Abstract
A unique evolutionary specialization of African weakly electric fish (Mormyridae) is their ability to produce and perceive electric signals. Mormyrids use their electric organs discharge (EOD) for electrolocation and electrocommunication. Here we discuss the adaptive significance of the EOD in foraging (electric prey detection) in light of recent results demonstrating that mormyrid fish mate assortatively according to EOD waveform characteristics (electric mate choice). Therefore the EOD as a single trait pleiotropically combines natural divergent selection and reproductive isolation. Consequently we postulate the EOD as a “magic trait” promoting the diversification of African weakly electric fish.
Key words: Campylomormyrus, Mormyridae, radiation, mate choice, reproductive isolation, ecological speciation
Novel evolutionary specializations are thought to be one of the major driving forces of diversification.1,2 Within African weakly electric fish (Mormyridae) the evolution of the electric organ discharge (EOD) may represent a key innovation promoting the radiation in this group of fish. Supporting evidence for the importance of the EOD for diversification has been identified in two well-studied mormyrid species flocks exhibiting a high degree of interspecific EOD variation: the Paramormyrops species flock from Gabon3–5 and the Campylomormyrus species flock from the lower Congo River6–8 (Fig. 1). These fish use their ability to produce and perceive electric signals to sense objects (active electrolocation9,10) and in a social context for electrocommunication.11,12 In this paper we scrutinize support for the idea that divergent EOD types evolved to allow different ecotypes to exploit dissimilar food sources (leading to trophic niche segregation), and that EOD simultaneously also plays an important role for conspecific mate attraction. Hence, EOD may be an example of a “magic trait”,13 which is a trait shaped by disruptive natural selection but pleiotropically also affects pre-zygotic reproductive isolation, facilitating rapid speciation in African weakly electric fish.
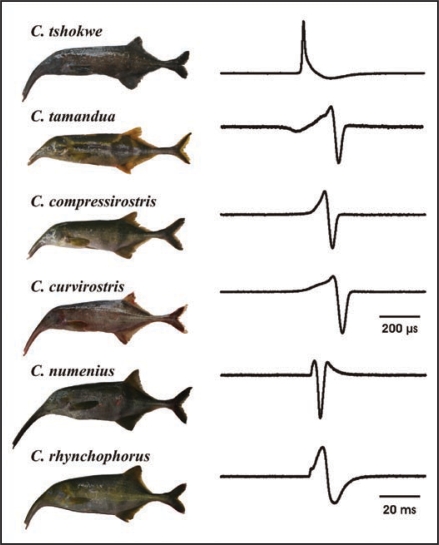
Figure 1.
Interspecific variation in Campylomormyrus species of the lower Congo River. Morphological differentiation as demonstrated in the images is pronounced in the trunk-like elongated snout, the corresponding EOD vary largely in their waveform but also dramatically in the duration (note different scales indicated).
Electric Prey Detection
Besides their ability to produce electric signals, mormyrid fish are well known for their remarkable diversity in viscerocranial morphology (Fig. 2). Campylomormyrus species possess a trunk-like snout with a terminal opening and feed on insect larvae hidden in the sediment.14 Within the sympatric Campylomormyrus species from the lower Congo River the shape of the rostrum shows the strongest degree of divergence in a morphometric analysis.6 This points towards a role for disruptive natural selection driving ecological differentiation between closely related species, leading to trophic niche segregation.
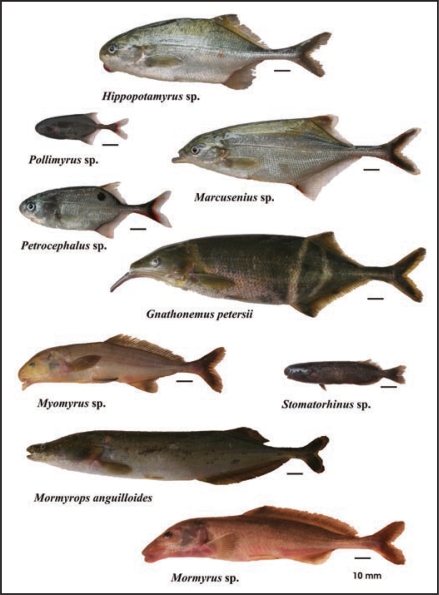
Figure 2.
Diversification of snout morphology in the Mormyridae of the lower Congo River.
A behavioral study highlighted the importance of active electrolocation for prey detection especially in the dark.15 Mormyrids are nocturnal and often occur in turbid rivers, so the electric sense of these fish is likely to play a crucial role during food detection. As electrolocation is a frequency dependent process, the duration of the EOD affects the kind of prey items that can be detected best.16 Mormyrid species with higher frequency components in their EODs, i.e., short duration EODs, should thus be able to detect smaller prey items. Indeed, sister species like C. compressirostris and C. rhynchophorus, which show marked differences in their EOD duration also show pronounced differences in their snout morphology, indicating the exploitation of different prey spectra. A recent study on the molecular evolution of sodium channel genes potentially shaping the electric signal, found support for positive selection acting on NaV1.4a within lineages of electric fish.17 This gene lost its expression in the muscle tissue of electric fish and is solely expressed in the electric organ.
