Abstract
Background Obesity is a risk factor for several cancers although appears to have an inverse association with cancers strongly related to tobacco. Studying obesity is difficult due to numerous biases and confounding.
Methods To avoid these biases we used a Mendelian randomization approach incorporating an analysis of variants in the FTO gene that are strongly associated with BMI levels among 7000 subjects from a study of lung, kidney and upper-aerodigestive cancer.
Results The FTO A allele which is linked with increased BMI was associated with a decreased risk of lung cancer (allelic odds ratio (OR) = 0.92, 95% confidence interval (CI) 0.84–1.00). It was also associated with a weak increased risk of kidney cancer, which was more apparent before the age of 50 (OR = 1.44, CI 1.09–1.90).
Conclusion Our results highlight the potential for genetic variation to act as an unconfounded marker of environmentally modifiable factors, and offer the potential to obtain estimates of the causal effect of obesity. However, far larger sample sizes than studied here will be required to undertake this with precision.
Keywords: Obesity, cancer, Mendelian randomization
Introduction
Obesity is implicated in the aetiology of several cancers including kidney, breast, colon and gallbladder, and may contribute to an important and increasing number of incident cancer cases in Western societies.1,2 Conversely, a consistent protective effect of higher body mass index (BMI) has been observed for some tobacco-related cancers, in particular for lung cancer.1 Evaluating a causal role of obesity and specific cancers is difficult for a number of reasons. Obesity is likely to be correlated with numerous other lifestyle factors, some of which may be unknown or unmeasured, and removing all potential confounding is not possible. Epidemiological studies of obesity and cancer also usually include measures of weight and height obtained at one point in time, and some level of measurement error is inevitable. Further, a one-off measure may not be representative of lifetime patterns of body composition, including during childhood and early adulthood. Measures of BMI also confuse weight change due to muscle formation as opposed to increased fat. Finally, early stages of cancer may result in weight loss, which may introduce bias due to reverse causation into studies of obesity and cancer.
A Mendelian randomization approach can help overcome these problems by using genetic variation as an instrument or proxy for BMI and obesity.3 Genetic variants that result in increased weight would have several advantages over traditional measures of obesity in that they will not be related to potential confounding factors, can be measured with little or no error, are not affected by disease or recall bias, and they may act as a lifelong marker of increased body weight. The principal restriction of this approach is to identify genetic markers that have a sufficiently important effect on weight for this to translate into a detectable increase (or decrease) in cancer risk.
Recent results provide compelling evidence that variants in the FTO gene can have an important effect on BMI and risk of obesity.4,5 One variant located in the first intron of FTO (rs9939609) resulted in an average increase of ∼3 kg in weight, or one BMI unit, between the two homozygous genotype groups A/A and T/T and an odds ratio (OR) of obesity of 1.67 for a BMI >30. This association was detectable among children as young as 7, implying a long-term effect starting in childhood. Furthermore, the A/A homozygote genetic variant that is associated with this increase is common, with approximately one in six individuals of European origin being homozygous for the variant allele. We have therefore assessed the potential for this gene to act as an unconfounded marker of BMI and obesity in a large study of 4000 cases of lung, aero-digestive and kidney cancer, and 3000 controls.
Materials and methods
Study details have been described in detail elsewhere.6 Briefly, 15 centres in six countries of central and eastern Europe (Czech Republic, Hungary, Poland, Romania, Russia and Slovakia) followed an identical protocol and sought to recruit a consecutive group of newly diagnosed cases of primary lung cancer, and, in some centres, upper aero-digestive cancer and/or kidney cancer, as well as a comparable group of population or hospital controls. All participants were recruited between 1998 and 2003. The total number of potential cases for this study was 2250 lung, 811 aero-digestive (168 mouth, 113 pharynx, 326 larynx and 176 oesophagus, with 28 having overlapping sites) and 954 kidney cancer cases. All cases had been histologically confirmed locally, using standard WHO criteria. Controls in all centres except Warsaw were in-patients or out-patients with non-tobacco-related conditions at the same hospital as the cases, and were frequency matched with the cases by sex, age (within 3 years), centre and area of residence. No diagnostic category made up >20% of the overall control group in each centre. In Warsaw, population controls were recruited instead of hospital controls. In total there were 3052 participating controls. The same interviewer-administered questionnaire was used for both cases and controls that included extensive information on reported height and weight 2 years prior to interview.
The mean BMI among all controls was 26.89, significantly greater than among both lung cancer cases [mean BMI difference adjusted for country and sex (diffadj) = 1.45, P < 0.0001] and aero-digestive cancer cases (diffadj = 1.84, P < 0.0001), but less than that observed among kidney cancer cases (diffadj = −0.65, P = 0.0003). When we compared cases and controls with a BMI of ≤25, with 5 BMI unit categories above this limit, a strong increased risk was observed for kidney cancer and increasing obesity levels (P = 1×10−4), with even stronger inverse risks for lung cancer (P = 5×10−18) and head and neck cancers (P = 6×10−21) (Table 1).
