Abstract
Objective To identify risk factors for HIV infection among young women aged 15–24 years reporting one lifetime partner in South Africa.
Design In 2003, we conducted a nationally representative household survey of sexual behaviour and HIV testing among 11 904 young people aged 15–24 years in South Africa. This analysis focuses on the subset of sexually experienced young women with only one reported lifetime sex partner (n = 1708).
Methods Using the proximate determinants framework and the published literature we identified factors associated with HIV in young women. The associations between these factors and HIV infection were explored in multivariable logistic regression models.
Results Of the young women, 15% reporting one lifetime partner were HIV positive. In multivariable analyses, young women who had not completed high school were more likely to be infected with HIV compared with those that had completed high school (AOR 3.75; 95% CI 1.34–10.46).
Conclusions Young South African women in this population were at high risk of HIV infection despite reporting only having one lifetime partner. Few individual level factors were associated with HIV infection, emphasizing the importance of developing HIV prevention interventions that address structural and partner level risk factors.
Keywords: HIV, South Africa, women, education, prevention
Background
Throughout sub-Saharan Africa, young women are at very high risk of HIV acquisition; in many African countries >30% of young women are infected with HIV.1,2 In 2003, we conducted a nationally representative household survey of close to 12 000 young people aged 15–24 years in South Africa. HIV prevalence increased from 4% among 15-year-old women to >30% by the age of 21 years.1 These high levels of infection were observed in the absence of high-risk sexual behaviours. Not unlike risk behaviours observed among adolescent women in the United States, the mean age of coital debut was 17 years, 45% of women reported having one lifetime partner and 48% reported condom use at last sex.3 Similarly, in Kisumu, Kenya, Glynn et al.4 observed an HIV prevalence of 30% among young women reporting one lifetime partner and only 1–5 lifetime sexual contacts.
Why these young women are at such an increased risk of HIV acquisition is not entirely clear. There are multiple factors that could increase young women's risk of infection including biological, behavioural, contextual and structural factors. Young women are biologically more susceptible to HIV acquisition than their male counterparts due to a larger mucosal surface area,5 cervical ectopy6 and a larger volume of inoculum.5 In addition, it is possible that hormonal or immunological changes associated with pregnancy or use of hormonal contraceptive methods could also increase women's risk of HIV acquisition.7,8 Structural/contextual and behavioural factors that could increase young women's risk of HIV infection include poverty and limited educational opportunities that may result in young women choosing older male partners.9 Finally, greater gender-power inequities may lead to reduced condom use, unwanted sexual experiences10,11 and transactional sex.12
Objective
In an attempt to better understand those factors that may increase young South African women's risk of HIV infection, we intentionally focused on risk factors among young women reporting one lifetime sexual partner.
Methods
Design
In 2003, we conducted a nationally representative, household survey of young people aged 15–24 years in South Africa. Details of the study methodology, including information on the sampling and survey design, are described elsewhere.1 Briefly, the survey's sample was identified using a three stage, disproportionate, stratified design. The first stage of selection was the 2001 census enumeration areas (EAs), the second stage involved selecting segments within EAs and the final stage involved randomly selecting one young person per household using the Kish grid method. All selected young people were asked to take part in a comprehensive questionnaire on sexual behaviour and attitudes. In addition, young people were asked to provide an anonymous oral fluid sample that was tested for HIV infection. In total, 11 904 interviews were completed so that 77.2% of enumerated and eligible youth completed an interview. The analyses reported here focus on a subset of sexually experienced young women with only one reported lifetime sex partner (n = 1708). Restricting the sample to those with one partner allowed us to examine risk factors among a seemingly low-risk sample. In addition, these young women had complete information on the characteristics of their first and only sex partner.
Informed consent was obtained from all young people and parental consent was also obtained for those 15–17 years of age. The study was approved by the Human Research Ethics Committee, University of the Witwatersrand, South Africa.
