Abstract
To determine whether angiotensin-converting enzyme (ACE) gene insertion/deletion (I/D) polymorphism is associated with the development and clinical features of systemic sclerosis (SSc) in Korean, we studied seventy two Korean patients with SSc fulfilling the ACR preliminary classification criteria. The controls were 114 healthy, disease free Koreans. ACE I/D genotypes were determined by PCR method using oligonucleotides. Sixty eight patients (94.4%) were women and age at diagnosis was 43.5±12.6 yr old (mean±SD). Thirty nine patients (54.2%) had a diffuse type of SSc. There were no statistical differences in the frequencies of all ACE I/D genotypes and D allele between patients and controls, and neither between diffuse and limited types of SSc. ACE I/D gene polymorphism was not associated with the development of SSc in Korea. The investigation for the pathogenesis of SSc requires more studies about the role of other candidate genes such as endothelin, TGF-β, nitric oxide, or angiotensin II receptor in addition to the ACE genes.
Keywords: Scleroderma, Systemic; Angiotensin-converting Enzyme; Peptidyl-Dipeptidase A; Polymorphism, Genetic; Korea
INTRODUCTION
Systemic sclerosis (SSc) is a systemic autoimmune disease of unknown etiology, characterized by microcirculatory dysfunction and accumulation of excessive extracellular matrices. Among suggested mechanisms, endothelial derangement is important in the pathogenesis of SSc, resulting in some features of vasculopathy such as Raynaud phenomenon and pulmonary hypertension (1).
Vascular injury is associated with increased local production of angiotensin-converting enzyme (ACE) and angiotensin II (2). ACE is a key enzyme in renin-angiotensin system (RAS) and widely distributed in human tissues including the lung, vascular endothelium, kidney, heart, and testes (3). ACE converts angiotensin I to angiotensin II, and inactivates bradykinin through the kallikrein-kininogen system. Angiotensin II may play a role in vascular diseases through vascular smooth muscle cell contraction and proliferation, monocyte adhesion, platelet aggregation, mediated either directly or via various factors such as endothelin, nitric oxide, and prostacyclin (4, 5).
The ACE gene is located in the long arm of chromosome 17 (17q 23), and an insertion/deletion (I/D) polymorphism, determined by the presence or absence of a 287 base pair (bp) Alu repeat within intron 16, was identified (6). The DD genotype has about two-fold higher serum level of ACE than the II genotype, and the ID genotype has intermediate level (7). The DD genotype was associated with endothelial dysfunction, blunting the stimulated endothelial or donated nitric oxide response in normal population (8). Some epidemiologic studies have found that the D allele was associated with increased cardiovascular and renal risk (9).
The association of ACE I/D genes has been studied in other rheumatic diseases with vascular involvement, and showed inconsistent data in systemic lupus erythematosus (3, 10), association of II genotypes in Kawasaki disease with coronary aneurysm (11), and irrelevance in Korean Behcet's disease (12).
Although ACE D genotype was reported as a risk factor of SSc in Italian (13), it has not been evaluated in other ethnic groups. We studied the association of ACE I/D genotypes with the development and clinical features of SSc in Korean.
MATERIALS AND METHODS
Seventy two patients (68 women, 4 men) with SSc fulfilling the criteria proposed by the American College of Rheumatology (14) were enrolled. The age at diagnosis was 43.5±12.6 yr old (mean±SD). The controls were 114 healthy, disease free Koreans (41 women, 73 men). The age at enrollment was 37.2±4.6 yr old. The medical records of patients were reviewed retrospectively to collect clinical and laboratory data at the time of diagnosis and follow up. Anti-topoisomerase I autoantibody (Anti-topo I) was measured by double immunodiffusion (Immunothink, Seoul, Korea) and anti-centromere autoantibody (ACA) by a characteristic discrete speckled nuclear staining pattern on indirect immunofluorescent stains using IT-1 cell (Immunothink, Seoul, Korea). For ACE genotyping, every patient's genomic DNA was isolated from peripheral blood (Puregene kit, Gentra systems, Minneapolis, MN, U.S.A.) after getting the written informed consent.
ACE I/D genotype determination
ACE I/D genotype was determined by PCR method using oligonucleotides (sense 5'CTGGAGACCACTCCCATCCTTTCT 3', and antisense 5'GATGTGGCCATCACATTCGTCAGAT 3'). The reaction was carried out using 250 ng of genomic DNA, 10 mM Tris-HCl, 50 mM KCl, 3 mM MgCl2, 0.5 mM of each dNTP, and 1 unit of Taq DNA polymerase (Boehringer Mannheim, Germany) at a final volume of 40 µL under the following conditions: 30 cycles of denaturation at 94℃ for 1 min, annealing at 58℃ for 1 min, and extension at 72℃ for 2 min by Gene Amp system 9600 (Perkin Elmer Biosystem, Norwalk, CT, U.S.A.). The PCR products (190 and 490 bp) were electrophoresed on ethidium bromide stained 2% agarose gel and visualized under UV transilluminator (GelDoc 2000, Bio-Rad, CA, U.S.A.). On electrophoresis, ACE genotype I/I showed a 490 bp band, genotype D/D showed a 190 bp band, and I/D genotype showed both of 490 and 190 bp bands (10). We tried to confirm the genotypes by modification of PCR; annealing temperature at 60℃, and addition of the 5% dimethylsulfoxide in reaction mixture (15). In addition, each DD genotype was subjected to a PCR with primer pair that recognizes an insertion specific sequence using same PCR condition with first PCR, except an annealing temperatue of 67℃ to reduce overestimation of DD genotype (15).