Table 1.
Association between increasing BMI and cancers of the lung, head and neck and kidney, in the IARC Central Europe study
| BMI | Cases | Controls | ORa | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| Lung | ⩽25 | 1180 | 1074 | 1.00 | ref | . |
| 26–30 | 719 | 1192 | 0.60 | (0.52–0.69) | 4 × 10−13 | |
| 31–35 | 231 | 449 | 0.47 | (0.39–0.58) | 3 × 10−13 | |
| 36–40 | 50 | 89 | 0.54 | (0.36–0.81) | 0.003 | |
| 41+ | 11 | 29 | 0.30 | (0.14–0.66) | 0.003 | |
| P-value for trend | 5 × 10−18 | |||||
| Head and Neck | ⩽25 | 492 | 986 | 1.00 | ref | . |
| 26–30 | 256 | 1118 | 0.52 | (0.43–0.63) | 1 × 10−11 | |
| 31–35 | 47 | 407 | 0.27 | (0.19–0.37) | 6 × 10−14 | |
| 36–40 | 11 | 77 | 0.35 | (0.18–0.71) | 0.003 | |
| 41+ | 0 | 25 | – | – | ||
| P-value for trend | 6 × 10−21 | |||||
| Kidney | ⩽25 | 282 | 917 | 1.00 | ref | . |
| 26–30 | 412 | 1018 | 1.22 | (1.02–1.47) | 0.033 | |
| 31–35 | 206 | 368 | 1.59 | (1.27–2.01) | 7 × 10−5 | |
| 36–40 | 43 | 69 | 1.59 | (1.04–2.43) | 0.034 | |
| 41+ | 11 | 21 | 1.34 | (0.62–2.90) | 0.458 | |
| P-value for trend | 1 × 10−4 | |||||
aOR: Adjusted for age, sex, cumulative tobacco consumption, years of drinking consumption and country.
After DNA extraction from lymphocyte samples, genotyping was performed by Taqman for three gene variants of the FTO gene that were reported to be strongly associated with BMI.4 All three genotypes were in very strong linkage disequilibrium, providing almost identical data, and results are presented for rs9939609 only.
Results
Similar to previous reports, controls with the A/A genotype had a higher BMI than controls with the T/T genotype (diffadj = 1.14 kg/m2, P < 0.00001) (Table 2). No particular heterogeneity was seen between the six different countries (P > 0.1 for all three cancer sites). A greater effect of the FTO gene on BMI was seen for those aged <50 [diffadj = 2.26, 95% confidence interval (CI) 1.14–3.38] compared with those aged ⩾50 (diffadj = 0.94, 95% CI 0.42–1.46) (Pheterogeneity = 0.04) No effect was observed between the FTO gene and a variety of potential confounders including tobacco and alcohol consumption (data not shown).
Table 2.
Mean BMI (kg/m2) in controls by genotype of the rs9939609 variant in the FTO gene, overall and by country, age and sex
| Mean BMIa |
||||||
|---|---|---|---|---|---|---|
| TT | TA | AA | Differenceb | 95% CIb | Pb | |
| Overall | 26.65 | 27.05 | 27.79 | 1.14 | (0.66–1.61) | |
| By country | ||||||
| Romania | 26.17 | 27.72 | 27.15 | 0.98 | (−0.89 to 2.86) | 0.905 |
| Hungary | 25.77 | 27.40 | 27.86 | 2.09 | (0.15–4.03) | |
| Poland | 26.11 | 26.61 | 27.21 | 1.1 | (0.27–1.93) | |
| Russia | 27.06 | 26.95 | 28.02 | 0.97 | (0.08–1.85) | |
| Slovakia | 27.91 | 28.05 | 28.66 | 0.75 | (−0.96 to 2.46) | |
| Czech rep | 26.80 | 27.26 | 28.22 | 1.42 | (0.42–2.42) | |
| By sex | ||||||
| Men | 26.24 | 26.52 | 27.10 | 0.86 | (0.34–1.38) | 0.11 |
| Women | 27.12 | 27.90 | 28.96 | 1.84 | (0.83–2.84) | |
| By age | ||||||
| Young (⩽50) | 25.21 | 25.54 | 27.47 | 2.26 | (1.14–3.38) | 0.038 |
| Old (>50) | 26.99 | 27.41 | 27.93 | 0.94 | (0.42–1.46) | |
aMeans BMI adjusted for age, sex, country and tobacco consumption (pack-years).
bDifference and 95% CI compare data for rs9939609-AA to rs9939609-TT genotypes. P is for test of heterogeneity between differences.