Conceptual model
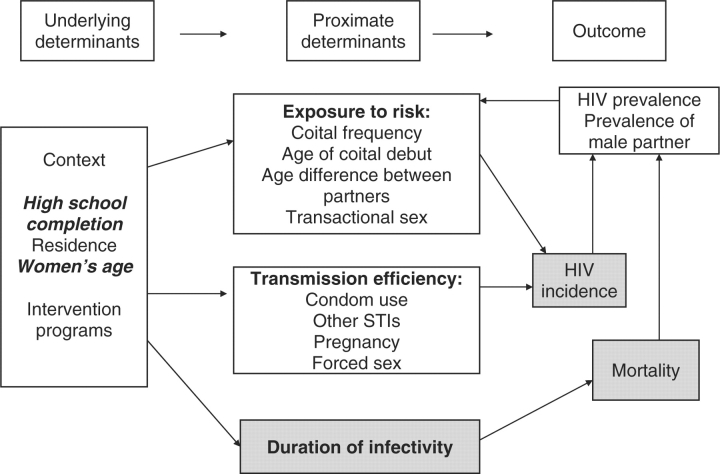
We used the proximate determinants conceptual model to help frame our analyses13,14 and to disentangle the multiple risk factors that function at various levels (i.e. biological, behavioural, contextual/structural), and which place young women at risk of HIV infection. An important feature of this model is that it accounts not only for the biological drivers of HIV infection (exposure of an uninfected individual to an HIV-infected partner, the efficiency of transmission per sexual contact and the duration of infection) but it also considers underlying determinants such as the context in which sexual behaviours take place as well as proximate determinants such as condom use and coital frequency, which directly impact the biological drivers of infection. In addition to this model, we also considered the background prevalence of HIV infection within a community as high-risk sexual behaviours in the absence of disease will not increase the risk of HIV acquisition.13
We identified the following factors from the literature that have been cited as increasing young women's risk of infection and that we measured in the survey: socio-demographic factors (age, education, location of residence),1,15 condom use,1,16 coital frequency,9 age difference between partners,1,9,17 sexually transmitted infections,18 pregnancy,7 forced sex,10,11 early sexual debut,17,19 transactional sex12,20 and prevalence of HIV in male partners.13 We then classified these factors as either underlying determinants or proximate determinants of HIV risk. For underlying determinants, we classified the following as contextual variables: education, place of residence and the woman's age. Within the proximate determinants category, we distinguished risk factors as those that increase the risk of exposure to HIV (e.g. age of sexual debut, age difference with the sex partner, coital frequency within a relationship and transactional sex) and those that increase the efficiency of HIV infection once exposed (e.g. condom use, STIs, pregnancy and forced sex) (Figure 1). We did not measure factors that influence the duration of infectivity (i.e. access to care, use of ARVs), nor did we have information on HIV incidence in the population or mortality. We estimated the prevalence of HIV in the partners of the young women by using the prevalence of HIV in South African men who were the same age as the women's partner.
Figure 1.
Determining risk factors for HIV acquisition among South African young women, using the proximate-determinants framework. Biological determinants are indicated in bold type within the proximate determinate boxes. Risk factors in bold italics are significant predictors in the logistic regression models. The greyed boxes indicate concepts not included in the analysis presented
Measures
Main outcome measure
Infection with HIV was the main outcome for the study and for this analysis. Oral fluid samples were collected using the Orasure® HIV-1 Oral Specimen Collection Device (Orasure Technologies Inc., Bethlehem, PA) and tested for HIV-1/2 antibodies using the Vironostika Uni-Form II HIV-1/2 plus O MicroElisa System (Biomerieux, Durham, NC).
