Abstract
During haptic exploration of surfaces, complex mechanical oscillations—of surface displacement and air pressure—are generated, which are then transduced by receptors in the skin and in the inner ear. Tactile and auditory signals thus convey redundant information about texture, partially carried in the spectral content of these signals. It is no surprise, then, that the representation of temporal frequency is linked in the auditory and somatosensory systems. An emergent hypothesis is that there exists a supramodal representation of temporal frequency, and by extension texture.
Key words: texture, somatosensory, audition, interaction, temporal frequency, multisensory integration
When exploring an object tactually, our ability to recognize the object depends a great deal on our perception of its surface texture and material properties. The perception of coarse surface texture is determined by the spatial pattern of deformation it produces in the skin.1 However, the perception of fine surface texture has been shown to rely on the transduction and processing of vibrations produced in the finger during exploration.2 In fact, fingerprint skin may play a role in enhancing texture-elicited vibrations to increase the salience of surface microtexture.3 First, in the absence of movement between texture and surface, the ability to discriminate between fine surfaces is abolished.4 Second, when mechanoreceptive afferents that are sensitive to vibrations are desensitized, the ability to distinguish finely textured surfaces is severely impaired.5 Third, the surreptitious delivery of vibrations during the exploration of surfaces affects their perceived texture.6 Fourth, the perceived roughness of a texture can be predicted from the intensity of the vibrations it produces.7,8
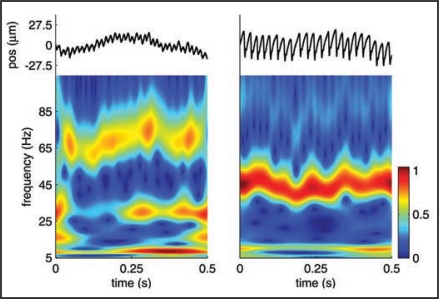
The intensity of the vibrations determines the perceived roughness of the surface that elicits them, but their temporal properties also play an important role in determining perceived texture. Indeed surfaces not only vary along several continua, notably roughness/smoothness, hardness/softness, stickiness/slipperiness and warmth/coolness,9 they are also imbued with characteristic properties that are not captured by these dimensions and that seem to involve a temporal element. The periodicity of corduroy, for instance, is not captured by any textural dimension. The overall percept evoked by a texture seems to be determined not only by the intensity of the vibrations it produces in the skin, but also by their spectral content10 (Fig. 1). Specifically, vibrations elicited during the exploration of a surface have a characteristic spectral content that determines its perceived texture, a sort of “textural timbre.” Our ability to perceive surface texture indirectly through tools constitutes further evidence that textural information is conveyed through vibratory cues.11,12
Figure 1.
Vibrations elicited as two etched silicon surfaces, consisting of an array of truncated pyramids, with spatial periods of 276 (left) and 416 microns (right), are scanned across the finger at 20 mm/s. Vibrations (top plots) were recorded using a Hall Effect Transducer.7 As can be seen, both from the raw traces and the spectrograms, the two surfaces elicit vibrations that differ in their spectral content. The peak frequency of the vibrations is linearly related to the scanning speed and inversely related to the spatial period of the surface (approximately 72 and 48 Hz for the two surfaces at the nominal scanning speed).
That vibrations convey textural information is an idea that has been embraced in rodent somatosensory research.13 Rodents actively explore their environment using stereotyped vibrissal sensing strategies,14 which result in micromotions of the vibrissae. Textural information can be derived from the characteristic temporal patterns of whisker movement15 or, according to the “resonance hypothesis,”16 from the spatial pattern of activated whiskers, each sensitive to a particular frequency or range of frequencies. Although the features of whisker motion that are relevant to texture encoding are under dispute, a consensus is emerging that rodents, like primates, rely on the spectral analysis of mechanical vibrations to distinguish textured surfaces.13
Our ability to discriminate textures solely on the basis of auditory cues can be interpreted as evidence for the role of spectral analysis in texture perception.17 In fact, the auditory and somatosensory systems have been shown to interact in texture perception. In the “Parchment-skin illusion”, the perceived texture of skin depends on the frequency content of simultaneously heard touch-related sounds.18 The perception of textured surfaces is similarly modified by manipulation of auditory cues.19 Results from these studies, along with other demonstrations of audiotactile interactions,20 strongly suggest that the auditory and somatosensory systems are perceptually linked, although these interactions remained, until recently, to be systematically explored in the temporal domain (as pointed out by Soto-Faraco and Deco20).
