Abstract
Churg-Strauss syndrome (CSS) is a rare multi-system vasculitis; some cases have been reported in Korea. The aim of this study is to describe the clinical features, treatment outcome, and long-term follow-up of CSS from a single Korean medical center. Between 1995 and 2004, seventeen patients were diagnosed with CSS at the Department of Medicine of the Samsung Medical Center, Sungkyunkwan University School of Medicine. The diagnosis of CSS is based on the classification criteria of the American Collage of Rheumatology. All patients had asthma. As in other case series, the lung, peripheral nervous system, and skin were the most commonly involved organs. During the active stage of the disease, most of the patients exhibited peripheral blood eosinophilia and an elevated serum eosinophil cationic protein level. Ten patients were treated with pulses of methylprednisolone followed by tapering and cyclophosphamide, and the others were treated with corticosteroids alone. The outcomes after long-term follow-up were generally good. One patient who was refractory to initial treatment died of heart failure during the follow-up period. CSS was highly variable in its presentation and course. The manifestations may range from mild symptoms to life-threatening conditions. The outcome after long-term follow-up was as good as that of previous studies.
Keywords: Churg-Strauss Syndrome, Treatment Outcome, Follow-up Studies
INTRODUCTION
Churg-Strauss syndrome (CSS) is a necrotizing systemic vasculitis which affects the small- to medium-sized blood vessels and is characterized by asthma, eosinophilia and extravascular eosinophilic granulomas (1). This disorder occurs in all age groups but has been reported most commonly in the third to fifth decades of life. The mean estimated incidence has been reported to be 2.4-6.8/1,000,000 in the general population, and 64.4/1,000,000 in asthma patients (2, 3). The prevalence is 13/1,000,000 in the general population, compared with 33/1,000,000 for polyarteritis nodosa (PAN) and 53/1,000,000 for Wegener's granulomatosis (WG) (4).
Most of previous reports regarding CSS in Korea have been case reports (5-8). Although the effect of intravenous pulse cyclophosphamide (CPM) in the treatment of CSS with refractory neuropathy to high-dose steroid treatment was reported (9), there have been no large single center studies of CSS patients reported in Korea.
The presentation and course of CSS are highly variable; manifestations may range from mild (asthma, sinusitis, cutaneous lesions) to life-threatening conditions (severe gastrointestinal [GI] involvement, heart failure, disabling neuropathy). Furthermore, diagnosis of CSS can be difficult because the manifestations vary with the disease stage. CSS is manifested in three consecutive phases: a prodromal phase consisting of allergic manifestations; a second phase of peripheral blood eosinophilia and eosinophilic tissue infiltrates; and a third phase consisting of vasculitic systemic manifestations (10). Treatment delay in the vasculitic phase may lead to irreversible major organ damage.
Here, we described 17 patients with CSS diagnosed at our Department of Medicine between 1995 and 2004. We analyze the clinical features, responsiveness to treatment, and long-term outcome.
MATERIALS AND METHODS
Subjects
Seventeen patients with CSS were selected from the Department of Medicine of the Samsung Medical Center, Sungkyunkwan University School of Medicine, between 1995 and 2004. Clinical data were obtained from outpatient and/or inpatient medical records.
The diagnosis was made when four or more of the American College of Rheumatology classification criteria were fulfilled. These criteria include asthma, peripheral blood eosinophilia (>10% on differential white blood cell count), mononeuropathy or polyneuropathy, pulmonary infiltrates, paranasal sinus abnormality, and a biopsy finding containing a blood vessel with extravascular eosinophils.
Asthma was diagnosed on the basis of symptoms, measurements of reversibility of lung function abnormalities, and airway hyperresponsiveness by methacholine bronchoprovocation test.
Peripheral neuropathy consisted of mononeuropathy including mononeuritis multiplex and polyneuropathy. Central nervous system (CNS) involvement was considered when central neurologic manifestations occurred and could not be attributed to metabolic disorders or stroke.
Myocardial involvement was considered to be due to CSS when cardiac insufficiency occurred in the absence of preexistent risk factors such as coronary heart disease, valvular disease, hypertension, diabetes, and toxic or infectious agents and/or when histologically proven by examination of a myocardial biopsy.