Risk factors
The following variables were assessed as risk factors for HIV infection: underlying determinants: education level (completed high school or not), location of residence (rural, urban), current age (15–19, 20–24 years); and proximate determinants: frequency of condom use (always, less than always), age difference with first sexual partner (0–4, 5+ years), self-reported unusual vaginal discharge in past 12 months (yes, no), ever pregnant (yes, no), ever experienced forced sex (yes, no), age of coital debut (≤14, 15+ years), age of male partner (13–17, 18+ years) and ever engaged in transactional sex (yes, no). Categorical variable cut points were created based on the distribution of the data and convention in the literature. Partnership duration was calculated by asking participants the month and year that they first and last had sex with their first partner (if the relationship was ongoing at the time of the survey then the date the questionnaire was administered was substituted for the last time that they had sex). Duration of the relationship was categorized into 1, 2–6, 7–12 months and >1 year. The frequency of sexual intercourse was assessed by asking participants who reported having had sex in the past 12 months to report the number of times they had sex with their partner in the past month; it was categorized as 0, 1, 2–4 and 5 or more contacts. HIV prevalence estimates for men aged 15–24 years were derived from this survey and for men over the age of 24 years from another nationally representative household survey21 and were estimated based on the prevalence in men the same age as the woman's partner. We categorized prevalence into three levels, <4.8%, 4.8–13.7%, 13.8–23.3%.
Statistical analysis
We reported data using unweighted counts and weighted percentages, except where indicated. Bivariate analyses were conducted to examine the association between HIV prevalence and socio-demographic and HIV risk factors. Multivariable logistic regression analyses were conducted using a full model with all potential covariates. Factors were kept in the model based on a priori hypotheses and statistical significance. Analyses were conducted using the svy and subpop commands in STATA version 9.2.
Results
HIV prevalence among young women reporting one lifetime sexual partner was 15.0% (95% CI 8.6–21.5%). The majority of women reported not completing high school (77.5%) (Table 1). The median age of coital debut was 17 years and just under one quarter (22.4%) reported that their partner was 5 or more years older (Table 1). Only 3.9% of women reported being married. Under one-third (28.6%) reported always using a condom with their partner (Table 1). The median length of relationship was 23.3 months and the median number of acts of vaginal sexual intercourse in the past month was only 1; 44.7% reported not having had sex with their partner in the past month (Table 1).
Table 1.
Unweighted counts, weighted proportions and weighted HIV prevalence of 1708 sexually experienced women ages 15–24 reporting one lifetime sex partner by key behavioural and demographic characteristics, South Africa, 2003
| Characteristic | n(%) | HIV prevalence (%) | Median (range) |
|---|---|---|---|
| Underlying determinants | |||
| Education levela | – | ||
| Not completed high school | 1338 (77.5) | 16.9** | |
| Completed high school | 369 (22.5) | 8.6 | |
| Residence | – | ||
| Rural | 913 (53.9) | 16.5 | |
| Urban | 795 (46.1) | 13.4 | |
| Current age | 19 (15–24) | ||
| 15–19 years | 930 (45.8) | 7.5** | |
| 20–24 years | 778 (54.2) | 21.4 | |
| Proximate determinants | |||
| Condom useb | – | ||
| Always | 485 (28.6) | 7.2 | |
| Sometimes/never/do not know | 1212 (71.4) | 18.2 | |
| Length of first sexual partnershipc | 23.3 (1–123) | ||
| 1 month | 202 (14.7) | 5.8 | |
| 2–6 months | 305 (13.2) | 10.7 | |
| 7–12 months | 245 (15.5) | 7.6 | |
| 13 months or more | 904 (56.6) | 20.0 | |
| Number of sexual contacts in past monthd | 1 (0–90) | ||
| 0 contacts | 514 (44.7) | 11.0 | |
| 1 contact | 249 (14.4) | 10.4 | |
| 2–4 contacts | 423 (29.2) | 12.8 | |
| 5 or more contacts | 141 (11.6) | 5.5 | |
| Frequency of sexual contacts per relationshipe | 14.5 (0–2914) | ||
| 0–5 contacts | 314 (28.4) | 5.4 | |
| 6–50 contacts | 595 (43.0) | 11.9 | |
| 51 or more contacts | 380 (28.7) | 12.2 | |
| Average age difference with first sexual partnerf | 3 (−3 to 32) | ||
| 0–4 years | 1271 (77.6) | 15.0 | |
| 5 or more years | 410 (22.4) | 15.7 | |
| Self–reported vaginal dischargea | – | ||
| No | 1478 (86.7) | 14.2 | |
| Yes | 224 (13.1) | 20.4 | |
| Ever pregnant a | – | ||
| No | 956 (57.9) | 15.3 | |
| Yes | 745 (42.1) | 14.7 | |
| Forced sex | – | ||
| No | 1622 (95.4) | 15.1 | |
| Yes | 86 (4.6) | 13.0 | |
| Coital debuta | 17 (5–24) | ||
| ≤14 years | 107 (5.1) | 10.8 | |
| >14 years | 1599 (94.9) | 15.3 | |
| Transactional sex | – | ||
| No | 1689 (98.6) | 14.9 | |
| Yes | 19 (1.4) | 21.7 |
aMissing data for ≤10 observations.