In a first such attempt, we recently showed that the percepts of auditory and tactile stimuli interact in a frequency-dependent manner.21 Indeed, auditory distractors affected the ability of human subjects to discriminate the frequency of tactile stimuli to the extent that the frequency content of the auditory and tactile stimuli was similar. The same auditory distractors did not affect participants' ability to discriminate the intensity of tactile vibrations, suggesting a specificity of auditory interference on tactile perception to the frequency domain. Furthermore, we found the distracting effects of band-pass noise stimuli to be comparable to their pure tone counterparts. This last result is especially relevant to the present discussion given that the signals conveying textural information are spectrally complex.10
The cortical representation of vibrotactile frequency has been a matter of debate, but it is generally accepted that “flutter” (<80 Hz) and vibration are processed along distinct channels.22,23 Although the cortical representation of “flutter” has been extensively studied,24 its high frequency counterpart remains to be elucidated. In light of the specific audio-tactile interactions described above and given that the auditory system is specialized for spectral analysis, one intriguing possibility is that the spectral analysis of tactile signals is mediated by an area traditionally believed to be auditory. A promising candidate is the caudomedial auditory belt area (area CM),25–27 which has been shown to receive somatosensory input28,29 and is sensitive to both auditory pure tones and band-pass noise.30 The hypothetical role of area CM in texture perception is readily testable: manipulation of CM activity using microstimulation (in the case of animal studies) or transcranial magnetic stimulation (TMS) should modify or disrupt textural percepts.
During haptic exploration of surfaces, then, complex mechanical oscillations are generated, which are tranduced by receptors in the skin and in the inner ear. Tactile and auditory signals thus convey redundant information about texture, in part carried in the spectral content of these signals. It is no surprise that the representation of temporal frequency is linked in the auditory and somatosensory systems. An emergent hypothesis is that there exists a supramodal representation of temporal frequency, and by extension texture.
Acknowledgements
We would like to thank James C. Craig for comments on an earlier version of the manuscript. We also thank Manuel Gomez- Ramirez for technical assistance. This research supported by NIH grant NS18787 and NS062511.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/8551
References
- 1.Johnson KO, Hsaio SS, Yoshioka T. Neural coding and the basic law of psychophysics. Neuroscientist. 2002;8:111–121. doi: 10.1177/107385840200800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollins M, Bensmaãa SJ, Risner R, Grondin S, Lacouture Y. Fechner Day 98: Proceedings of the Fourteenth Annual Meeting of the International Society for Psychophysics. QC, CA: The International Society for Psychophysics; 1998. The duplex theory of tactile texture perception; pp. 115–120. [Google Scholar]
- 3.Scheibert J, Leurent S, Prevost A, Debregeas G. The role of fingerprints in the coding of tactile information probed with a biomimetic sensor. Science. 2009;323:1503–1506. doi: 10.1126/science.1166467. [DOI] [PubMed] [Google Scholar]
- 4.Hollins M, Risner SR. Evidence for the duplex theory of tactile texture perception. Percept Psychophys. 2000;62:695–705. doi: 10.3758/bf03206916. [DOI] [PubMed] [Google Scholar]
- 5.Hollins M, Bensmaia SJ, Washburn S. Vibrotactile adaptation impairs discrimination of fine, but not coarse, textures. Somatosens Motor Res. 2001;18:253–262. doi: 10.1080/01421590120089640. [DOI] [PubMed] [Google Scholar]
- 6.Hollins M, Fox A, Bishop C. Imposed vibration influences perceived tactile smoothness. Perception. 2000;29:1455–1465. doi: 10.1068/p3044. [DOI] [PubMed] [Google Scholar]
- 7.Bensmaia SJ, Hollins M. The vibrations of texture. Somatosens Motor Res. 2003;20:33–43. doi: 10.1080/0899022031000083825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollins M, Bensmaia SJ. The coding of roughness. Can J Exp Psychol. 2007;61:184–195. doi: 10.1037/cjep2007020. [DOI] [PubMed] [Google Scholar]
