Abstract
Objectives
Patients suffering from invasive mycoses often receive concomitant antifungal therapy and antibacterial agents. Ciprofloxacin, a carboxyfluoroquinolone, was previously observed to demonstrate the pharmacodynamic interactions with antifungal agents by altering their growth inhibitory activity against Candida albicans and Aspergillus fumigatus. However, little is known about the interaction between other extended-spectrum fluoroquinolones, such as levofloxacin and moxifloxacin, and antifungal agents against C. albicans and A. fumigatus.
Methods
Using a microdilution chequerboard technique, we employed isobolographic analysis adapted to incorporate a non-active agent in order to analyse the potential in vitro interaction between ciprofloxacin, levofloxacin or moxifloxacin and the following representative antifungal agents: amphotericin B, fluconazole or voriconazole and caspofungin.
Results
Synergistic interactions [interaction indices (Iis) 0.69–0.83, P < 0.05] were observed between amphotericin B (0.07–0.31 mg/L) and either ciprofloxacin (0.19–7.65 mg/L) or levofloxacin (0.41–32.88 mg/L) against C. albicans and A. fumigatus. Synergy (Iis 0.56–0.87, P < 0.05) also was found between voriconazole (0.09–0.14 mg/L) and ciprofloxacin (0.22–11.41 mg/L) as well as between caspofungin (8.94–22.07 mg/L) and levofloxacin (0.14–5.17 mg/L) against A. fumigatus. Some antagonistic (Iis 1.16–1.29, P < 0.05) interactions were observed between fluoroquinolones and fluconazole against C. albicans. In general, ciprofloxacin enhanced the activity of antifungal agents more than moxifloxacin and levofloxacin against both C. albicans and A. fumigatus.
Conclusions
The knowledge of the pharmacodynamic interactions between fluoroquinolones and antifungal agents may guide selection and potentially improve the outcome of immunosuppressed patients with concurrent bacterial and fungal infections.
Keywords: C. albicans, A. fumigatus, fluoroquinolones
Introduction
Patients at risk for invasive fungal infections are also at risk for developing bacterial infections. Therefore, these patients often receive antifungal therapy concomitantly with antibacterial agents such as the fluoroquinolones.1,2
Ciprofloxacin, a carboxyfluoroquinolone,3 was previously found to interact pharmacodynamically with antifungal agents by altering their growth inhibitory activity against Candida albicans and Aspergillus fumigatus.4 In vivo studies also have demonstrated enhancement of the efficacy of antifungal agents when they combined with fluoroquinolones against experimental invasive candidiasis and aspergillosis.5–7 Thus, fluoroquinolones may interact with antifungal agents by altering their activity against fungal pathogens. This interaction may have beneficial implications for the outcome of antifungal therapy.
Little is known, however, about the interaction of other fluoroquinolones, such as moxifloxacin and levofloxacin, and antifungal agents against C. albicans and A. fumigatus. We therefore studied the comparative in vitro pharmacodynamic interactions between ciprofloxacin, levofloxacin or moxifloxacin with amphotericin B, fluconazole, voriconazole and caspofungin against C. albicans and A. fumigatus, adapting the isobolographic analysis to incorporate a non-active agent. In the present study, this analysis was used in order to describe the concentration–effect curves of antifungal agents alone and in combination with fluoroquinolones to obtain interaction indices (Iis) quantifying the magnitude of synergy or antagonism and to compare these indices among the three quinolones for each antifungal agent.