bMissing data for 11 observations.
cMissing data for 52 observations.
dMissing data for 381 observations.
eMissing data for 419 observations.
fMissing data for 27 observations.
**Significantly different at an alpha level of 0.05.
Bivariate analyses were conducted to examine the association between these key behavioural characteristics and HIV infection. Only educational level and age were significantly associated with HIV infection (Table 2).
Table 2.
Unadjusted and adjusted ORs for factors associated with HIV infection among women aged 15–24 with one lifetime sex partner, South Africa, 2003
| Covariates | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Underlying determinants | ||
| Education | ||
| Completed high school | 1.0 | 1.0 |
| Did not complete high school | 2.15 (1.09–4.26) | 3.75 (1.34–10.46) |
| Woman's age | ||
| 15–19 | 1.0 | 1.0 |
| 20–24 | 3.37 (1.10–10.26) | 4.16 (1.52–11.41) |
| Proximate determinants | ||
| Condom use | ||
| Always | 1.0 | 1.0 |
| Less than always | 2.88 (0.94–8.83) | 2.55 (0.89–7.34) |
| Difference in age between women and partner | ||
| 0–4 years | 1.0 | 1.0 |
| ≥5 years | 1.05 (0.50–2.24) | 0.86 (0.46–1.60) |
| Self-reported vaginal discharge in past 12 months | ||
| No | 1.0 | 1.0 |
| Yes | 1.54 (0.64–3.73) | 1.64 (0.78–3.44) |
| Transactional sex | ||
| No | 1.0 | – |
| Yes | 1.57 (0.19–12.79) | – |
| Ever pregnant | ||
| No | 1.0 | 1.0 |
| Yes | 0.95 (0.36–2.50) | 0.48 (0.16–1.51) |
| Ever experienced forced sex | ||
| No | 1.0 | – |
| Yes | 0.84 (0.37–1.92) | – |
| Age of coital debut | ||
| 15+ years | 1.0 | 1.0 |
| ≤14 years | 0.67 (0.19–2.37) | 1.44 (0.64–3.24) |
| Age of male partner | ||
| 13–17 years | 1.0 | 1.0 |
| 18+ years | 2.47 (0.53–11.56) | – |
| Estimated mean HIV prevalence of male partner (%) | ||
| 2.1–4.7 | 1.0 | 1.0 |
| 4.8–13.7 | 1.73 (0.41–7.36) | 1.52 (0.58–3.97) |
| 13.8–23.3 | 1.15 (0.57–2.32) | 1.67 (0.89–3.14) |
In a multivariable analysis controlling for women's age, age difference between partners, self-reported vaginal discharge, ever being pregnant, age of coital debut and estimated HIV prevalence of their partner, young women who had not completed high school had a 3.75 (95% CI 1.34–10.46) greater odds of being HIV infected compared with those young women who had completed high school (Table 2). Young women aged 20–24 years were also more likely to be infected with HIV compared with younger females aged 15–19 years (OR 4.16 95% CI 1.52–11.41). Although not statistically significant, young women who reported inconsistent condom use, unusual vaginal discharge in the past 12 months, early age of first sex and who had a partner with a higher estimated HIV prevalence were more likely to be infected with HIV.
