Abstract
Recent live cell imaging analyzing the components required for endocytosis has elucidated that endocytosis actively occurs at the hyphal tip region in filamentous fungi. To examine further the physiological roles of endocytosis we investigated a conditional mutant of endocytosis in Aspergillus oryzae. Endocytosis-deficient hyphae displayed retarded apical growth, abnormal hyphal morphology, mislocalization of a vesicle- SNARE, which is thought to undergo endocytic recycling to the tip region, and aberrant accumulation of cell wall components at large invaginated structures. These results suggest that endocytosis is crucial for apical growth and for recycling components, which should be re-transported to the tip region. In this report, we discuss the endocytic recycling pathway and present its possible mechanism in filamentous fungi.
Key words: apical growth, endocytosis, filamentous fungi, recycling
Endocytosis is highly conserved in eukaryotic cells, and is an important cellular process that occurs, for example, in acquisition of nutrients from the extracellular environment, uptake of plasma membrane proteins and reconstruction of cell polarity. In the endocytic process, many proteins are involved and regulated spatiotemporally, and the mechanism of endocytosis has been studied in detail in the model organisms such as unicellular yeast Saccharomyces cerevisiae.1 In contrast, in filamentous fungi, the existence of endocytosis was recently confirmed using the fluorescent dye FM4-64 and the plasma membrane protein AoUapC (uric acid-xanthine permease) fused with EGFP which were internalized from the plasma membrane into cells.2,3 Filamentous fungi undergo polarized apical growth in almost all of their life cycle, which is critically different from S. cerevisiae. Endocytosis is thought to be the counterpart of exocytosis, which is required for hyphal growth in the fungal tip region. To achieve continuous tip elongation, filamentous fungi need to re-transport certain components, such as cell wall-building enzymes, to the tip region by endocytic recycling. Thus it is speculated that endocytosis occurs more actively in A. oryzae than in S. cerevisiae. Recently, the localization of components required for endocytosis has been analyzed in living hyphal tip region.4–6 However, the physiological importance of endocytosis in filamentous fungi still remains largely unaddressed.
To investigate the roles of endocytosis in filamentous fungi, we analyzed a conditional mutant of endocytosis in A. oryzae.7 Because it is likely that Aoend4, the A. oryzae ortholog of S. cerevisiae END4/SLA2, is essential for hyphal growth, we generated a strain in which Aoend4 was expressed from its original locus under the control of a regulatable thiA promoter. End4p/Sla2p functions as an adaptor that connects the invaginated plasma membrane and actin cytoskeleton.8 In the Aoend4-repressed condition endocytosis was impaired, which was confirmed by the analyses using FM4-64 and AoUapC-EGFP. Hyphae that showed the endocytic defect displayed aberrant hyphal morphology, presumably due to the lack of endocytosis. Hyphal tip elongation requires many components, some of which are thought to be recycled to the tip region by endocytosis. AoSnc1, a vesicle-SNARE (v-SNARE) protein localized both in the plasma membrane and endosome, is a candidate component for endocytic recycling in the tip region.9 We demonstrated that endocytic recycling of AoSnc1 occurs at the tip region; AoSnc1 is normally localized to Spitzenkörper, a structure containing a large number of secretory vesicles at the tip region, but it mislocalized to the whole plasma membrane in endocytosis- deficient hyphae. These results suggest that endocytosis is essential for apical growth and plays a role in recycling of certain components, such as v-SNARE, occurring mainly at the tip region in filamentous fungi. Moreover, in endocytosis-deficient hyphae, large invaginated structures filled with cell wall components were observed, suggesting that cell wall synthases, which should undergo endocytic recycling, failed to be internalized by endocytosis (Fig. 1A).
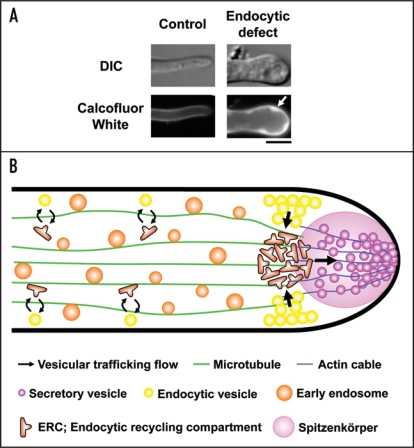
Figure 1.
A model of endocytic recycling at the tip region in A. oryzae. (A) In endocytosis-deficient hyphae, large invaginated structures are stained with Calcofluor White, which binds to chitin, one of major cell wall components in filamentous fungi (arrow). Bar, 5 µm. (B) In this figure, motor proteins, such as kinesins and dynein, which drive early endosomes moving on microtubules bidirectionally, are omitted. Components, which should be re-transported to the tip region, are internalized from the subapical tip region of plasma membrane where endocytosis actively occurs, to ERC, and secretory vesicles are subsequently transported to Spitzenkörper. Although there is no evidence, ERC possibly exists not only in the apical region but also, to some extent, in the subapical and basal region (the latter two strategies are technically represented by the opposite of the highlighted arrows shown above).