Materials and methods
Isolates and medium
Three clinical isolates each of C. albicans (CA 362, CA 8621 and CA 5685) and A. fumigatus [AF 2025, AF 4215 (ATCC MYA-3626) and AF 2350] were used in this study. The strains were stored on potato dextrose agar slants at −70°C. Candida and Aspergillus conidia were collected with a wet swab from 1- to 2- and 5- to 7-day-old cultures in Sabouraud dextrose agar, respectively. Conidial suspensions were adjusted spectrophotometrically at 530 nm to 75% to 77% and 80% to 82% transmittance, respectively. Conidial suspensions were diluted in order to obtain two times the final inoculum, which ranged from 5×102 to 2.5 × 103 cfu/mL for Candida isolates and from 0.4 × 104 to 5 × 104 cfu/mL for Aspergillus isolates in a medium consisting of RPMI 1640 medium buffered at pH 7 with 0.165 M MOPS (BioWhittaker, Walkerville, MD, USA). Candida parapsilosis (ATCC 22019), Candida krusei (ATCC 6258), A. fumigatus (ATCC MYA-3626) and Escherichia coli (ATCC 259222) were used as quality controls.
Antimicrobial compounds and combination microtitration plates
Ciprofloxacin (Bayer AG, Leverkusen, Germany), moxifloxacin (Bayer AG), levofloxacin (Bayer HealthCare AG, Germany), amphotericin B (Ben Venue Laboratories, Inc., Bedford, OH, USA), caspofungin (Merck and Company, Rahway, NJ, USA), fluconazole (Pfizer Pharmaceuticals, New York, NY, USA) and voriconazole (Pfizer Pharmaceuticals) were provided as clinical formulations and prepared according to the manufacturer's guidelines in order to obtain working solutions of 200, 200, 200, 8, 2040, 8 and 10 mg/L, respectively, in the assay medium. The drugs were serially diluted 2-fold in the medium in order to obtain a 1:4 dilution, which ranged from 0.05 to 50 mg/L ciprofloxacin, 0.032 to 2.0 mg/L amphotericin B, 0.015 to 1 mg/L and 8 to 512 mg/L caspofungin for C. albicans and A. fumigatus isolates, respectively, 0.03–2 mg/L fluconazole for C. albicans isolates and 0.03 to 2 mg/L voriconazole for A. fumigatus isolates. The ranges of the antifungal drug concentrations were chosen in order to be around the MICs. The ranges of the concentrations of the fluoroquinolones were selected in order to represent achievable concentrations in the plasma.8,9 Fifty microlitres of each antifungal agent concentration and its drug-free control were combined with 50 µL of each concentration of the fluoroquinolone and its drug-free control in order to obtain a 12 × 8 chequerboard in 96-well flat-bottom microtitration plates (Corning Inc., Corning, NY, USA). The plates were stored at −70°C and thawed on the day of the experiment.
Susceptibility testing
Microtitration plates were thawed and 100 µL of conidial suspensions were inoculated into each well. Plates were incubated at 37°C for 24 h and fungal growth in each well was assessed visually with the aid of a magnifying mirror. The MICs of amphotericin B, caspofungin and voriconazole were defined as the lowest drug concentration that showed no visible growth. The MIC of fluconazole was defined as the lowest drug concentration showing slight growth (20% compared with the drug-free control). Fungal growth was also assessed spectrophotometrically at 405 nm with a spectrophotometer (ELX808, Biotek Instruments, Winooski, VT, USA) and the percentage of growth in each well was calculated based on the following formula: (A405 of a well − background A405)/(A405 of the drug-free well − background A405 of the drug-free well)×100%, where the background A405 was measured from a plate inoculated with a conidia-free inoculum and handled in the same way as the inoculated plates with the conidia-containing inocula. All studies for each strain were replicated three times.