Discussion
In this study of young South African women reporting only one lifetime sexual partner, 15% were HIV positive and only two factors were significantly associated with prevalent HIV infection in multivariable analyses. Young women who had not completed high school were more likely to be infected with HIV compared with those that had completed high school. In addition, women who were 20- to 24-years-old were more likely to be infected compared with those ages 15–19 years. Although not statistically significant, women who reported having had an unusual vaginal discharge in the past month, early age of first sex, who did not always use condoms with their main partners and women whose partners had a higher estimated prevalence of HIV were also more likely to be infected.
Education can significantly reduce young women's vulnerability to HIV infection. Compared with less educated peers, better educated women are more likely to delay marriage and childbearing, have fewer children, earn better incomes and have greater decision maker power within relationships.22 In 17 countries in Africa and 4 in Latin America, better-educated girls were found to delay age of first sex and were more likely to use condoms.23 In Zambia, young women with more education were less likely to be HIV infected than those with less education and declines in infection rates from 1995 to 2003 were greatest in young women with the most education.24 In Uganda, HIV infection rates declined the most in young women with a secondary school education.23
Nevertheless, girls face numerous barriers to education. Direct and indirect costs associated with education often prevent young women from attending school.22 In a country such as South Africa where many people live on <1$ a day, the costs of school fees, school uniforms, transportation and books make school attendance an economic impossibility. The primary reason for not completing high school in South Africa is lack of affordability followed second by pregnancy for young women.25 Even in the absence of economic barriers to school attendance, young people, especially young women, are often taken out of school to financially contribute to the household or to take care of sick family members or younger siblings.
Reducing economic barriers to education has been found to increase school attendance and may reduce HIV risk. Children in South African households that receive government social welfare grants are more likely to attend school.26 Cash transfers to families that are conditional upon engaging in behaviours deemed socially beneficial have been used in other parts of the world to encourage children to stay in school. In Mexico, the Progresa program, which provides conditional cash transfers to poor families to send their children to school, has found that the programme increases school enrolment, particularly for girls.27 Reducing the costs associated with school attendance also may reduce risky sexual behaviour. A study in Kenya found that reducing economic barriers to school attendance by paying for girl's school uniforms reduced pregnancy levels and school drop-out rates in those girls.28 Programmes that aim to keep girls in school should be further explored as potential HIV prevention interventions.
As with other studies from sub-Saharan Africa that have aimed to identify behavioural factors associated with HIV infection, we found very few individual level factors that were associated with HIV infection. Access to education is a structural level factor that is not easily modified by the individual, as lack of schools, financial resources and cultural norms often prevent young women from attending school. Other factors identified as increasing the risk of HIV infection, other than the age of the woman, were not individual level factors but rather factors that operate at the level of the woman's partner. Inconsistent condom use and the estimated HIV prevalence of the woman's partner (which increased with the age of her partner) increased the odds that a young woman would be HIV infected, although the associations were not statistically significant. The smaller sample size and analysis for this subgroup within the complex survey framework may have contributed to the imprecision of these estimates. Nevertheless, these factors emphasize the importance of male partners in increasing a woman's risk of infection.
Women aged 20–24 years were significantly more likely to be infected with HIV compared with women aged 15–19 years. Given that women in both age-groups reported having only one lifetime partner, older women might be at increased risk because they reported having sex more frequently than younger women (mean of 2.4 times/month vs 1.6 times/month, respectively) and were in relationship of longer duration than younger women (77% had been in a relationship for >12 months compared with 34% of 15–19-year-old women). Although the age difference between younger and older women and their male partners were similar (the majority had partner 0–4 years older) (data not shown), women who are aged 20–24 years would be more likely to have male partners in an age-group with a higher prevalence of infection. Finally, it is also possible that older women were less truthful about their partner numbers and actually had more lifetime partners and thus were at greater risk.