GI involvement was considered when unexplained abdominal pain, intestinal bleeding, intestinal perforation, cholecystitis or pancreatitis was present without other pathogenic explanation.
Renal involvement was defined as the presence of unexplained increase in serum creatinine, proteinuria >1 g/day, abnormal urinary sediment and/or histologic findings in a renal biopsy consistent with systemic vasculitis.
The therapeutic regimen
In cases of severe, life-threatening, multi-system involvement (e.g., heart failure, severe GI involvement or disabling neuropathy), the patient treated with pulses of methylprednisolone followed by tapering and CPM. Intravenous pulses of CPM (500-750 mg/m2) were administered at a total of six pulses at one-month interval. Depending on the clinical status of the patient, further pulses could be administered. At 2 weeks after every pulse CPM, routine blood tests were performed to examine cytopenia and presence or absence of liver and renal dysfunction. For maintenance therapy, low-dose prednisolone was orally administered according to the clinical manifestations and results of laboratory testing.
A patient was considered to be in remission when the clinical manifestations disappeared for a period of at least 6 months. However, neurologic sequelae could persist. A relapse was defined as the recurrence of clinical manifestations of CSS other than asthma or isolated eosinophilia.
RESULTS
Demographic data
Between 1995 and 2004, 17 patients (9 men, 8 women) were diagnosed with CSS in our department. The mean age at the time of diagnosis was 36.7 (range from 24 to 53) yr. The patients were followed for a mean period of 42.5 (range tremities. Skin biopsies were performed in 9 patients. Vasculitis was found in 6 biopsies, eosinophil infiltrates in 2 biopsies, and lymphocyte infiltrates in 1 biopsy.
GI involvement was seen in 3 patients (17.6%). In the first case (case 4) with recurrent epigastric pain, hemorrhagic gastritis was found on gastroendoscopy. In the second case (case 7), which presented with diarrhea, hemorrhagic erosions were found on colonoscopy and, on mesenteric angiography, thrombosis was found in the inferior mesenteric vein. In the third case (case 12) with severe acute abdominal pain, emergency laparotomy was performed and revealed peritonitis and perforation of the small intestine. Intestinal biopsy in the third case showed ulcer with granulation tissue, congested vessels, and mixed infiltration of inflammatory cells with a predominance of eosinophils.
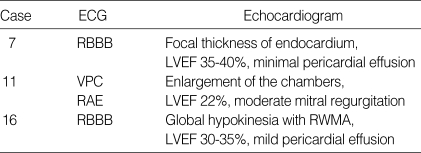
Three patients (17.6%) presented with congestive heart failure with 2 patients showing a small amount of pericardial effusion (Table 4). A case 16, young woman was treated with corticosteroids alone with consideration of the possibility of pregnancy; the other 2 patients were treated with corticosteroids and CPM. A case 16 underwent coronary angiography with endomyocardial biopsy. No evidence of coronary artery disease was detected, but hypokinesia of the apex and anterolateral wall was noted. Histology showed focal myocarditis with prominent interstitial eosinophilic infiltration without vasculitis.
Table 4.
Electrocardiography and echocardiogram in 3 Churg-Strauss syndrome patients with heart involvement
ECG, electrocardiography; RBBB, right bundle branch block; LVEF, left ventricle ejection fraction; VPC, ventricular premature complex; RAE, right atrial enlargement; RWMA, regional wall motion abnormality.
Laboratory findings
At the time of diagnosis, peripheral blood eosinophilia was present in 15 patients. Those without blood eosinophilia were already undergoing treatment with corticosteroids. The mean blood eosinophil count of all patients was 5085.2/µL (reference value <500/µL). Eosinophil cationic protein (ECP) level was checked in the sera of 13 patients, all of whom exhibited elevated serum ECP level at the time of diagnosis; the mean value was 279.6 ng/mL (reference value<16 ng/mL). Anti-neutrophil cytoplasmic antibodies (ANCA) were tested in all patients and myeloperoxidase-ANCA were detected in 1 patient at the time of diagnosis.