Isobolographic drug interaction analysis
The interactions between the antifungal agents and the fluoroquinolones were assessed using the isobolographic analysis of Loewe additivity as described previously.4 Initially, the Emax model was fitted with a non-linear non-weighted regression analysis (Graph Pad Prism 4.0 Software, San Diego, CA, USA) to the concentration–effect data of each antifungal drug alone and in combination with each fluoroquinolone at mixtures with different proportions (P) of antifungal agents (P = ECAA/ECMIX, where ECAA is the concentration of antifungal agent and ECMIX is the concentration of antifungal agents plus the concentration of the fluoroquinolone). The proportions of amphotericin B, fluconazole and voriconazole in the combination mixtures with each fluoroquinolone were 0.02, 0.04, 0.07, 0.14, 0.24 and 0.39. The proportions of caspofungin in the mixtures were 0.02, 0.07, 0.14, 0.24, 0.39 and 0.56 for C. albicans isolates and 0.84, 0.91, 0.95, 0.98 and 0.99 for A. fumigatus isolates. For each mixture, the ECMIX corresponding to 50% of growth (EC50,MIX) obtained with the Emax model was compared with the isoeffective theoretical additive total concentration (EC50,THE) calculated as EC50,THE = EC50,AA/(PAA + PFQ × EC50,AA/EC50,FQ), where EC50,AA and EC50,FQ are the concentrations of the antifungal drug and the fluoroquinolone alone, respectively, corresponding to 50% of growth and obtained from the Emax model of the concentration–effect curves of the drugs alone, and PAA and PQ are the proportions of the antifungal agent and the fluoroquinolone in the mixture, respectively. However, since fluoroquinolones have no direct antifungal activity, i.e. EC50,FQ≫EC50,AA, the above equation becomes:
An Ii for each mixture was then calculated as the ratio EC50,MIX/EC50,THE for each replicate. The interactions were analysed based on EC50 because this endpoint is the most representative endpoint to describe concentration–effect curves and it is commonly used to quantify pharmacological actions. The analysis also was performed with other endpoints corresponding to 15% and 85% growth inhibition to ensure that the nature of interactions corresponds with those found at 50% growth inhibition (data not shown). Finally, the concentrations of antifungal agents and fluoroquinolones, where significant interactions were observed, were determined and the median and range were calculated for each combination and species.
Statistical analysis
Deviation from 1 of Iis obtained for all mixtures and isolates was assessed statistically using the Wilcoxon signed rank test for each species and combination. Significant synergy and antagonism were concluded when the Iis were statistically significantly (P < 0.05) lower or higher than 1, respectively. In any other case, indifference was concluded. Furthermore, the Iis between the three fluoroquinolones and each antifungal agent were compared statistically using the Kruskal–Wallis test followed by Dunn's multiple comparison test (P < 0.05) for all mixtures and isolates of each species.
Results
MICs and EC50s of single antifungal agents
The MICs of amphotericin B, fluconazole and caspofungin for C. albicans strains were 0.125–0.5, 0.125–0.25 and 1.0–2.0 mg/L, respectively. The median (range) EC50s of amphotericin B, fluconazole and caspofungin as determined by the Emax model fitted to concentration–effect data of each drug individually were 0.12 (0.11–0.16), 0.09 (0.05–0.14) and 0.60 (0.07–1.94) mg/L, respectively, for C. albicans isolates (Table 1).
Table 1.
Antifungal and fluoroquinolone drug concentrations against C. albicans (median and range among replicates and different mixtures) for the combinations that showed statistically significant interactions
| Drug combination | Antifungal agent (mg/L) | Fluoroquinolone (mg/L) |
|---|---|---|
| CIP + AMB | 0.12 (0.11–0.16) | 1.12 (0.19–7.65) |
| CIP + FLC | NDa | ND |
| CIP + CAS | ND | ND |
| LVX + AMB | 0.26 (0.26–0.29) | 1.62 (0.41–7.36) |
| LVX + FLC | 0.27 (0.26–0.35)b | 2.58 (0.41–16.6)b |
| LVX + CAS | 0.64 (0.58–0.7) | 7.58 (1.81–32.88) |
| MXF + AMB | ND | ND |
| MXF + FLC | 0.11 (0.09–0.12)b | 1.08 (0.16–5.3)b |
| MXF + CAS | 0.75 (0.67–0.94) | 6.9 (2.93–16.69) |
AMB, amphotericin B; CAS, caspofungin; CIP, ciprofloxacin; FLC, fluconazole; LVX, levofloxacin; MXF, moxifloxacin.
aND, not determined because no statistically significant interactions were observed.
bThere were statistically significant antagonistic interactions for these combinations.