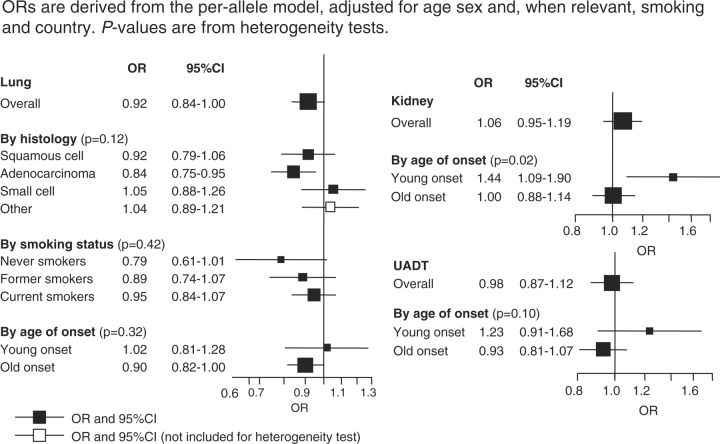
When controls were compared with the three different case groups, the A/A genotype was associated with a significantly reduced risk of lung cancer (OR = 0.83, 95% CI 0.69–0.99) with a more pronounced effect for squamous cell tumours (OR = 0.69, 95% CI 0.53–0.89) and among never-smokers (OR = 0.53, 95% CI 0.30–0.93) (Figure 1). A marginal increase in risk was observed for kidney cancer (OR = 1.13, 95% CI 0.90–1.43), which was restricted to those aged <50 (OR = 2.08, 95% CI 1.19–2.54) (Pheterogeneity = 0.02). No effect was observed for upper aero-digestive cancers.
Figure 1.
Odds ratio (OR) and 95% confidence interval (CI) for lung, upper-aerodigestive (UADT) and kidney cancer by FTO genotype
Discussion
These results provide tentative support for an association between the FTO gene and both lung and kidney cancer and, if replicated in further large studies, would appear to confirm an independent and unconfounded role of obesity for these two cancers (one positive and one negative association). The observed effect size for the FTO gene and kidney cancer is potentially greater than that expected based on previous epidemiological findings, a feature expected to some degree given that the FTO gene is likely to correlate with obesity over the lifecourse. Our results on lung cancer would appear to support the hypothesis that weight change is inversely associated with risk of this cancer, independent of smoking and weight loss due to early disease. Further, the mechanism for any effect, and whether it differs for lung and kidney cancer, is not clear.
This current analysis was based on a large sample size comprising over 7000 subjects, although still only provided evidence that was borderline significant for an association with lung cancer (P = 0.05) and a non-significant association with kidney cancer. Assuming that the difference in BMI with each A allele is ∼0.6 BMI units (as in Table 2), and that the effect of increasing 1 BMI unit is to decrease risk of lung cancer by ∼10%, and increase kidney cancer by 5% (as in Table 1) then one would expect an allele OR associated with FTO of 0.94 for lung cancer and 1.03 for kidney cancer (compared with the observed OR of 0.92 and 1.06, respectively). Based on these expected values, we had only a 34% power to detect a significant (P = 0.05) effect for lung cancer and a 10% power for kidney cancer. A larger sample size of 10 000 case–control pairs will result in a power of close to 90% for lung cancer, although still only a moderate power for kidney cancer (34%).
In conclusion, genetic markers of obesity (such as the FTO gene, but increasingly others), allow for an unbiased assessment of the role of obesity in cancer. However, effects are likely to be moderate and very large-scale coordinated initiatives to determine the true causal effect of obesity-related genes on cancer risk are required.
Funding
European Commission's INCO-COPERNICUS Programme (Contract No. IC15-CT96-0313); National Cancer Institute R01 grant (contract no. CA 092039-01A2).
Conflict of interest: None declared.
References
- 1.Lyon: IARC Press; 2002. IARC Handbooks of Cancer Prevention: Weight Control and Physical Activity. [Google Scholar]
- 2.Scélo G, Brennan P. The epidemiology of bladder and kidney cancer. Nat Clin Pract Urol. 2007;4:205–17. doi: 10.1038/ncpuro0760. [DOI] [PubMed] [Google Scholar]
- 3.Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 4.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:89–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dina C, Meyre D, Gallina S, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–26. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 6.Brennan P, McKay J, Moore L, et al. Uncommon CHEK2 mis-sense variant and reduced risk of tobacco-related cancers: case-control study. Hum Mol Genet. 2007;16:1–8. doi: 10.1093/hmg/ddm127. [DOI] [PubMed] [Google Scholar]