Abstract
Vesicular secretion is a fundamental process in the body with vesicle fusion releasing vesicle contents to the outside. This process called exocytosis is usually thought of as leading to an all-or-none release of content; regulation of secretory output dependent on regulating the numbers of fused vesicles. However, it is well established that the fusion pore that forms when the vesicle membrane fuses with the cell membrane is dynamic. More recent evidence indicates the dynamic opening and closing, and the size of the fusion pore, are limiting factors to the release of vesicle content. What remains unclear is whether these fusion pore behaviors are under cellular control and therefore relevant to cell physiology.
Accumulating evidence over the last two years points to myosin 2 as one regulator of fusion pore behavior. This is interesting since myosin 2 activity is in turn controlled by kinases and phosphatases, well known to be under cellular control. We conclude that fusion pore behavior is likely a genuine control point for vesicle content release. This leads to a model for secretion with secretory output controlled not only by the numbers of vesicles fused but also by the regulation of the behavior of individual vesicles.
Key words: secretion, exocytosis, vesicle, fusion pore, calcium, myosin 2, actin
Vesicular secretion, exocytosis, is fundamental to normal body function and health. It is the key process in neurotransmission, endocrine, paracrine, or autocrine signaling, and protein secretion from cells. As such it plays a pivotal role in almost every aspect of animal. Furthermore, secretory dysfunction is central in many diseases, such as type 2 diabetes and pancreatitis1–3 and the mechanisms of secretory control the target for many drugs. While some of the core molecular components regulating secretion have been identified4,5 it is still largely unknown how these are orchestrated to control secretion.
Graded Secretion as a New Model for Secretory Control
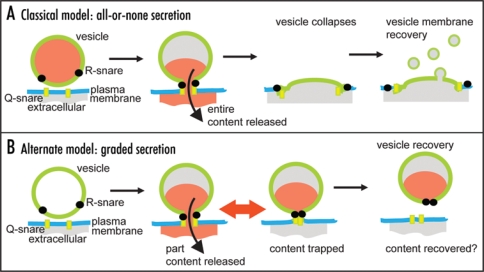
Neurotransmitters, hormones and peptides are packed inside secretory vesicles and classical models for secretion propose that these vesicles fuse with the cell membrane and then collapse releasing their entire hormone content (Fig. 1A). This is therefore an all-or-none model of release and in terms of neurotransmission is the corollary of the classical descriptions of quantal neurotransmitter release.6 However, there is evidence that after vesicle fusion the fusion pore can open and close over time.7–10 While this behavior can still eventuate in vesicle collapse this does not necessarily happen. In the neuronal cell field, the mechanisms of subsequent vesicle recovery are hotly contested,11,12 but in endocrine cells mounting evidence shows whole vesicles can be recaptured back into the cell in a processes termed cavicapture13 (Fig. 1B). Significantly these recaptured vesicles can contain residual quantities of peptide hormones13–15 suggesting that fusion pore dynamics is a limiting factor in the release of peptides. Some papers now suggest that fusion pore dynamics might specifically regulate the loss of low molecular weight (<200 Da) neurotransmitters.14,16,17 In this new model, we here term graded secretion, the dynamics and size of the fusion pore lead to partial release of vesicle content (Fig. 1B). The differences between the models are fundamental to our understanding of secretory control. In the all-or-none model secretory output is adjusted by changing the numbers of vesicles fusing. In contrast, the new graded model places regulation of vesicle behavior as central to controlling secretory output.
Figure 1.
Models for secretory control. (A) in the classical model the entire vesicle content is released, the vesicle collapses and membrane is recovered. (B) in the new model, fusion pore opening and closing regulates content release then either the entire or part of the vesicle membrane is recovered via an unknown mechanism. In some cases it has been shown that part vesicle content can be recovered.