The MICs of amphotericin B, voriconazole and caspofungin for A. fumigatus isolates were 0.5–1.0, 0.5 and 128 mg/L, respectively. The median (range) EC50s of amphotericin B, voriconazole and caspofungin were 0.19 (0.09–0.54), 0.14 (0.03–0.24) and 15.54 (10.43–9.85) mg/L, respectively, for A. fumigatus isolates (Table 2).
Table 2.
Antifungal and fluoroquinolone drug concentrations against A. fumigatus (median and range among replicates and different mixtures) for the combinations that showed statistically significant synergistic interactions
| Drug combination | Antifungal agent (mg/L) | Fluoroquinolone (mg/L) |
|---|---|---|
| CIP + AMB | 0.28 (0.23–0.31) | 2.69 (0.81–7.78) |
| CIP + VRC | 0.14 (0.09–0.14) | 11.41 (0.31–11.41) |
| CIP + CAS | 17.92 (16.53–22.07) | 0.97 (0.22–3.95) |
| LVX + AMB | 0.14 (0.07–0.21) | 1.36 (0.41–5.17) |
| LVX + VRC | NDa | ND |
| LVX + CAS | 10.35 (8.94–15.85) | 0.51 (0.14–3.1) |
| MXF + AMB | ND | ND |
| MXF + VRC | ND | ND |
| MXF + CAS | ND | ND |
AMB, amphotericin B; CAS, caspofungin; CIP, ciprofloxacin; LVX, levofloxacin; MXF, moxifloxacin; VRC, voriconazole.
aND, not determined because no statistically significant interactions were observed.
Pharmacodynamic interactions for C. albicans
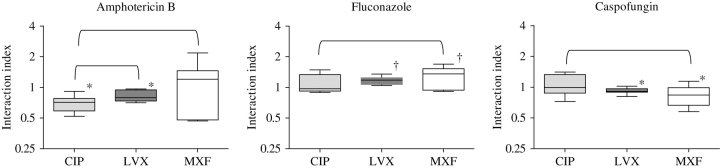
Different interactive patterns were observed between ciprofloxacin, levofloxacin or moxifloxacin and antifungal agents (Table 1). Significant synergy was found for the combination of ciprofloxacin + amphotericin B (Ii ± statistical error = 0.69 ± 0.03) and levofloxacin + amphotericin B (0.83 ± 0.02) (Figure 1). Significant antagonistic interactions were observed for the combinations levofloxacin + fluconazole (Ii = 1.16 ± 0.02) and moxifloxacin + fluconazole (Ii = 1.29 ± 0.07) (Figure 1). The levofloxacin + caspofungin (Ii = 0.91 ± 0.01) and moxifloxacin + caspofungin (Ii = 0.84 ± 0.05) combinations were also synergistic (Figure 1). The synergistic interactions were observed at 0.19–7.65 mg/L ciprofloxacin, 0.41–32.88 mg/L levofloxacin and 2.93–16.69 mg/L moxifloxacin. The amphotericin B and caspofungin concentrations, where the synergistic interactions were observed, were 0.11–0.29 and 0.58–0.94 mg/L, respectively. The concentrations of levofloxacin and moxifloxacin where antagonistic interactions were observed when combined with fluconazole were 0.41–16.6 and 0.16–5.3 mg/L, respectively.
Figure 1.
Box-whisker plots of the Iis for the combination between ciprofloxacin (CIP), levofloxacin (LVX) and moxifloxacin (MXF) and (i) amphotericin B, (ii) fluconazole or (iii) caspofungin against three C. albicans isolates. The symbols * and † indicate statistically significant synergistic and antagonistic interactions, respectively, using Wilcoxon's rank test. The brackets at the top of the plots join the combinations for which statistically significant comparative results (P < 0.05) were observed using the Kruskal–Wallis test.