Statistical analysis
The chi-square test was used for comparison of nonparametric data and Fisher's exact test was used when the number was small. For comparison of parametric data, Student's t-test was used. The p-value lower than 0.05 (two-tailed) was considered statistically significant. We employed SPSS for Windows version 11.0.
RESULTS
Clinical characteristics of patients with SSc
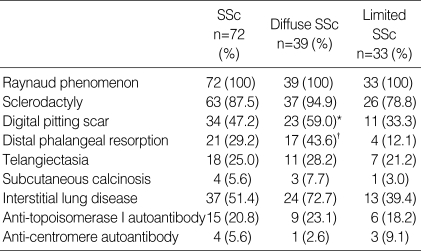
All patients had Raynaud phenomenon and the other manifestations were sclerodactyly (87.5%), digital pitting scar (47.2%), distal phalangeal resorption (29.2%), telangiectasia (25.0%), and subcutaneous calcinosis (5.6%). Interstitial lung disease was found in 37 patients (51.4%). Anti-topo I was positive in 15 patients (20.8%) and ACA in 4 patients (5.6%) (Table 1).
Table 1.
Clinical characteristics in patients with systemic sclerosis (SSc)
Diffuse vs. limited SSc. *OR=2.875, 95% CI: 1.095-7.545, p=0.030. †OR=5.602, 95%CI: 1.651-19.015, p=0.004.
The diffuse type of SSc was observed in 39 patients (54.2 %), showing more digital pitting scar (odds ratio [OR]=2.875, 95% confidence interval [95% CI]: 1.095-7.545, p=0.030) and distal phalangeal resorption (OR=5.602, 95% CI: 1.651-19.015, p=0.004) than the limited type (Table 1).
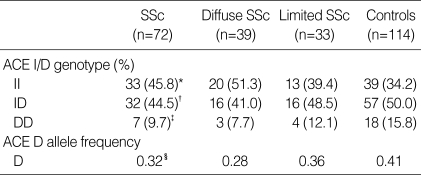
Distribution of ACE I/D genotypes in patients with SSc and controls
The frequencies of II, ID, and DD genotypes were 45.8%, 44.5%, and 9.7% in patients, and 34.2%, 50.0%, and 15.8% in normal controls, respectively (Table 2). Also the frequency of D allele was 0.32 in patients, and 0.41 in normal controls. There was no significant difference in the distribution of genotypes and alleles between patients and controls. In addition there was no important difference in the gene frequency between diffuse and limited types of SSc.
Table 2.
Distribution of ACE I/D genotypes between patients with systemic sclerosis (SSc) and controls
SSc vs. Controls. *II genotype compared with ID and DD genotypes, p corr=0.226. †ID compared with II and DD, p corr=0.920. ‡DD compared with II and ID, p corr=0.474. §D allele compared with I allele, p=0.186. ACE, angiotensin-converting enzyme.
ACE I/D genotypes and clinical features of SSc
There was no significant difference in the frequency of D allele according to the presence of individual clinical parameters of SSc (data not shown).
DISCUSSION
The main pathology of SSc is endothelial dysfunction. Higher ACE levels, related to the ACE D allele, may promote the production of angiotensin II and the degradation of bradykinin (16). However the ACE activity, which has been reported in SSc, was rather lower than normal even in those patients with very early disease, and without pulmonary hypertension resulting from endothelial dysfunction by chronic injury (17).
ACE I/D genotypes in 73 Italian patients with SSc and 112 controls showed that ACE D allele was associated with an increased risk of SSc, but not with the clinical features and disease subsets (13). In contrast, we did not find an association of ACE I/D genotypes with Korean SSc. The diffuse type of SSc showed more digital pitting scar and distal phalangeal resorption than limited type, but the distribution of ACE I/D genotypes was not different between both types. When we compared the frequencies of genotypes in SSc patients between Italian data (13) and ours, the frequency of D allele and DD genotype was much lower in this study (32% vs. 64% for D allele, and 9.7% vs. 38% for DD genotype). The exact reason why two studies showed different result requires more careful evaluation. Compared with Italian data (13), we have more women (94% vs. 85%) and younger (mean age, 44 vs. 62) patients. Although, it is still uncertain whether demographic discrepancy such as age or female predominance may influence the distribution of ACE I/D genes, there was no meaningful difference of gene frequency between Korean and Italian controls despite different sex and age ratios. The frequency of DD genotype, linked to higher ACE level in normal, was lower than the other genotypes in Korean SSc patients, but the difference did not show statistical significance. The exact correlation between ACE I/D genotype and serum levels of ACE in patients with SSc has not been performed yet. The exact reason of this discrepancy in the association with SSc is still unclear, and the racial differences in the genetic background, or interaction between genes controlling vascular tone may play a role.