Possible Regulators of Post-Fusion Vesicle Behavior
It could be argued that these complex post-fusion vesicle behaviors are essentially random, inherent in the nature of the protein and lipid interactions that underlie vesicle fusion and fission. So if fusion pore dynamics really were a control point for vesicle content release then we would expect to see regulatory mechanisms. Gathering evidence now supports this idea of cellular control. It has been shown, in some cell types, that complexin II, Munc18, dynamin and cysteine string proteins can affect pore dynamics19 although it is not clear whether these are regulatory factors or necessary, static components in a macromolecular pore complex. More direct evidence for second messenger control shows that calcium,20 and protein kinase C21 can affect fusion pore opening possibly acting on calcium-sensitive targets like synaptotagmin.22 Further, a growing number of recent reports, from a wide range of cell types, are reaching a consensus that F-actin and myosin 2 are dynamic regulators of complex vesicle behavior. Work shows that actin polymerization is triggered immediately after vesicle fusion forming an F-actin network around the vesicle23–28 that keeps the fusion pore open27 and stabilizes the vesicle shape.23,24,29,30 In the last year two reports show that myosin 2 phosphorylation directly regulates fusion pore opening.31,32
Myosin 2 Maintains an Open Fusion Pore
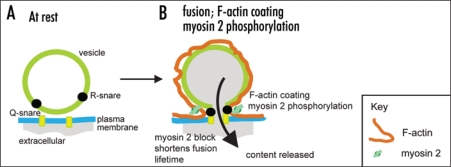
Bhat and Thorn (2009) adds to this body of evidence showing that in epithelial cells myosin 2 effects are specific to post-fusion vesicle dynamics. The time course of myosin 2 phosphorylation and the localization of the myosin 2A isoform are consistent with an action at the secretory vesicle. Imaging experiments identify the opening of the fusion pore as at least one target of myosin 2 action with both the direct myosin 2 inhibitor (-)-blebbistatin and an inhibitor of myosin light chain kinase (the likely regulatory kinase) ML-9, causing a closure of the fusion pore. This work then leads us to conclude myosin 2 acts, probably with F-actin, to keep the fusion pore open (Fig. 2). Given that myosin light chain kinase is calcium dependent, it supports the idea that fusion pore dynamics are under direct cellular control.
Figure 2.
Myosin 2 action in secretion. F-actin coating and myosin 2 phosphorylation appear after fusion. Together we hypothesize these act to maintain the structural integrity of the vesicle and to keep the fusion pore open to allow content loss.
Concluding Remarks
We conclude that for many cell types the regulation of the postfusion behavior of secretory vesicles is important in the control of secretory output. It is now suggested that dysfunction of this behavior, in type 2 diabetics, may lead to premature closure of the fusion pore and decrease vesicle content release, leading to the insufficient insulin secretion often seen in the disease.33,34 Given the potential importance for our understanding of secretory control in health and disease further work is needed to unravel the complexities of these processes.
Acknowledgements
This work is funded by an Australian Research Council Grant (DP0771481) and a Research Infrastructure Block Grant from The University of Queensland to Peter Thorn.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/8262
References
- 1.Porte D. Banting lecture Beta cells in type II diabetes mellitus. Diabetes. 1991;40:166–180. doi: 10.2337/diab.40.2.166. [DOI] [PubMed] [Google Scholar]
- 2.Farret A, Lugo-Garcia L, Galtier F, Gross R, Petit P. Pharmacological interventions that directly stimulate or modulate insulin secretion from pancreatic β-cell: implications for the treatment of type 2 diabetes. Fund Clin Pharm. 2005;19:647–656. doi: 10.1111/j.1472-8206.2005.00375.x. [DOI] [PubMed] [Google Scholar]
- 3.Gaisano HY, Lutz MP, Láser J, Sheu L, Lynch G, Tang L, et al. Supramaximal cholecystokinin displaces Munc18c from the pancreatic acinar basal surface, redirecting apical exocytosis to the basal membrane. J Clin Invest. 2001;108:1597–1611. doi: 10.1172/JCI9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rettig J, Neher E. Emerging roles of presynaptic proteins in Ca-triggered exocytosis. Science. 2002;298:781–785. doi: 10.1126/science.1075375. [DOI] [PubMed] [Google Scholar]
- 5.Sudhof TC. The synaptic vesicle cycle. Ann Rev Neuro. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 6.Del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez JM, Neher E, Gomperts BD. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature. 1984;312:453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- 8.Spruce AE, Breckenridge LJ, Lee AK, Almers W. Properties of the fusion pore that forms during exocytosis of a mast-cell secretory vesicle. Neuron. 1990;4:642–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerberg J, Curran M, Cohen FS, Brodwick M. Simultaneous electrical and optical measurements show that membrane-fusion precedes secretory granule swelling during exocytosis of beige mouse mast-cells. Proc Natl Acad Sci USA. 1987;84:1585–1589. doi: 10.1073/pnas.84.