Disease course and long-term follow-up
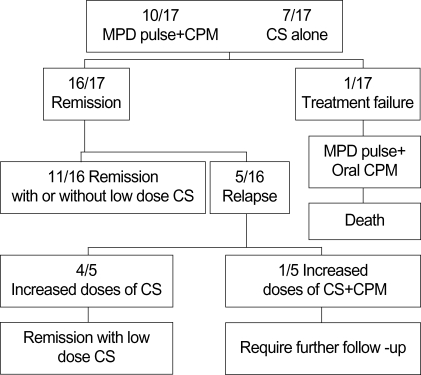
Fig. 1 summarizes the therapeutic modalities and disease course of 17 patients with CSS.
Fig. 1.
Therapeutic modalities and disease course of 17 patients with Churg-Strauss syndrome. MPD, methylprednisolone; CPM, cyclophosphamide; CS, corticosteroids.
Ten patients were treated with pulses of methylprednisolone followed by tapering and CPM. The other patients were treated with corticosteroids alone. Nine patients were administered on average 7.3 intravenous pulses of CPM; one patient was administered oral CPM. The serious adverse effects of CPM, such as hemorrhagic cystitis, bone marrow suppression, and neoplasm, were not documented during the follow-up period. One patient was refractory to the initial treatment and was subsequently placed on alternative pulses of methylprednisolone and oral CPM, but the patient did not comply with the treatment regimen and eventually died of heart failure. Five patients suffered relapses during the follow-up period and the clinical manifestations noted at the time of relapse were similar to those at the time of diagnosis. A suspected triggering factor for the relapses was the rapid tapering or discontinuation of corticosteroids treatment in 4 patients. At the time of the last visit, 13 of the 16 survivors in this study still required low doses of oral prednisolone (mean dose of 7.4 mg/day), 2 patients sustained remission without corticosteroids, and 1 patient required further follow-up.
DISCUSSION
CSS is a rare disorder characterized by eosinophilia and systemic vasculitis and almost invariably occurs in patients with asthma. Vasculitis commonly affects the lung, peripheral nerves, skin, heart, and GI tract. Renal involvement is not a necessarily characteristic of this disease, but it may occur in some cases (11).
The clinical spectrum of PAN or microscopic polyangiitis is similar to that of CSS, but the patients with PAN have no asthma, eosinophilia, or history of atopy. Additionally, patients with PAN have a higher frequency of nephritis, bowel vasculitis, and hypertension than do patients with CSS (12). The differentiation of these diseases may be important because CSS has a better rate of long-term remission than PAN when corticosteroid treatment was introduced (12, 13).
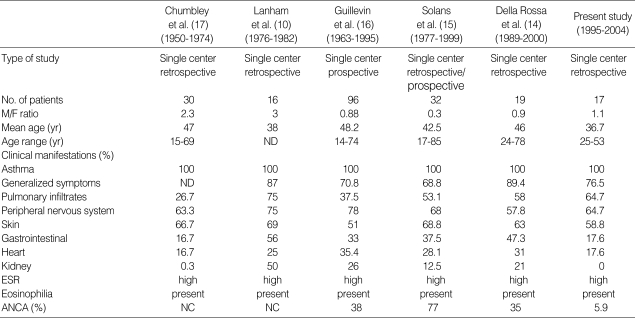
This is the first study to observe the clinical characteristics of CSS patients based on Korean experience in a single center over a 10-yr period. Table 5 shows data from the present study in comparison with those from previous studies.
Table 5.
Comparison of data from the present study with data from previous studies
ESR, erythrocyte sedimentation rate; ANCA, anti-neutrophil cytoplasmic antibodies; ND, not documented; NC, not checked.
The mean age of patients at disease onset was a little younger than that documented in previous studies (10, 14-17). The clinical and laboratory features seen in the present study was similar to that in the largest previous studies except that renal involvement and ANCA were demonstrated less frequently (10, 14-17).
