Abstract
Changes in behavioral state are accompanied by coordinated changes in the information processing mode in the hippocampus and neocortex of the brain. We review here the recent progress in the knowledge of behavioral state-dependent changes in the information processing mode in the central olfactory system. Olfactory cortex shows state-dependent gating of afferent sensory inputs. In the olfactory bulb, granule-to-mitral dendrodendritic synaptic inhibition is enhanced and the frequency of synchronized oscillatory activity of bulbar output neurons decreases during slow-wave sleep or deeply anesthetized state. These results suggest that the information processing mode in the whole olfactory system changes in a behavioral state-dependent manner to keep the neuronal circuits functioning optimally in each behavioral state.
Key words: behavioral state, olfactory bulb, dendrodendritic synapses, olfactory cortex, cholinergic input, slow-wave sleep
Mammalian brains have a remarkable ability to use sensory information about the external world and the interoceptive state to choose an appropriate behavior from among a wide repertoire of behavioral responses. Changes in behavioral state are accompanied by internally coordinated changes in the information processing mode of local neuronal circuits, including those in the cerebral neocortex and hippocampus.1
Sleep-wake alternation is a characteristic change in behavioral state that is reflected in the information processing mode in the brain. When animals explore for food, for example, they are in the awake exploratory behavior state and the neocortex processes sensory information from the external world. During the exploratory behavior, the neocortical EEG shows fast-wave activity, whereas the hippocampal EEG is characterized by theta-wave oscillations.2,3 During the slow-wave sleep state, in contrast, the neocortex shows large slow-wave oscillations, and sensory gating at the level of the thalamus prevents most of the sensory information from reaching the neocortex.4,5 The functional meaning of the slow-wave activity during slow-wave sleep is a topic of active research and debate. One hypothesis is that slow waves function to reconstruct neuronal circuits and to consolidate memory traces acquired during wakefulness.1,6,7 Another hypothesis is that slow waves downscale the strengths of synapses that were both strengthened and not strengthened during wakefulness.8 This is called synaptic homeostasis hypothesis.
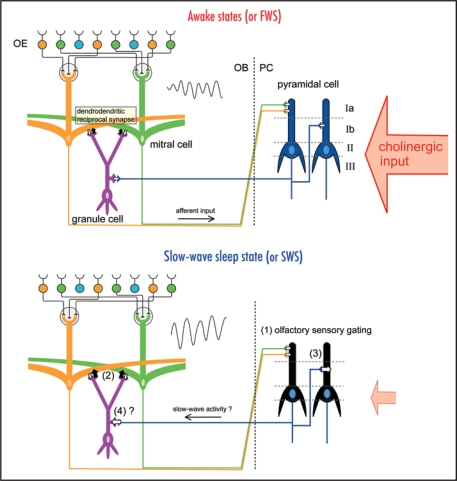
In the mammalian olfactory system, odor signals detected by sensory neurons in the olfactory epithelium are sent via olfactory axons to the olfactory bulb, synaptically relayed to mitral cells, and then sent via mitral cell axons to pyramidal cells in the olfactory cortex (Fig. 1). Thus, the olfactory sensory pathway to the olfactory cortex (olfactory epithelium → olfactory bulb → olfactory cortex) does not contain a thalamic relay. This is in a striking contrast with the visual, auditory, and somatosensory pathways, in which sensory information reaches the neocortex via the thalamus and receives thalamic gating,9–12 and it was only recently that researchers began to understand the behavioral state-dependent changes in the information processing mode of the central olfactory system. In 2005, Murakami and colleagues demonstrated state-dependent sensory gating in the rat olfactory cortex that occurs in synchrony with the thalamic gating of other sensory systems.13 During the fast-wave state (FWS; lightly anesthetized) of urethane-anesthetized rats, each olfactory cortex neuron shows robust spike responses to specific odorants, whereas they show only weak responses during the slow-wave state (SWS; deeply anesthetized). The finding of state-dependent sensory gating in the olfactory sensory pathway, which lacks a thalamic relay, indicates that neuronal circuits within the olfactory cortex have a mechanism for the state-dependent gating of olfactory information.
Figure 1.
Behavioral state-dependent change in information flow in the olfactory system. Schematic diagrams of the neuronal circuit of the olfactory system. The odor information is detected by the olfactory sensory neurons in the olfactory epithelium (OE). Mitral cells in the olfactory bulb (OB) receive excitatory synaptic input from the olfactory sensory neurons and send their axons to the olfactory cortex, including the piriform cortex (PC). The afferent axons from mitral cells of the OB terminate on the dendrites of the pyramidal cells of the PC within layer Ia, whereas the associational fibers from the olfactory cortex terminate in layer Ib or layer III. During awake states or the fast-wave state (FWS) in urethane-anesthetized animals (upper diagram), cholinergic input from the basal forebrain is strong. During the slow-wave sleep state or slow-wave state (SWS) in urethaneanesthetized animals (lower diagram), cholinergic input is weak. During the slow-wave sleep state, (1) olfactory sensory gating occurs in the olfactory cortex, (2) granule-to-mitral dendrodendritic synaptic inhibition is enhanced in the OB and the frequency of gamma oscillation of local field potential (sinusoidal waves, inset) in the OB decreases, (3) the associational input to the PC is enhanced, and (4) the centrifugal input from the PC to the OB granule cells might be enhanced. These state-dependent changes occur coordinately in the whole brain to keep the neuronal circuits functioning optimally in each behavioral state.