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larina O, Bhat P, Pickett JA, Launikonis BS, Shah A, Kruger WA, et al. Dynamic regulation of the large exocytotic fusion pore in pancreatic acinar cells. Mol Biol Cell. 2007;18:3502–3511. doi: 10.1091/mbc.E07-01-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atluri PP, Ryan TA. The kinetics of synaptic veicle reacidification at hippocampal nerve terminals. J Neurosci. 2006;26:2313–2320. doi: 10.1523/JNEUROSCI.4425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with nanoparticles. Science. 2009 doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrais D, Kleppe IC, Taraska JW, Almers W. Recapture after exocytosis causes differential retention of protein in granules of bovine chromaffin cells. J Physiol. 2004;560:413–428. doi: 10.1113/jphysiol.2004.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obermuller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S. Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci. 2005;118:4271–4282. doi: 10.1242/jcs.02549. [DOI] [PubMed] [Google Scholar]
- 15.Bauer RA, Overlease RL, Lieber JL, Angleson JK. Retention and stimulus-dependent recycling of dense core vesicle content in neuroendocrine cells. J Cell Sci. 2004;117:2193–2202. doi: 10.1242/jcs.01093. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez de Toledo G, Fernández-Chacón R, Fernández JM. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- 17.Albillos A, Dernick G, Horstmann H, Alers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- 18.Graham ME, O'Callaghan DW, McMahon HT, Burgoyne RD. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc Natl Acad Sci USA. 2002;99:7124–7129. doi: 10.1073/pnas.102645099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson MB, Chapman ER. The fusion pores of Ca2+-triggered exocytosis. Nat Struct Mol Biol. 2008;15:684–689. doi: 10.1038/nsmb.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alés E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol. 1999;1:40–44. doi: 10.1038/9012. [DOI] [PubMed] [Google Scholar]
- 21.Scepek S, Coorssen JR, Lindau M. Fusion pore expansion in horse eosinophils is modulated by calcium and protein kinase C via distinct mechanisms. EMBO J. 1998;17:4340–4345. doi: 10.1093/emboj/17.15.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C-T, Lu JC, Bai J, Chang PY, Martin TFJ, Chapman ER, et al. Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature. 2003;424:943–947. doi: 10.1038/nature01857. [DOI] [PubMed] [Google Scholar]
- 23.Nemoto T, Kojima T, Oshima A, Bito H, Kasai H. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. J Biol Chem. 2004;279:37544–37550. doi: 10.1074/jbc.M403976200. [DOI] [PubMed] [Google Scholar]
- 24.Sokac AM, Bement WM. Kiss-and-coat and compartment mixing, coupling exocytosis to signal generation and local actin assembly. Mol Biol Cell. 2006;17:1495–1502. doi: 10.1091/mbc.E05-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jerdeva GV, Wu K, Yarber FA, Rhodes CJ, Kalman D, Schechter JE, et al. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J Cell Sci. 2005;118:4797–4812. doi: 10.1242/jcs.02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson JR, Ludowyke R, Biden TJ. A redistribution of actin and myosin IIA accompanies Ca-dependent insulin secretion. FEBS Letts. 2001;492:101–106. doi: 10.1016/s0014-5793(01)02241-4. [DOI] [PubMed] [Google Scholar]
- 27.Turvey MR, Thorn P. Lysine-fixable dye tracing of exocytosis shows F-actin coating is a step that follows vesicle fusion in pancreatic acinar cells. Pflugers Arch Eur J Physiol. 2004;448:552–555. doi: 10.1007/s00424-004-1288-z. [DOI] [PubMed] [Google Scholar]
- 28.Malacombe M, Bader M-F, Gasman S. Exocytosis in neuroendocrine cells: new tasks for actin. Biochem Biophys Acta. 2006;1763:1175–1183. doi: 10.1016/j.bbamcr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Felmy F. Modulation of cargo release from dense core granules by size and actin network. Traffic. 2007;8:983–997. doi: 10.1111/j.1600-0854.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 30.Giner D, Neco P, Frances MDM, Lopez I, Viniegra S, Gutierrez LM. Real time dynamics of the F-actin cytoskeleton during secretion from chromaffin cells. J Cell Sci. 2005;118:2871–2880. doi: 10.1242/jcs.02419. [DOI] [PubMed] [Google Scholar]
- 31.Doreian BW, Fulop TG, Smith CB. Myosin II activation and actin reorganization regulate the mode of quantal exocytosis in mouse adrenal chromaffin cells. J Neurosci. 2008;28:4470–4478. doi: 10.1523/JNEUROSCI.0008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neco P, Fernandez-Peruchena C, Navas S, Gutierrez LM, Alvarez de Toledo G, Ales E. Myosin 2 contributes to fusion pore expansion during exocytosis. J Biol Chem. 2008;16:10949–10957. doi: 10.1074/jbc.M709058200. [DOI] [PubMed] [Google Scholar]
- 33.Rutter GA, Hill EV. Insulin vesicle release: walk, kiss, pause… then run. Physiology. 2006;21:189–196. doi: 10.1152/physiol.00002.2006. [DOI] [PubMed] [Google Scholar]
- 34.Olofsson CS, Collins S, Bengtsson M, Eliasson L, Salehi A, Shimomura K, et al. Longterm exposure to glucose and lipids inhibits glucose-induced insulin secretion downstream of granule fusion with plasma membrane. Diabetes. 2007;56:1888–1897. doi: 10.2337/db06-1150. [DOI] [PubMed] [Google Scholar]