As in previous studies, asthma was present in all the study patients and preceded CSS development in all patients but one, in whom asthma began simultaneously with CSS. A pulmonary infiltrates was present in 64.7% of patients. The most frequent findings of HRCT were consolidations in patchy or diffuse distribution and bronchial wall thickening. Worthy et al. (18) reviewed the HRCT findings in 17 patients with CSS. The most common abnormality consisted of bilateral consolidation or ground-glass attenuation in either patchy or predominantly peripheral distribution. Choi et al. (19) reviewed the HRCT findings in 9 patients with CSS. The most common findings included bilateral ground-glass opacities, centrilobular nodules mostly within the ground-glass opacities, and bronchial wall thickening.
Peripheral nerve involvement, present in 64.7% of patients, was the most frequent manifestation; its frequency in the present study was comparable to those seen in previous studies. Also as in previous studies, mononeuritis multiplex constituted the most frequent manifestation of peripheral nerve involvement and the peroneal nerve was the most frequently involved (20-22). All patients responded well to corticosteroids and CPM therapy, although 4 patients had residual symptoms, such as hypesthesia or neuropathic pain, sometimes associated with mild motor deficit. However, the symptoms were not severe in any of these cases. No involvement of the CNS was noted in the study subjects. CNS involvement may include palsies of the cranial nerves, cerebral hemorrhage or infarction, convulsions, and psychosis, but these occurrences are atypical. When CNS involvement dose occur with CSS, it causes significant morbidity and mortality (10, 23).
Skin involvement was present in 58.8% of patients, with palpable purpura presenting as the most frequent manifestation, as in previous studies (10, 14-17).
GI involvement was less frequent than in previous studies. Pathophysiology of GI involvement in CSS is known ischemic change from vasculitis and bowel wall infiltration with eosinophils (24). Churg and Strauss (1) described 12 of 13 patients with CSS with abdominal pain or diarrhea, one of whom died of intestinal perforation from ulceration. GI involvement in CSS, such as abdominal pain, diarrhea, intestinal bleeding, and intestinal obstruction was present in 37-62% of patients, as reported by other authors (10, 16, 17, 25).
In 17.6% of patients, cardiac manifestation was present. A case 16 showed striking improvement in left ventricular function (LVEF 54%) with improvement of hypokinesia on initial treatment. A case 11 relapsed at the eighth month and responded to increased doses of corticosteroids with conservative management, but case 7 was refractory to treatment and died of heart failure. Cardiac involvement includes eosinophilic endomyocarditis, coronary vasculitis, valvular heart disease, congestive heart failure, hypertension, and pericarditis (1, 11, 23, 26). In previously reported cases, treatment ranged from relatively high doses of corticosteroids to low doses combined with digoxin and diuretics. However, when immunosuppressive agents, especially CPM, were added to the treatment, it was found to be effective and to improve the from 8 to 113) months.
Clinical features and histologic findings
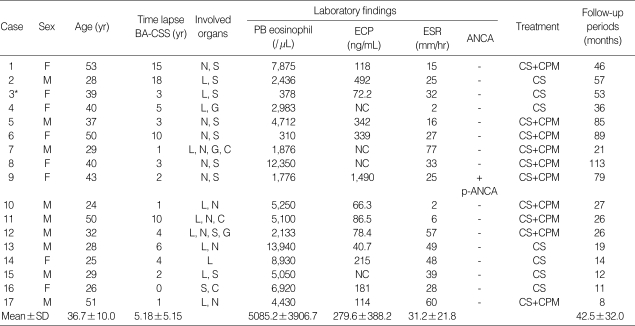
Clinical and laboratory features of 17 patients with CSS are listed in Table 1 and the histologic findings in biopsies of 14 patients with CSS are detailed in Table 2.
Table 1.
Clinical and laboratory features in 17 patients with Churg-Strauss syndrome
BA, bronchial asthma; CSS, Churg-Strauss syndrome; PB, peripheral blood; ECP, eosinophil cationic protein; ESR, erythrocyte sedimentation rate; ANCA, anti-neutrophil cytoplasmic antibodies; L, lung; N, nervous system; S, skin; G, gastrointestinal system; C, cardiovascular system; NC, not checked; p, perinuclear; CS, corticosteroids; CPM, cyclophosphamide. *These cases were already undergoing treatment with corticosteroids. Data are expressed as Mean±SD.
Table 2.