In conclusion, the ACE I/D polymorphism was not associated with the development of SSc in Korea. The genetic susceptibility in SSc needs more studies about the role of the other candidate genes such as endothelin-1, endothelin receptor, TGF-β, nitric oxide, or angiotensin II receptor, in addition to the ACE genes.
References
- 1.LeRoy EC. Systemic sclerosis: a vascular perspective. Rheum Dis Clin North Am. 1996;22:675–694. doi: 10.1016/s0889-857x(05)70295-7. [DOI] [PubMed] [Google Scholar]
- 2.Rakugi H, Kim DK, Krieger JE, Wang DS, Dzau VJ, Pratt RE. Induction of angiotensin converting enzyme in the neointima after vascular injury: possible role in restenosis. J Clin Invest. 1994;93:339–346. doi: 10.1172/JCI116965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman KM, Kelly J, Gray-McGuire C, Asundi N, Yu H, Reid J, Baird T, Hutchings D, Bruner G, Scofield RH, Moser K, Harley JB. Linkage analysis of angiotensin-converting enzyme (ACE) insertion/deletion polymorphism and systemic lupus erythematosus. Mol Cell Endocrinol. 2001;177:81–85. doi: 10.1016/s0303-7207(01)00424-5. [DOI] [PubMed] [Google Scholar]
- 4.Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J Clin Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaughan DE. Fibrinolytic balance, the renin-angiotensin system and atherosclerotic disease. Eur Heart J. 1998;19(Suppl G):G9–G12. [PubMed] [Google Scholar]
- 6.Rigat B, Hubert C, Corvol P, Soubrier F. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1) Nucleic Acids Res. 1992;20:1433. doi: 10.1093/nar/20.6.1433-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler R, Morris AD, Burchell B, Struthers AD. DD angiotensin-converting enzyme gene polymorphism is associated with endothelial dysfunction in normal humans. Hypertension. 1999;33:1164–1168. doi: 10.1161/01.hyp.33.5.1164. [DOI] [PubMed] [Google Scholar]
- 9.Miller JA, Scholey JW. The impact of renin-angiotensin system polymorphisms on physiological and pathophysiological processes in humans. Curr Opin Nephrol Hypertens. 2004;13:101–106. doi: 10.1097/00041552-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Uhm WS, Lee HS, Chung YH, Kim TH, Bae SC, Joo KB, Kim TY, Yoo DH. Angiotensin-converting enzyme gene polymorphism and vascular manifestations in Korean patients with SLE. Lupus. 2002;11:227–233. doi: 10.1191/0961203302lu174oa. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi K, Yamamoto K, Kataoka S, Kakihara T, Tanaka A, Sato S, Uchiyama M. High incidence of angiotensin I converting enzyme genotype II in Kawasaki disease patients with coronary aneurysm. Eur J Pediatr. 1997;156:266–268. doi: 10.1007/s004310050597. [DOI] [PubMed] [Google Scholar]
- 12.Chang HK, Kim JU, Lee SS, Yoo DH. Lack of association between angiotensin converting enzyme polymorphism and Korean Behcet's disease. Ann Rheum Dis. 2004;63:106–107. doi: 10.1136/ard.2003.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fatini C, Gensini F, Sticchi E, Battaglini B, Angotti C, Conforti ML, Generini S, Pignone A, Abbate R, Matucci-Cerinic M. High prevalence of polymorphisms of angiotensin-converting enzyme (I/D) and endothelial nitric oxide synthase (Glu298Asp) in patients with systemic sclerosis. Am J Med. 2002;112:540–544. doi: 10.1016/s0002-9343(02)01069-0. [DOI] [PubMed] [Google Scholar]
- 14.Masi AT, Rodman GP, Medsger TA. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 15.Ribichini F, Steffenino G, Dellavalle A, Matullo G, Colajanni E, Camilla T, Vado A, Benetton G, Uslenghi E, Piazza A. Plasma activity and insertion/deletion polymorphism of angiotensin I-converting enzyme: a major risk factor and a marker of risk for coronary stent restenosis. Circulation. 1998;97:147–154. doi: 10.1161/01.cir.97.2.147. [DOI] [PubMed] [Google Scholar]
- 16.Brown NJ, Nadeau J, Vaughan DE. Stimulation of tissue-type plasminogen activator in vivo by infusion of bradykinin. Thromb Haemost. 1997;77:522–525. [PubMed] [Google Scholar]
- 17.Orfanos SE, Psevdi E, Stratigis N, Langleben D, Catravas JD, Kyriakidis M, Moutsopoulos HM, Roussos C, Vlachoyiannopoulos PG. Pulmonary capillary endothelial dysfunction in early systemic sclerosis. Arthritis Rheum. 2001;44:902–911. doi: 10.1002/1529-0131(200104)44:4<902::AID-ANR147>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]