Interestingly, our recent study on Campylomormyrus18 did not find any differences between the sexes in EOD even when only mature, reproducing specimens were considered. This finding is compatible with the idea that ecological adaptation rather than sexual selection drives divergence in EOD. However, our results contrast with studies on various other mormyrid genera where sex-specific differences in the EOD were found—especially during the breeding season.19–21
Electric Mate Choice
Species specificity of EOD types along with the marked differentiation between closely related sympatric species led us to ask whether EOD differentiation affects reproductive isolation.5,7 In a recent study18 we showed, using dichotomous choice tests, that sexually mature Campylomormyrus females exhibit a preference to associate with conspecific males. Although females could not mate during experiments association preferences, as measured in our study,18 provides a good proxy to mate choice and has been shown to be a good indicator of mating preferences in other fish species [e.g., blennies (Salaria pavo),22 mollies (Poecilia mexicana)23 and gobies (Pomatoschistus minutus)24]. Even if the preferences we measured were not entirely sexually motivated, phenotype-assortative social preferences still promote species segregation and thus can facilitate reproductive isolation.25,26
Using electric playback experiments we confirmed that female decision-making is indeed based on EOD waveform characteristics.18 During playback experiments, we kept the amplitude of EODs and the sequence of pulse intervals (SPI) constant, so any behavioral response in females was due to differences in EOD waveforms. The SPI varies in different contexts, such as foraging, agonistic as well as non-agonistic interactions, while the EOD waveform is determined by the anatomy and physiology of the electric organ.27,28 Females in our study did discriminate when EOD waveforms differed, preferring the conspecific waveform but did not discriminate between males or playback signals of species with very similar EOD waveforms.18 Knollenorgan receptors exhibit distinctive responses to different EOD waveforms as shown by a study on the Paramormyrops species from Gabon.29 This indicates that signal discrimination based on EOD waveform characteristics does not require change in receptor response properties.
Magic Trait EOD
During ecological speciation adaptive trait divergence is caused by disruptive natural selection.30 Especially in systems, in which diverging morphotypes face gene flow, reproductive isolation is crucial to complete speciation.
Considering the radiation in mormyrid fish, if divergence in snout morphology was the driving force of speciation, then the ecological trait (snout morphology) and traits responsible for reproductive isolation (EOD waveform) would need to be genetically linked to each other. However, in an alternative scenario where the trait under divergent natural selection is at the same time also responsible for reproductive isolation, e.g., the EOD being adaptive in foraging and simultaneously causing reproductive isolation via assortative mating, no linkage between traits is required. We provide arguments that pronounced differences in EOD both likely affect the food spectrum and are used for mate recognition. Hence, we propose that the EOD of mormyrid fish—with its dual function—could be a “magic trait” combining disruptive natural selection and assortative mating on a single trait.
Acknowledgements
K. Manteuffel and A. Schneider provided technical assistance. Financial support is acknowledged from the German Science Foundation (DFG; Priority Program SPP-1127 “Adaptive Radiation—Origin of Biological Diversity”; TI 349/1). We appreciated the discussion with Matthew E. Arnegard, Jessica Stapley and Carole Smadja.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/8386
References
- 1.Schluter D. The Ecology of Adaptive Radiation. Oxford: Oxford University Press; 2000. [Google Scholar]
- 2.Simpson GG. The Major Features of Evolution. New York: Columbia University Press; 1953. [Google Scholar]
- 3.Arnegard ME, Bogdanowicz SM, Hopkins CD. Multiple cases of striking genetic similarity between alternate electric fish signal morphs in sympatry. Evolution. 2005;59:324–343. [PubMed] [Google Scholar]
- 4.Sullivan JP, Lavoué S, Arnegard ME, Hopkins CD. AFLPs resolve phylogeny and reveal mitochondrial introgression within a species flock of African electric fish (Mormyroidea: Teleostei) Evolution. 2004;58:825–841. doi: 10.1111/j.0014-3820.2004.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 5.Arnegard ME, Hopkins CD. Electric signal variation among seven blunt-snouted Brienomyrus species (Teleostei: Mormyridae) from a riverine species flock in Gabon, Central Africa. Environ Biol Fishes. 2003;67:321–339. [Google Scholar]
- 6.Feulner PG, Kirschbaum F, Mamonekene V, Ketmaier V, Tiedemann R. Adaptive radiation in African weakly electric fish (Teleostei: Mormyridae: Campylomormyrus): a combined molecular and morphological approach. J Evol Biol. 2007;20:403–414. doi: 10.1111/j.1420-9101.2006.01181.x. [DOI] [PubMed] [Google Scholar]