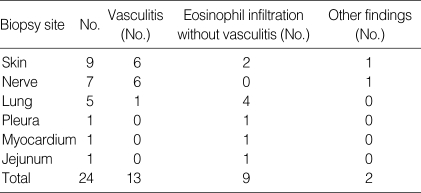
Histologic findings in biopsies of the 14 patients with Churg-Struass syndrome
Non-specific symptoms consisting of malaise, weight loss, fever, and arthromyalgia marked the early stages of vasculitis in 13 of 17 patients (76.5%).
All patients had asthma. The mean time lapse between the appearance of asthma and the onset of vasculitis was 5.18 (range from 0 to 18) yr. In 15 patients, asthma began when the patient was an adult (mean age 32.4 yr). Two patients had recent-onset asthma of less than 6 months, 1 of whom had asthma as presenting symptom of CSS. In 8 of 17 patients (47.1%), asthma was severe and had necessitated oral corticosteroids frequently. Mechanical ventilation was needed for status asthmaticus at the presentation of CSS in 1 patient. The upper respiratory tract was affected in 11 patients (64.7%). These patients complained rhinorrhea, nasal obstruction, and postnasal drip; sinusitis was demonstrated in paranasal sinus radiography. Three of these patients required endoscopic sinus surgery. The lower respiratory tract was also affected in 11 patients (64.7%). Transient pulmonary infiltrates were detected by chest radiography or high-resolution computed tomography (HRCT) scan. The infiltrates were various such as nodules, ground glass opacities, consolidations, and bronchial wall thickening in patchy or diffuse distribution with no preferred location. Three patients had hemoptysis. Alveolar hemorrhage was detected by the findings of bronchoalveolar lavage in 2 patients and 1 patient presented with acute respiratory failure. One patient had polymorphonuclear cell-dominant exudative pleural effusion and the finding upon pleural biopsy was mesothelial hyperplasia with infiltration of numerous eosinophils and plasma cells. Transbronchial lung biopsies or video-assisted thoracoscopic biopsies were performed in 5 patients. Eosinophilic vasculitis was found in 1 biopsy and vascular and interstitial eosinophil infiltrates were found in 4 biopsies.
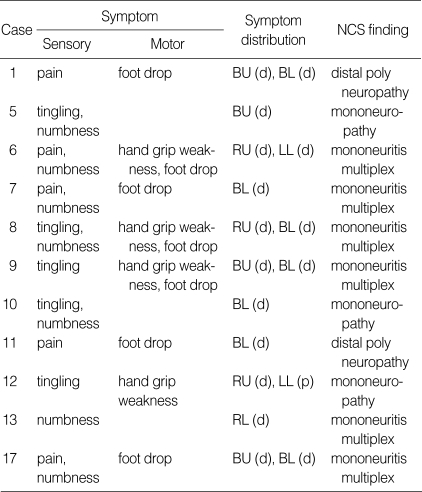
Eleven patients (64.7%) showed signs of peripheral nerve involvement without involvement of the CNS (Table 3). This manifestation was asymmetric and distal-dominant fashion. Electroneurography revealed mononeuritis multiplex most commonly. The most frequently involved nerve was the peroneal nerve, followed by tibial, sural, median, and ulnar nerves. Sural nerve biopsies were performed in 7 of 11 patients. Six showed vasculitis (one with fibrinoid necrosis, five with perivascular infiltrates) and the other showed axonal degeneration without vasculitis.
Table 3.
Clinical features and electrodiagnostic findings in 11 Churg-Strauss syndrome patients with peripheral nerve involvement
NCS, nerve conduction study; RU, right upper extremity; RL, right lower extremity; LL, left lower extremity; BU, both upper extremity; BL, both lower extremity; d, distal; p, proximal.
Ten patients (58.8%) experienced cutaneous manifestations: palpable purpura, papules, urticarial rash, vesicles, aseptic pustules, and Raynaud phenomenon. This manifestation typically involves the distal limbs, most frequently the lower exprognosis (15).
Renal involvement was absent in our patients. Although it has been reported in CSS with variation across studies (0.3-67%) (10, 12, 14-17), unlike other necrotizing vasculitis such as WG or microscopic polyangiitis, renal failure is rare (11).