Mitral cells in the olfactory bulb project their lateral dendrites over a long distance and form numerous dendrodendritic synaptic connections with spines of granule cells (Fig. 1).14 A dendrodendritic reciprocal synapse consists of a mitral-to-granule excitatory synapse and a granule-to-mitral inhibitory synapse. We showed that granule-to-mitral dendrodendritic synaptic inhibition is much lower during FWS than during SWS, and this state-dependent change is regulated by cholinergic input.15 The dendrodendritic synaptic interactions are thought to be responsible for the generation of synchronized oscillatory discharges of mitral cells that account for the gamma-range oscillation of local field potentials in the olfactory bulb.16–18 We showed that the frequencies of the oscillatory discharges of mitral cells and the oscillatory local field potential are higher during FWS than during SWS.15 This state-dependent change of granule-to-mitral inhibition is also observed in freely behaving animals. Granule-to-mitral inhibition is the smallest during the awake moving state and gets gradually larger during the awake immobility state, light sleep state, and slow-wave sleep state.
These results indicate that the information processing mode in the olfactory system changes in a state-dependent manner. Gating in the olfactory cortex and the enhancement of granule-to-mitral inhibition in the olfactory bulb during SWS may reflect a global and coordinated change of information processing across the whole brain. The olfactory bulb and cortex receive neuromodulatory inputs from cholinergic neurons in the basal forebrain that shows behavioral state-dependent changes in their activity (Fig. 1).19,20 Previous studies in slice preparations showed that association fiber inputs in the piriform cortex and the olfactory tubercle receive presynaptic cholinergic inhibition, whereas the cholinergic system has little influence on afferent inputs from the olfactory bulb to the olfactory cortex.21,22 In addition, the muscarinic cholinergic system enables long-term potentiation of association fiber inputs in the piriform cortex by reducing the dendritic inhibitory postsynaptic potential that follows association fiber stimulation.23,24
During awake states with high cholinergic input, granule-to-mitral inhibition is set to an appropriate level, and mitral cells can fire in synchrony at high frequency in response to odor stimulation. Therefore, the olfactory information is effectively transmitted to the piriform cortex at the same time that the associational fiber inputs within the olfactory cortex are partially inhibited. The cholinergic system regulates the dendritic inhibition in the piriform cortex to enable long-term plasticity of association fiber inputs. During sleep states, especially during the slow-wave sleep state when cholinergic input is low, granule-to-mitral inhibition is enhanced, and the frequency of the synchronized oscillatory firing of mitral cells decreases. Although the activity of mitral cells is transmitted to the olfactory cortex, the olfactory cortex neurons do not respond to the afferent inputs, because of the olfactory sensory gating. During this slow-wave sleep state, the suppression of associational fiber inputs within the olfactory cortex may be released, and centrally generated slow-wave activity may travel from the olfactory cortex to granule cells in the olfactory bulb via centrifugal input. In agreement with this idea, our preliminary data suggest that the centrifugal input from the olfactory cortex to granule cells in the olfactory bulb also changes depending on state (Tsuno Y, unpublished observation). Thus, the state-dependent change in the centrifugal input to the olfactory bulb may be coordinated with the change in the information processing mode in the entire olfactory system.
Changes in the information processing mode occur even within the awake or sleep state. Within the awake state, animals show distinct sub-states that include the exploratory behavior state, awake resting state, and consummatory behavior state. The brain's information processing modes change according to the awake behavioral sub-state. The respiration rate also changes in a sub-state-dependent manner. During consummatory behavior or resting, respiration is slow. In contrast, during exploratory behavior, rats perform theta-frequency sniffing, and theta-wave oscillations are observed in the hippocampus.3 There might be some coordination between sniffing-related signals in the olfactory system and hippocampal theta-wave activity.25 During exploratory behavior, the central olfactory system is effectively processing odor signals from the external world, and animals can discriminate odorants in only one sniff.26,27 The central olfactory system may therefore show the behavioral sub-state-dependent changes in the information processing modes.
The molecular mechanisms of the state-dependent changes in the information processing mode are not clear yet, but trafficking of neurotransmitter receptors changes with behavioral state. For example, excitatory inputs to rat cerebral cortex and hippocampus are regulated by trafficking of GluR1, an AMPA receptor subunit.28 Similarly, inhibitory inputs to rat visual cortex are regulated by trafficking of GABAA receptors.29 Behavioral state-dependent receptor trafficking might also occur in the olfactory bulb and the olfactory cortex. Further studies at the molecular, cellular and network levels should shed light on the functional implications of the behavioral state-dependent changes of the information processing mode in the central olfactory system.
