH, Choi YH, Kim HJ, Kang KH. Useful of LIFE in diagnosis of bronchogenic cancer. Tubercul Respir Dis. 1996;44:70–84. [Google Scholar]
- 13.Häuβinger K, Stanzel F, Huber RM, Pichler J, Stepp H. Autoflorescence detection of bronchial tumors with the D-light/AF. Diagn Therap Endosc. 1999;5:105–112. doi: 10.1155/DTE.5.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. WHO Pathology Panel: WHO. International histological classification of tumors. 3rd ed. Berlin: Springer Verlag; 1999. Histologic and graphical text sides for the histological typing of lung and pleural tumors. [Google Scholar]
- 15.Keith RL, Miller YE, Gemmill RM, Drabkin HA, Dempsey EC, Kennedy TC, Pridiville S, Fanklin WA. Angiogenic squamous dysplasia in bronchi of individual at high risk for lung cancer. Clin Cancer Res. 2000;6:1616–1625. [PubMed] [Google Scholar]
- 16.Woolner LB, Fontana RS, Cortese DA, Sanderson DR, Bernatz PE, Payne WS, Pairlero PC, Piehler JM, Taylor WF. Roentgenographically occult lung cancer: pathologic findings and frequency of multicentricity during a 10-yr period. Mayo Clin Proc. 1984;59:453–466. doi: 10.1016/s0025-6196(12)60434-0. [DOI] [PubMed] [Google Scholar]
- 17.Lam S, MacAulay C, Hung J, LeRiche J, Profio AE, Palcic B. Detection of dysplasia and carcinoma in situ with a lung imaging fluorescence endoscope device. J Thorac Cardiovasc Surg. 1993;105:1035–1040. [PubMed] [Google Scholar]
- 18.Lam S, Hung JY, Kenney SM, LeRiche JC, Vedal S, Nelems B, Mac-Aulay C, Palcic B. Detection of dysplasia and carcinoma in situ by ratio fluorometry. Am Rev Respir Dis. 1992;146:1458–1461. doi: 10.1164/ajrccm/146.6.1458. [DOI] [PubMed] [Google Scholar]
- 19.Herth FJ, Ernst A, Becker HD. Autofluorescence bronchoscopy: A comparison of two systems (LIFE and D-Light.) Respiration. 2003;70:395–398. doi: 10.1159/000072903. [DOI] [PubMed] [Google Scholar]
- 20.Häuβinger K, Becker H, Stanzel F, Kreuzer A, Schmidt B, Strausz J, Cavaliere S, Herth F, Kohlhäufl M, Müller KM, Huber RM, Pichlmeier U, Bolliger CT. Autofluorescence bronchoscopy with white light bronchoscopy compared with white light bronchoscopy alone for the detection of precancerous lesions: a European randomised controlled multicentre trial. Thorax. 2005;60:496–503. doi: 10.1136/thx.2005.041475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam S, MacAulay C, LeRiche JC, Palcic B. Detection and localization of early lung cancer by fluorescence bronchoscopy. Cancer. 2000;89:2468–2473. doi: 10.1002/1097-0142(20001201)89:11+<2468::aid-cncr25>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- 22.O'Neil KM, Johnson BE. Lights flicker on fluorescence bronchoscopy in patients at risk for lung cancer. J Natl Cancer Inst. 1998;90:953–955. doi: 10.1093/jnci/90.13.953. [DOI] [PubMed] [Google Scholar]
- 23.Kurie JM, Lee JS, Morice RC, Walsh GL, Khuri FR, Broxon A, Ro JY, Franklin WA, Yu R, Hong WK. Autofluorescence bronchoscopy in the detection of squamous metaplasia and dysplasia in current and former smokers. J Natl Cancer Inst. 1998;90:991–995. doi: 10.1093/jnci/90.13.991. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch FR, Prindiville SA, Miller YE, Franklin WA, Dempsey EC, Murphy JR, Bunn PA, Jr, Kennedy TC. Fluorescence versus white-light bronchoscopy for detection of preneoplastic lesions: a randomized study. J Natl Cancer Inst. 2001;93:1385–1391. doi: 10.1093/jnci/93.18.1385. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy TC, Lam S, Hirsch FR. Review of recent advances in fluorescence bronchoscopy in early localization of central airway lung cancer. Oncologist. 2001;6:257–262. doi: 10.1634/theoncologist.6-3-257. [DOI] [PubMed] [Google Scholar]
- 26.Wistuba II, Lam S, Behrens C, Virmani AK, Fong KM, LeRiche J, Samet JM, Srivastava S, Minna JD, Gazdar AF. Molecular damage in the bronchial epithelium of current and former smokers. J Natl Cancer Inst. 1997;89:1366–1373. doi: 10.1093/jnci/89.18.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]