A total of 24 biopsies were performed in 14 patients. Three patients did not undergo any biopsy because of typical clinical manifestation. Vasculitis was seen in 6 of 9 skin biopsies (66.7%) and 6 of 7 nerve biopsies (85.7%). Therefore, the skin and nerves seem to be useful as diagnostic biopsy sites.
Corticosteroids have been the cornerstone of treatment for CSS since Chumbley et al. (17) reported a 5-yr survival rate of 62% and a median survival of more than 9 yr after early administration of corticosteroids. With acute multi-organ involvement or poor predicted prognosis, treatment with 15 mg/kg intravenous methylprednisolone for 3 days followed by 40-60 mg of prednisolone daily with gradual taper, has been advocated (27-29). However, administration of high dose corticosteroids for a long period has been known to complicate treatment-related side effects. Immunosuppressive agents such as immunoglobulin (30) or interferon (31) have been added to the treatment of patients with severe multiorgan involvement, unresponsiveness to corticosteroids, or relapse. CPM, in particular, has improved prognosis and has been recommended as adjuvant therapy in these patients (11, 29, 32). The benefit of pulse intravenous administration of CPM versus oral CPM is debatable. A small clinical study of 25 patients with CSS compared daily oral CPM with monthly intravenous CPM. The efficacy was comparable in both groups and the side effect profile was twice as great in the group receiving oral administration; the researchers in this study recommended pulse intravenous therapy over daily oral therapy for CSS (33). In very severe cases of CSS, corticosteroids and CPM may be insufficient to induce remission. In these cases, anti-TNF blocking agents such as infliximab or etanercept, may be added for a limited period of time (34).
In the present study, initial clinical remission was achieved in 16 patients (94.1%) and one patient (5.9%) died during follow-up. Five patients (29.4%) suffered single relapse and no suffered multiple relapses. The outcome in the present study was similar to that in recent reports (14-16).
In conclusion, the main difference in manifestation of CSS in this study compared to that in the previous studies was the absence of renal involvement and lower detection rate for ANCA. The question of whether renal involvement and ANCA are less present in Korean CSS patients needs to be answered by in a large study. Additionally, favorable treatment outcome in this study certainly needs further long-term study.
References
- 1.Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol. 1951;27:277–301. [PMC free article] [PubMed] [Google Scholar]
- 2.Martin RM, Wilton LV, Mann RD. Prevalence of Churg-Strauss syndrome, vasculitis, eosinophilia and associated conditions: retrospective analysis of 58 prescription-event monitoring cohort studies. Pharmacoepidemiol Drug Saf. 1999;8:179–189. doi: 10.1002/(SICI)1099-1557(199905/06)8:3<179::AID-PDS414>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Watts RA, Lane SE, Bentham G, Scott DG. Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum. 2000;43:414–419. doi: 10.1002/1529-0131(200002)43:2<414::AID-ANR23>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Watts RA, Carruthers DM, Scott DG. Epidemiology of systemic vasculitis: changing incidence or definition? Semin Arthritis Rheum. 1995;25:28–34. doi: 10.1016/s0049-0172(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 5.Yang KJ, Moon HS, Lee WK, Song JS, Ro JC, Park SH, Pyun HW, Kim YW, Seo EJ. A case of allergic granulomatosis. Tuberc Respir Dis. 1986;33:247–251. [Google Scholar]