Although women in this sample reported having only one lifetime partner, 15% were HIV positive. High HIV prevalence levels have also been observed in young women in other sub-Saharan African countries who report one lifetime partner and few acts of sexual intercourse.4 Overall these women report relatively ‘low-risk’ behaviours with regard to factors normally considered ‘high-risk’ for acquiring HIV (e.g. multiple partners, transactional sex, etc.). Only 1% of women in this sample reported having ever engaged in transactional sex and 5% reported early coital debut. Relationship length among women in this sample was on average, close to 2 years, although only 4% of women reported being married (legal or traditional). These young women do not report frequent sex with their partners; almost 45% reported not having sex with their partner in the past month. This raises questions about the nature of these relationships and could indicate that if these young women are in long-term, non-cohabiting relationships where they infrequently have sex with their male partners, their partners may be engaging in concurrent relationships with other women. Concurrency has been found to increase the potential for disease transmission within populations.29 In addition, concurrent relationships also pose risk to the individual; if a male partner acquires HIV from a new partnership, he is significantly more likely to transmit the virus to his main partner during the acute phase of HIV infection than during the latent phase of infection.30
Despite the low risk behavioural profile reported by the women in this sample, a significant proportion acquired HIV in the short span of time that they have been sexually active. We recently reported that the per-partnership probability that a young South African woman would acquire HIV infection if her partner was HIV infected was between 70% and 100%.31 Previous studies from developed countries have found this to be on the average of 30–50%.32 It seems likely that biological factors that increase the efficiency of transmission and acquisition are important within this context. Better understanding the factors that so significantly increase young women's risk of HIV acquisition in sub-Saharan Africa is a critical step in preventing new infections from occurring.
There are several limitations to this analysis. Perhaps most importantly social desirability bias could have resulted in under-reporting of some sexual behaviours (i.e. number of sexual partners) and over-reporting of others (i.e. condom use). Collecting accurate information on self-reported behaviours currently plagues all HIV prevention research.33–35 From our previous research on this sample, there is likely under-reporting of sexual partners by young women.31 Nevertheless, the level of under-reporting at a population level is relatively small. Other studies on sexual behaviour have also reported similar under- and over-reporting.34,35 Methods to improve self-reported behaviours such as Audio Computer Assisted Self Interview (ACASI) or use of biomarkers such as prostate-specific antigen (PSA) may help collect more valid data.36,37 In addition, as these data are cross-sectional, we cannot draw conclusions as to the temporal association between exposures and the outcome of HIV infection. For example, it is possible that young women first acquired HIV infection and then dropped out of school due to illness as opposed to the other way around. This is unlikely, however, as few of the HIV infected individuals in the study knew their status and given their young age, it is probable that they were not infected long enough to be suffering from signs and symptoms related to late stage disease.
Conclusion
Young South African women in this population were at high risk of HIV infection despite reporting having only one lifetime partner. Factors found to increase the risk of HIV infection included not completing high school, older age, higher HIV prevalence in the male partner and inconsistent condom use. Few individual level factors were associated with HIV infection emphasizing the importance of developing HIV prevention interventions that address structural and partner level risk factors such as keeping girls in school and interventions targeted at changing behaviours in men in the age-group with the highest prevalence of infection.
Acknowledgements
Dr Pettifor's time was supported by the Developmental Awards Program of the National Institutes of Health NIAID Sexually Transmitted Infections and Topical Microbicide Cooperative Research Centers (STI-TM CRC) grants to the University of Washington (AI 31448) and the University of North Carolina (AI 31496).
Conflict of interest: None declared.