In Aspergillus nidulans, it has been recently suggested that endocytic recycling at the tip region occurs via a compartment termed dynein loading zone [or endocytic recycling compartment (ERC)], but its recycling pathway has been less clear.10 Based on the analyses using the endocytosis-deficient mutant in A. oryzae, a model of endocytic recycling at the tip region is presented as shown in Figure 1B. Components such as v-SNARE destined to be recycled to the tip by endocytosis are mainly internalized from the subapical tip region of plasma membrane to ERC, and thereafter are transported to Spitzenkörper. However, these hypotheses are postulated by analyses mainly using v-SNARE as an endocytic recycling cargo; therefore, analyses using cargoes besides v-SNARE, such as cell wall-related components which would go through endocytic recycling, are certainly needed because they are likely to undergo discrete endocytic recycling pathway. Of note, A. oryzae can secrete a large amount of proteins, for example α-amylase, to the medium and is regarded to be a safe host for the production of heterologous proteins.11 In A. oryzae, endocytosis occurring actively at the tip region probably permits a large amount of protein secretion by recycling of certain components required for exocytosis.
In summary, in filamentous fungi, endocytic recycling is closely associated with apical growth and exocytosis at the tip region. There is possibly a mechanism of endocytic recycling which is especially active and specific to filamentous fungi, unlike unicellular yeasts such as S. cerevisiae. Endocytic recycling at the tip region probably enables a large amount of protein secretion, which is assumed to occur notably at the tip region.
Acknowledgements
Y.H. was supported by research fellowships for young scientists from the Japan Society for the Promotion of Science. This study was supported by Grant-in-Aid for Scientific Research (S) and Grant-in-Aid for Scientific Research on Priority Areas “Applied Genomics” from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
- EGFP
enhanced green fluorescent protein
- ERC
endocytic recycling compartment
- FM4-64
N-(3-triethylammoniumpropyl)4-(p-diethyl-aminophenyl-hexatrienyl) pyridinium dibromide
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/8385
References
- 1.Toret CP, Drubin DG. The budding yeast endocytic pathway. J Cell Sci. 2006;119:4585–4587. doi: 10.1242/jcs.03251. Erratum in: J Cell Sci 2007; 120:1501. [DOI] [PubMed] [Google Scholar]
- 2.Peñalva MA. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fungal Genet Biol. 2005;42:963–975. doi: 10.1016/j.fgb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Higuchi Y, Nakahama T, Shoji JY, Arioka M, Kitamoto K. Visualization of the endocytic pathway in the filamentous fungus Aspergillus oryzae using an EGFP-fused plasma membrane protein. Biochem Biophys Res Commun. 2006;340:784–791. doi: 10.1016/j.bbrc.2005.12.077. [DOI] [PubMed] [Google Scholar]
- 4.Araujo-Bazán L, Peñalva MA, Espeso EA. Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in Aspergillus nidulans. Mol Microbiol. 2008;67:891–905. doi: 10.1111/j.1365-2958.2007.06102.x. [DOI] [PubMed] [Google Scholar]
- 5.Taheri-Talesh N, Horio T, Araujo-Bazán L, Dou X, Espeso EA, Peñalva MA, et al. The tip growth apparatus of Aspergillus nidulans. Mol Biol Cell. 2008;19:1439–1449. doi: 10.1091/mbc.E07-05-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Upadhyay S, Shaw BD. The role of actin, fimbrin and endocytosis in growth of hyphae in Aspergillus nidulans. Mol Microbiol. 2008;68:690–705. doi: 10.1111/j.1365-2958.2008.06178.x. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi Y, Shoji JY, Arioka M, Kitamoto K. Endocytosis is crucial for cell polarity and apical membrane recycling in the filamentous fungus Aspergillus oryzae. Eukaryot Cell. 2009;8:37–46. doi: 10.1128/EC.00207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smythe E, Ayscough KR. Actin regulation in endocytosis. J Cell Sci. 2006;119:4589–4598. doi: 10.1242/jcs.03247. [DOI] [PubMed] [Google Scholar]
- 9.Kuratsu M, Taura A, Shoji JY, Kikuchi S, Arioka M, Kitamoto K. Systematic analysis of SNARE localization in the filamentous fungus Aspergillus oryzae. Fungal Genet Biol. 2007;44:1310–1323. doi: 10.1016/j.fgb.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Abenza JF, Pantazopoulou A, Rodríguez JM, Galindo A, Peñalva MA. Long-distance movement of Aspergillus nidulans early endosomes on microtubule tracks. Traffic. 2009;10:57–75. doi: 10.1111/j.1600-0854.2008.00848.x. [DOI] [PubMed] [Google Scholar]
- 11.Shoji JY, Arioka M, Kitamoto K. Dissecting cellular components of the secretory pathway in filamentous fungi: insights into their application for protein production. Biotechnol Lett. 2008;30:7–14. doi: 10.1007/s10529-007-9516-1. [DOI] [PubMed] [Google Scholar]