- 6.Jung SH, Kim KH, Nam SM, Park HC, Chu HK, Whang IS, Kim JH, Jun HS, Park SH, Lee SH, Kim HY. A case of Churg - Strauss syndrome with manifestations of esophageal ulcer, acute acalculous cholecystitis and ischemic colitis. Korean J Med. 1993;45:369–375. [Google Scholar]
- 7.Kim HG, Park SH, Choi MH, Kang MJ, Ko DH, Cho CS, Kim HY, Kim J. A case of Churg-Strauss syndrome: Presented as mimic of rheumatoid arthritis. J Korean Rheum Assoc. 1998;5:139–145. [Google Scholar]
- 8.Kim SH, Park HW, Yeon TJ, Kim SH, Chang YH, Jeong H, Lee BJ, Kim YK, Cho SH, Min KU, Kim YY. A case of Churg-Strauss syndrome with heart and coronary artery involvement confirmed by coronary angiography and endomyocardial biopsy. J Asthma Allergy Clin Immunol. 2000;20:248–254. [Google Scholar]
- 9.Lim YH, Lee SP, Koh EM, Choi DC. Effect of intravenous pulse cyclophosphamide in the treatment of Churg-Strauss syndrome with refractory neuropathy to high-dose steroid treatment. J Asthma Allergy Clin Immunol. 2000;20:113–121. [Google Scholar]
- 10.Lanham JG, Elkon KB, Pusey CD, Hughes GR. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine (Baltimore) 1984;63:65–81. doi: 10.1097/00005792-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Noth I, Strek ME, Leff AR. Churg-Strauss syndrome. Lancet. 2003;361:587–594. doi: 10.1016/S0140-6736(03)12518-4. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Shakra M, Smythe H, Lewtas J, Badley E, Weber D, Keystone E. Outcome of polyarteritis nodosa and Churg-Strauss syndrome. An analysis of twenty-five patients. Arthritis Rheum. 1994;37:1798–1803. doi: 10.1002/art.1780371214. [DOI] [PubMed] [Google Scholar]
- 13.Guillevin L, Lhote F, Gayraud M, Cohen P, Jarrousse B, Lortholary O, Thibult N, Casassus P. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore) 1996;75:17–28. doi: 10.1097/00005792-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Della Rossa A, Baldini C, Tavoni A, Tognetti A, Neglia D, Sambuceti G, Puccini R, Colangelo C, Bombardieri S. Churg-Strauss syndrome: clinical and serological features of 19 patients from a single Italian centre. Rheumatology (Oxford) 2002;41:1286–1294. doi: 10.1093/rheumatology/41.11.1286. [DOI] [PubMed] [Google Scholar]
- 15.Solans R, Bosch JA, Perez-Bocanegra C, Selva A, Huguet P, Alijotas J, Orriols R, Armadans L, Vilardell M. Churg-Strauss syndrome: outcome and long-term follow-up of 32 patients. Rheumatology (Oxford) 2001;40:763–771. doi: 10.1093/rheumatology/40.7.763. [DOI] [PubMed] [Google Scholar]
- 16.Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine (Baltimore) 1999;78:26–37. doi: 10.1097/00005792-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Chumbley LC, Harrison EG, Jr, DeRemee RA. Allergic granulomatosis and angiitis (Churg-Strauss syndrome). Report and analysis of 30 cases. Mayo Clin Proc. 1977;52:477–484. [PubMed] [Google Scholar]
- 18.Worthy SA, Muller NL, Hansell DM, Flower CD. Churg-Strauss syndrome: the spectrum of pulmonary CT findings in 17 patients. AJR Am J Roentgenol. 1998;170:297–300. doi: 10.2214/ajr.170.2.9456932. [DOI] [PubMed] [Google Scholar]
- 19.Choi YH, Im JG, Han BK, Kim JH, Lee KY, Myoung NH. Thoracic manifestation of Churg-Strauss syndrome: radiologic and clinical findings. Chest. 2000;117:117–124. doi: 10.1378/chest.117.1.117. [DOI] [PubMed] [Google Scholar]
- 20.Sehgal M, Swanson JW, DeRemee RA, Colby TV. Neurologic manifestations of Churg-Strauss syndrome. Mayo Clin Proc. 1995;70:337–341. doi: 10.4065/70.4.337. [DOI] [PubMed] [Google Scholar]