References
- 1.Pettifor A, Rees H, Kleinschmidt I, et al. Young people's sexual health in South Africa: HIV prevalence and sexual behaviours from a nationally representative household survey. AIDS. 2005;19:1525–34. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. Report on the Global AIDS Epidemic 2006. Geneva: UNAIDS; 2006. [Google Scholar]
- 3.Santelli JS, Lindberg LD, Abma J, McNeely CS, Resnick M. Adolescent sexual behavior: estimates and trends from four nationally representative surveys. Fam Plann Perspect. 2000;32:156–65, 194. [PubMed] [Google Scholar]
- 4.Glynn J, Carael M, Auvert B, et al. Why do young women have a much higher prevalence of HIV than young men? A study in Kisumu, Kenya and Ndola, Zambia. AIDS. 2001;15(Suppl 4):S51–60. doi: 10.1097/00002030-200108004-00006. [DOI] [PubMed] [Google Scholar]
- 5.Fideli U, Allen S, Musonda M, et al. Virologic and Immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17:901–10. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson DL, Peralta L, Graham NM, Zenilman J. Histologic development of cervical ectopy: relationship to reproductive hormones. Sex Transm Dis. 2000;27:252–58. doi: 10.1097/00007435-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–88. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 8.Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science. 2005;308:1582–83. doi: 10.1126/science.1112489. [DOI] [PubMed] [Google Scholar]
- 9.Gregson S, Nyamukapa C, Garnett G, et al. Sexual mixing patterns and sex-differentials in teenage exposure to HIV infection in Zimbabwe. Lancet. 2002;359:1896–903. doi: 10.1016/S0140-6736(02)08780-9. [DOI] [PubMed] [Google Scholar]
- 10.Pettifor A, Measham D, Rees H, Padian N. Sexual power and HIV risk, South Africa. Emerg Infect Dis. 2004;10:1996–2004. doi: 10.3201/eid1011.040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunkle KL, Jewkes RK, Brown HC, Gray GE, McIntryre JA, Harlow SD. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. Lancet. 2004;363:1415–21. doi: 10.1016/S0140-6736(04)16098-4. [DOI] [PubMed] [Google Scholar]
- 12.Dunkle KL, Jewkes RK, Brown HC, Gray GE, McIntryre JA, Harlow SD. Transactional sex among women in Soweto, South Africa: prevalence, risk factors and association with HIV infection. Soc Sci Med. 2004;59:1581–92. doi: 10.1016/j.socscimed.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Zaba B, Slaymaker E, Urassa M, Boerma JT. The role of behavioral data in HIV surveillance. AIDS. 2005;19(Suppl 2):S39–52. doi: 10.1097/01.aids.0000172876.74886.86. [DOI] [PubMed] [Google Scholar]
- 14.Boerma JT, Weir SS. Integrating demographic and epidemiological approaches to research on HIV/AIDS: the proximate-determinants framework. J Infect Dis. 2005;191(Suppl 1):S61–67. doi: 10.1086/425282. [DOI] [PubMed] [Google Scholar]
- 15.Hargreaves JR, Glynn JR. Educational attainment and HIV-1 infection in developing countries: a systematic review. Trop Med Int Health. 2002;7:489–98. doi: 10.1046/j.1365-3156.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed S, Lutalo T, Wawer M, et al. HIV incidence and sexually transmitted disease prevalence associated with condom use: a population study in Rakai, Uganda. AIDS. 2001;15:2171–79. doi: 10.1097/00002030-200111090-00013. [DOI] [PubMed] [Google Scholar]
- 17.Laga M, Schwartlander B, Pisani E, Salif Sow P, Carael M. To stem HIV in Africa, prevent transmission to young women. AIDS. 2001;15:931–34. doi: 10.1097/00002030-200105040-00014. [DOI] [PubMed] [Google Scholar]
- 18.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 19.Pettifor AE, van der Straten A, Dunbar MS, Shiboski SC, Padian NS. Early age of first sex: a risk factor for HIV infection among women in Zimbabwe. AIDS. 2004;18:1435–42. doi: 10.1097/01.aids.0000131338.61042.b8. [DOI] [PubMed] [Google Scholar]