- 7.Feulner PG, Kirschbaum F, Tiedemann R. Adaptive radiation in the Congo River: An ecological speciation scenario for African weakly electric fish (Teleostei; Mormyridae; Campylomormyrus) J Physiol Paris. 2008;102:340–346. doi: 10.1016/j.jphysparis.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Feulner PGD, Kirschbaum F, Schugardt C, Ketmaier V, Tiedemann R. Electrophysiological and molecular genetic evidence for sympatrically occuring cryptic species in African weakly electric fishes (Teleostei: Mormyridae: Campylomormyrus) Mol Phylogenet Evol. 2006;39:198–208. doi: 10.1016/j.ympev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Lissmann HW, Machin KE. The mechanism of object location in Gymnarchus niloticus and similar fish. J Exp Biol. 1958;35:451–486. [Google Scholar]
- 10.von der Emde G. Active electrolocation of objects in weakly electric fish. J Exp Biol. 1999;202:1205–1215. doi: 10.1242/jeb.202.10.1205. [DOI] [PubMed] [Google Scholar]
- 11.Ladich F, Collin SP, Moller P, Kapoor BG. Communication in Fishes. Enfield, NH: Science Publisher; 2006. [Google Scholar]
- 12.Moller P. Electric Fishes History and Behavior. London: Chapman & Hall; 1995. [Google Scholar]
- 13.Gavrilets S. Fitness landscapes and the origin of species. Princeton, NJ: Princeton University Press; 2004. [Google Scholar]
- 14.Marrero C, Winemiller KO. Tube-snouted gymnotiform and mormyriform fishes—convergence of a specialized foraging mode in teleosts. Environ Biol Fishes. 1993;38:299–309. [Google Scholar]
- 15.von der Emde G, Bleckmann H. Finding food: senses involved in foraging for insect larvae in the electric fish Gnathonemus petersii. J Exp Biol. 1998;201:969–980. doi: 10.1242/jeb.201.7.969. [DOI] [PubMed] [Google Scholar]
- 16.Meyer JH. Behavioral-responses of weakly electric fish to complex impedances. J Comp Physiol. 1982;145:459–470. [Google Scholar]
- 17.Zakon HH, Lu Y, Zwickl DJ, Hillis DM. Sodium channel genes and the evolution of diversity in communication signals of electric fishes: convergent molecular evolution. Proc Natl Acad Sci USA. 2006;103:3675–3680. doi: 10.1073/pnas.0600160103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feulner PG, Plath M, Engelmann J, Kirschbaum F, Tiedemann R. Electrifying love: electric fish use species-specific discharge for mate recognition. Biol Lett. 2009;5:225–228. doi: 10.1098/rsbl.2008.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landsman RE. Sex-differences in external morphology and electric organ discharges in imported Gnathonemus petersii (Mormyriformes) Anim Behav. 1993;46:417–429. [Google Scholar]
- 20.Machnik P, Kramer B. Female choice by electric pulse duration: attractiveness of the males' communication signal assessed by female bulldog fish, Marcusenius pongolensis (Mormyridae, Teleostei) J Exp Biol. 2008;211:1969–1977. doi: 10.1242/jeb.016949. [DOI] [PubMed] [Google Scholar]
- 21.Westby GWM, Kirschbaum F. Sex-differences in the waveform of the pulse-type electric fish, Pollimyrus isidori (Mormyridae) J Comp Physiol. 1982;145:399–403. [Google Scholar]
- 22.Gonçalves D, Oliveira R. Time spent close to a sexual partner as a measure of female mate preference in a sex-role-reversed population of the blenny Salaria pavo (Risso) (Pisces: Blenniidae) Acta Ethol. 2003;6:1. [Google Scholar]
- 23.Plath M, Seggel U, Burmeister H, Heubel KU, Schlupp I. Choosy males from the underground: male mating preferences in surface- and cave-dwelling Atlantic mollies (Poecilia mexicana) Naturwissenschaften. 2006;93:103–109. doi: 10.1007/s00114-005-0072-z. [DOI] [PubMed] [Google Scholar]
- 24.Lehtonen TK, Lindstrom K. Repeatability of mating preferences in the sand goby. Anim Behav. 2008;75:55–61. [Google Scholar]
- 25.Bolnick DI, Svanback R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, et al. The ecology of individuals: Incidence and implications of individual specialization. Am Nat. 2003;161:1–28. doi: 10.1086/343878. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg ME, Sinervo B, Brown SP. Socially mediated speciation. Evolution. 2003;57:154–158. doi: 10.1111/j.0014-3820.2003.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 27.Bass AH. Species differences in electric organs of mormyrids: substrates for species-typical electric organ discharge waveforms. J Comp Neurol. 1986;244:313–330. doi: 10.1002/cne.902440305. [DOI] [PubMed] [Google Scholar]
- 28.Werneyer M, Kramer B. Intraspecific agonistic interactions in freely swimming mormyrid fish, Marcusenius macrolepidotus (South African form) J Ethol. 2002;20:107–121. [Google Scholar]
- 29.Arnegard ME, Jackson BS, Hopkins CD. Time-domain signal divergence and discrimination without receptor modification in sympatric morphs of electric fishes. J Exp Biol. 2006;209:2182–2198. doi: 10.1242/jeb.02239. [DOI] [PubMed] [Google Scholar]
- 30.Rundle HD, Nosil P. Ecological speciation. Ecol Lett. 2005;8:336–352. [Google Scholar]