Acknowledgements
We would like to thank Dr. Masahiro Yamaguchi, Dr. Hideki Kashiwadani, and all members of the Department of Physiology, the University of Tokyo. This work was supported by grants-in-aid for scientific research from MEXT, JAPAN (Kensaku Mori) and JSPS Research Fellowships (Yusuke Tsuno).
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/8719
References
- 1.Buzsáki G. Rhythms of the Brain. Oxford; New York: Oxford University Press; 2006. [Google Scholar]
- 2.Buzsáki G, Czopf J, Kondakor I, Kellenyi L. Laminar distribution of hippocampal rhythmic slow activity (RSA) in the behaving rat: current-source density analysis, effects of urethane and atropine. Brain Res. 1986;365:125–137. doi: 10.1016/0006-8993(86)90729-8. [DOI] [PubMed] [Google Scholar]
- 3.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 4.McCormick DA, Bal T. Sensory gating mechanisms of the thalamus. Curr Opin Neurobiol. 1994;4:550–556. doi: 10.1016/0959-4388(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 5.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 6.Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 7.Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 8.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Coenen AM, Vendrik AJ. Determination of the transfer ratio of cat's geniculate neurons through quasi-intracellular recordings and the relation with the level of alertness. Exp Brain Res. 1972;14:227–242. doi: 10.1007/BF00816160. [DOI] [PubMed] [Google Scholar]
- 10.Edeline JM, Manunta Y, Hennevin E. Auditory thalamus neurons during sleep: changes in frequency selectivity, threshold and receptive field size. J Neurophysiol. 2000;84:934–952. doi: 10.1152/jn.2000.84.2.934. [DOI] [PubMed] [Google Scholar]
- 11.Livingstone MS, Hubel DH. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981;291:554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- 12.Mariotti M, Formenti A, Mancia M. Responses of VPL thalamic neurones to peripheral stimulation in wakefulness and sleep. Neurosci Lett. 1989;102:70–75. doi: 10.1016/0304-3940(89)90309-1. [DOI] [PubMed] [Google Scholar]
- 13.Murakami M, Kashiwadani H, Kirino Y, Mori K. State-dependent sensory gating in olfactory cortex. Neuron. 2005;46:285–296. doi: 10.1016/j.neuron.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd GM, Chen WR, Greer CA. The Synaptic Organization of the Brain. New York: Oxford University Press; 2004. Olfactory Bulb; pp. 165–216. [Google Scholar]
- 15.Tsuno Y, Kashiwadani H, Mori K. Behavioral state regulation of dendrodendritic synaptic inhibition in the olfactory bulb. J Neurosci. 2008;28:9227–9238. doi: 10.1523/JNEUROSCI.1576-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashiwadani H, Sasaki YF, Uchida N, Mori K. Synchronized oscillatory discharges of mitral/tufted cells with different molecular receptive ranges in the rabbit olfactory bulb. J Neurophysiol. 1999;82:1786–1792. doi: 10.1152/jn.1999.82.4.1786. [DOI] [PubMed] [Google Scholar]
- 17.Schoppa NE. Synchronization of olfactory bulb mitral cells by precisely timed inhibitory inputs. Neuron. 2006;49:271–283. doi: 10.1016/j.neuron.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Rall W, Shepherd GM. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968;31:884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- 19.Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000;20:1505–1518. doi: 10.1523/JNEUROSCI.20-04-01505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasselmo ME, Bower JM. Cholinergic suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. J Neurophysiol. 1992;67:1222–1229. doi: 10.1152/jn.1992.67.5.1222. [DOI] [PubMed] [Google Scholar]
- 22.Owen GS, Halliwell JV. Electrophysiological characterization of laminar synaptic inputs to the olfactory tubercle of the rat studied in vitro: modulation of glutamatergic transmission by cholinergic agents is pathway-specific. Eur J Neurosci. 2001;13:1767–1780. doi: 10.1046/j.0953-816x.2001.01556.x. [DOI] [PubMed] [Google Scholar]
- 23.Patil MM, Hasselmo ME. Modulation of inhibitory synaptic potentials in the piriform cortex. J Neurophysiol. 1999;81:2103–2118. doi: 10.1152/jn.1999.81.5.2103. [DOI] [PubMed] [Google Scholar]
- 24.Patil MM, Linster C, Lubenov E, Hasselmo ME. Cholinergic agonist carbachol enables associative long-term potentiation in piriform cortex slices. J Neurophysiol. 1998;80:2467–2474. doi: 10.1152/jn.1998.80.5.2467. [DOI] [PubMed] [Google Scholar]
- 25.Macrides F, Eichenbaum HB, Forbes WB. Temporal relationship between sniffing and the limbic theta rhythm during odor discrimination reversal learning. J Neurosci. 1982;2:1705–1717. doi: 10.1523/JNEUROSCI.02-12-01705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- 27.Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem Senses. 2006;31:167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- 28.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 29.Kurotani T, Yamada K, Yoshimura Y, Crair MC, Komatsu Y. State-dependent bidirectional modification of somatic inhibition in neocortical pyramidal cells. Neuron. 2008;57:905–916. doi: 10.1016/j.neuron.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]