- 9.Hollins M, Bensmaãa SJ, Karlof K, Young F. Individual differences in perceptual space for tactile textures: Evidence from multidimensional scaling. Percept Psychophys. 2000;62:1534–1544. doi: 10.3758/bf03212154. [DOI] [PubMed] [Google Scholar]
- 10.Bensmaia SJ, Hollins M. Pacinian representations of fine surface texture. Percept Psychophys. 2005;67:842–854. doi: 10.3758/bf03193537. [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka T, Bensmaia SJ, Craig JC, Hsiao SS. Texture perception through direct and indirect touch: An analysis of perceptual space for tactile textures in two modes of exploration. Somatosens Mot Res. 2007;24:53–70. doi: 10.1080/08990220701318163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lederman SJ, Klatzky RL, Hamilton CL, Ramsay GI. Perceiving surface roughness via a rigid probe: Psychophysical effects of exploration speed and mode of touch. Electr J Hapt Res. 1999;1:1–20. [Google Scholar]
- 13.Diamond ME, von HM, Knutsen PM, Kleinfeld D, Ahissar E. ‘Where’ and ‘what’ in the whisker sensorimotor system. Nat Rev Neurosci. 2008;9:601–612. doi: 10.1038/nrn2411. [DOI] [PubMed] [Google Scholar]
- 14.Ritt JT, Andermann ML, Moore CI. Embodied information processing: vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron. 2008;57:599–613. doi: 10.1016/j.neuron.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfe J, Hill DN, Pahlavan S, Drew PJ, Kleinfeld D, Feldman DE. Texture coding in the rat whisker system: slip-stick versus differential resonance. PLoS Biol. 2008;6:215. doi: 10.1371/journal.pbio.0060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore CI. Frequency-dependent processing in the vibrissa sensory system. J Neurophysiol. 2004;91:2390–2399. doi: 10.1152/jn.00925.2003. [DOI] [PubMed] [Google Scholar]
- 17.Lederman SJ. Auditory texture perception. Perception. 1979;8:93–103. doi: 10.1068/p080093. [DOI] [PubMed] [Google Scholar]
- 18.Jousmaki V, Hari R. Parchment-skin illusion: sound-biased touch. Curr Biol. 1998;8:190. doi: 10.1016/s0960-9822(98)70120-4. [DOI] [PubMed] [Google Scholar]
- 19.Guest S, Catmur C, Lloyd D, Spence C. Audiotactile interactions in roughness perception. Exp Brain Res. 2002;146:161–171. doi: 10.1007/s00221-002-1164-z. [DOI] [PubMed] [Google Scholar]
- 20.Soto-Faraco S, Deco G. Multisensory contributions to the perception of vibrotactile events. Behav Brain Res. 2009;196:145–154. doi: 10.1016/j.bbr.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Yau JM, Olenczak JB, Dammann JF, Bensmaia SJ. Temporal frequency channels are linked across audition and touch. Curr Biol. 2009 doi: 10.1016/j.cub.2009.02.013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968;31:301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- 23.Tommerdahl M, Hester KD, Felix ER, Hollins M, Favorov OV, Quibrera PM, et al. Human vibrotactile frequency discriminative capacity after adaptation to 25 Hz or 200 Hz stimulation. Brain Res. 2005;1057:1–9. doi: 10.1016/j.brainres.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Romo R, Salinas E. Flutter discrimination: neural codes, perception, memory and decision making. Nat Rev Neurosci. 2003;4:203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder CE, Foxe J. Multisensory contributions to low-level, ‘unisensory’ processing. Curr Opin Neurobiol. 2005;15:454–458. doi: 10.1016/j.conb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Driver J, Noesselt T. Multisensory interplay reveals crossmodal influences on ‘sensory-specific’ brain regions, neural responses and judgments. Neuron. 2008;57:11–23. doi: 10.1016/j.neuron.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu KM, Johnston TA, Shah AS, Arnold L, Smiley J, Hackett TA, et al. Auditory cortical neurons respond to somatosensory stimulation. J Neurosci. 2003;23:7510–7515. doi: 10.1523/JNEUROSCI.23-20-07510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hackett TA, Smiley JF, Ulbert I, Karmos G, Lakatos P, de la Mothe LA, et al. Sources of somatosensory input to the caudal belt areas of auditory cortex. Perception. 2007;36:1419–1430. doi: 10.1068/p5841. [DOI] [PubMed] [Google Scholar]
- 30.Recanzone GH. Response profiles of auditory cortical neurons to tones and noise in behaving macaque monkeys. Hear Res. 2000;150:104–118. doi: 10.1016/s0378-5955(00)00194-5. [DOI] [PubMed] [Google Scholar]