- 21.Hattori N, Ichimura M, Nagamatsu M, Li M, Yamamoto K, Kumazawa K, Mitsuma T, Sobue G. Clinicopathological features of Churg-Strauss syndrome-associated neuropathy. Brain. 1999;122(Pt 3):427–439. doi: 10.1093/brain/122.3.427. [DOI] [PubMed] [Google Scholar]
- 22.Seok JI, Bae JS, Joo EY, Min TH, Choi DC, Kim BJ. Clinical and electrophysiologic features of peripheral neuropathy in Churg-Strauss Syndrome. J Korean Neurol Assoc. 2004;22:127–133. [Google Scholar]
- 23.Ramakrishna G, Midthun DE. Churg-Strauss syndrome. Ann Allergy Asthma Immunol. 2001;86:603–613. doi: 10.1016/S1081-1206(10)62286-7. quiz 13. [DOI] [PubMed] [Google Scholar]
- 24.Kaneki T, Kawashima A, Hayano T, Honda T, Kubo K, Koizumi T, Sekiguchi M, Ichikawa H, Matsuzawa K, Katsuyama T. Churg-Strauss syndrome (allergic granulomatous angitis) presenting with ileus caused by ischemic ileal ulcer. J Gastroenterol. 1998;33:112–116. doi: 10.1007/s005350050054. [DOI] [PubMed] [Google Scholar]
- 25.Modigliani R, Muschart JM, Galian A, Clauvel JP, Piel-Desruisseaux JL. Allergic granulomatous vasculitis (Churg-Strauss syndrome). Report of a case with widespread digestive involvement. Dig Dis Sci. 1981;26:264–270. doi: 10.1007/BF01391641. [DOI] [PubMed] [Google Scholar]
- 26.Hasley PB, Follansbee WP, Coulehan JL. Cardiac manifestations of Churg-Strauss syndrome: report of a case and review of the literature. Am Heart J. 1990;120:996–999. doi: 10.1016/0002-8703(90)90227-o. [DOI] [PubMed] [Google Scholar]
- 27.Lanham JG. Churg-Strauss syndrome. Br J Hosp Med. 1992;47:667–673. [PubMed] [Google Scholar]
- 28.Calabrese LH, Hoffman GS, Guillevin L. Therapy of resistant systemic necrotizing vasculitis. Polyarteritis, Churg-Strauss syndrome, Wegener's granulomatosis, and hypersensitivity vasculitis group disorders. Rheum Dis Clin North Am. 1995;21:41–57. [PubMed] [Google Scholar]
- 29.Guillevin L, Lhote F, Gherardi R. Polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome: clinical aspects, neurologic manifestations, and treatment. Neurol Clin. 1997;15:865–886. doi: 10.1016/s0733-8619(05)70352-2. [DOI] [PubMed] [Google Scholar]
- 30.Hamilos DL, Christensen J. Treatment of Churg-Strauss syndrome with high-dose intravenous immunoglobulin. J Allergy Clin Immunol. 1991;88:823–824. doi: 10.1016/0091-6749(91)90195-t. [DOI] [PubMed] [Google Scholar]
- 31.Tatsis E, Schnabel A, Gross WL. Interferon-alpha treatment of four patients with the Churg-Strauss syndrome. Ann Intern Med. 1998;129:370–374. doi: 10.7326/0003-4819-129-5-199809010-00004. [DOI] [PubMed] [Google Scholar]
- 32.Guillevin L, Lhote F, Cohen P, Jarrousse B, Lortholary O, Genereau T, Leon A, Bussel A. Corticosteroids plus pulse cyclophosphamide and plasma exchanges versus corticosteroids plus pulse cyclophosphamide alone in the treatment of polyarteritis nodosa and Churg-Strauss syndrome patients with factors predicting poor prognosis. A prospective, randomized trial in sixty-two patients. Arthritis Rheum. 1995;38:1638–1645. doi: 10.1002/art.1780381116. [DOI] [PubMed] [Google Scholar]
- 33.Gayraud M, Guillevin L, Cohen P, Lhote F, Cacoub P, Deblois P, Godeau B, Ruel M, Vidal E, Piontud M, Ducroix JP, Lassoued S, Christoforov B, Babinet P French Cooperative Study Group for Vasculitides. Treatment of good-prognosis polyarteritis nodosa and Churg-Strauss syndrome: comparison of steroids and oral or pulse cyclophosphamide in 25 patients. Br J Rheumatol. 1997;36:1290–1297. doi: 10.1093/rheumatology/36.12.1290. [DOI] [PubMed] [Google Scholar]
- 34.Hellmich B, Gross WL. Recent progress in the pharmacotherapy of Churg-Strauss syndrome. Expert Opin Pharmacother. 2004;5:25–35. doi: 10.1517/14656566.5.1.25. [DOI] [PubMed] [Google Scholar]