- 20.Luke N. Cross-generational and Transactional Sexual Relations in Sub-Saharan Africa: A Review of the Evidence on Prevalence and Implications for Negotiation of Safe Sexual Practices for Adolescent Girls. Philadelphia: International Center for Research on Women; 2001. [Google Scholar]
- 21.Shisana O, Simbayi L. Behavioural Risks and Mass Media Household Survey 2002. Cape Town: Human Sciences Research Council; 2002. Nelson Mandela/HSRC Study of HIV/AIDS South African National HIV Prevalence. [Google Scholar]
- 22.World Bank. Education and HIV/AIDS a Window of Hope. Washington, DC: World Bank; 2002. [Google Scholar]
- 23.UNAIDS. Report on the Global HIV/AIDS Epidemic. Geneva: UNAIDS; 2000. [Google Scholar]
- 24.Michelo C, Sandoy IF, Fylkesnes K. Marked HIV prevalence declines in higher educated young people: evidence from population-based surveys (1995–2003) in Zambia. AIDS. 2006;20:1031–38. doi: 10.1097/01.aids.0000222076.91114.95. [DOI] [PubMed] [Google Scholar]
- 25.South African Department of Education. Monitoring and Evaluation Report on the Impact and Outcomes of the Education System on South Africa's Population: Evidence from Household Surveys. Pretoria: Department of Education; 2006. [Google Scholar]
- 26.Samson M, Lee U, Ndlebe A, et al. Department of Social Development. 2004. The Social and Economic Impact of South Africa's Social Security System: Economics and Finance Directorate. [Google Scholar]
- 27.Schultz T. The Impact of PROGRESA on School Enrollments. Washington, DC: International Food Policy Research Institute; 2000. [Google Scholar]
- 28.Duflo E, Dupas P, Kremer M, Sinei S. Education and HIV/AIDS Prevention: evidence from a randomized evaluation in Western Kenya. World Bank Policy Research Working Paper. 2006. [Google Scholar]
- 29.Morris M. Sexual networks and HIV. AIDS. 1997;11(Suppl A):S209–Ss16. [PubMed] [Google Scholar]
- 30.Cohen MS, Pilcher CD. Amplified HIV transmission and new approaches to HIV prevention. J Infect Dis. 2005;191:1391–93. doi: 10.1086/429414. [DOI] [PubMed] [Google Scholar]
- 31.Pettifor AE, Hudgens MG, Levandowski BA, Rees HV, Cohen MS. Highly efficient HIV transmission to young women in South Africa. AIDS. 2007;21:861–65. doi: 10.1097/QAD.0b013e3280f00fb3. [DOI] [PubMed] [Google Scholar]
- 32.Baggaley R. PhD dissertation. University of London; 2006. The impact of antiretroviral use in resource-poor settings: insights from mathematical models. [Google Scholar]
- 33.Hewett PC, Mensch BS, Erulkar AS. Consistency in the reporting of sexual behaviour by adolescent girls in Kenya: a comparison of interviewing methods. Sex Transm Infect. 2004;80(Suppl 2):ii43–48. doi: 10.1136/sti.2004.013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregson S, Zhuwau T, Ndlovu J, Nyamukapa CA. Methods to reduce social desirability bias in sex surveys in low-development settings: experience in Zimbabwe. Sex Transm Dis. 2002;29:568–75. doi: 10.1097/00007435-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Nnko S, Boerma JT, Urassa M, Mwaluko G, Zaba B. Secretive females or swaggering males? An assessment of the quality of sexual partnership reporting in rural Tanzania. Soc Sci Med. 2004;59:299–310. doi: 10.1016/j.socscimed.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 36.NIMH Collaborative HIV/STD Prevention Trial Group. The feasibility of audio computer-assisted self-interviewing in international settings. AIDS. 2007;21(Suppl 2):S49–58. doi: 10.1097/01.aids.0000266457.11020.f0. [DOI] [PubMed] [Google Scholar]
- 37.Gallo MF, Behets FM, Steiner MJ, et al. Validity of self-reported ‘safe sex’ among female sex workers in Mombasa, Kenya. Int J STD AIDS. 2007;18:33–38. doi: 10.1258/095646207779949899. [DOI] [PubMed] [Google Scholar]