Comparative pharmacodynamic drug interaction analysis showed that the Iis of the ciprofloxacin + amphotericin B combination were significantly lower than the Iis of levofloxacin + amphotericin B and moxifloxacin + amphotericin B (P < 0.05), indicating that amphotericin B interacts synergistically with ciprofloxacin more strongly than with the other two fluoroquinolones against C. albicans (Figure 1). The Iis of fluconazole + moxifloxacin were slightly higher than the Iis of the combination fluconazole + ciprofloxacin (P < 0.05), indicating stronger antagonism between fluconazole and moxifloxacin than with ciprofloxacin (Figure 1). The strongest synergistic interaction between caspofungin and the three fluoroquinolones was found for moxifloxacin + caspofungin, with the Iis significantly lower than the Iis of the combination ciprofloxacin + caspofungin (Figure 1).
Pharmacodynamic interactions for A. fumigatus
Ciprofloxacin had more synergistic interaction with antifungal agents than moxifloxacin and levofloxacin did (Table 2). Synergy was found for the combinations ciprofloxacin + amphotericin B (Ii = 0.61 ± 0.03) and levofloxacin + amphotericin B (Ii =0.87 ± 0.06), for the combination ciprofloxacin + voriconazole (Ii = 0.56 ± 0.03) and for the combinations ciprofloxacin + caspofungin (Ii = 0.6 ± 0.04) and levofloxacin + caspofungin (Ii = 0.87 ± 0.04) (Figure 2). The synergistic interactions were observed at 0.22–11.41 mg/L ciprofloxacin and 0.14–5.17 mg/L levofloxacin. The concentrations of amphotericin B, voriconazole and caspofungin where these synergistic interactions were observed were 0.07–0.31, 0.09–0.14 and 8.94–22.07 mg/L, respectively.
Figure 2.
Box-whisker plots of the Iis for the combination between ciprofloxacin (CIP), levofloxacin (LVX) and moxifloxacin (MXF) and (i) amphotericin B, (ii) voriconazole or (iii) caspofungin against three A. fumigatus isolates. The symbol * indicates statistically significant deviation of Iis from 1 using Wilcoxon's rank test. The brackets at the top of the plots join the combinations for which statistically significant comparative results (P < 0.05) were observed using the Kruskal–Wallis test.
The comparative pharmacodynamic analysis showed that the Iis of combinations with ciprofloxacin and the three antifungal agents were significantly lower than the Iis of the combinations with the other two fluoroquinolones (P < 0.05) and all three antifungal agents, indicating that ciprofloxacin interacts synergistically with the antifungal agents more strongly than moxifloxacin and levofloxacin did against A. fumigatus isolates (Figure 2).
Discussion
Significant in vitro pharmacodynamic interactions were found between antifungal agents and fluoroquinolones against C. albicans and A. fumigatus. The synergistic activities that were found are: (i) ciprofloxacin or levofloxacin with amphotericin B against C. albicans and A. fumigatus; (ii) ciprofloxacin with voriconazole against A. fumigatus; (iii) levofloxacin or moxifloxacin with caspofungin against C. albicans; and (iv) ciprofloxacin or levofloxacin with caspofungin against A. fumigatus. For both C. albicans and A. fumigatus, ciprofloxacin had stronger synergistic interactions with antifungal agents than levofloxacin and moxifloxacin did. Antagonistic interactions were found between levofloxacin or moxifloxacin with fluconazole against C. albicans.
Most in vitro combination studies have failed to find a significant interaction between fluoroquinolones and antifungal agents, despite in vivo data demonstrating enhancement of antifungal therapy with fluoroquinolone therapy against experimental candidiasis.5–7 The data of such studies were usually analysed by the fractional inhibitory concentration index. Previously, using isobolographic analysis, we were able to find a significant in vitro synergistic interaction between ciprofloxacin and antifungals against C. albicans and A. fumigatus, indicating the sensitivity of isobolographic analysis in detecting in vitro pharmacodynamic interactions.4 However, there were no reported studies of the interactions between antifungal agents and other fluoroquinolones that are administered concomitantly with antifungal therapy. We therefore analysed the interaction between ciprofloxacin, levofloxacin and moxifloxacin and antifungal agents using isobolographic analysis mainly demonstrating significant synergistic interactions.
The mechanisms of these interactions are not well understood. Fluoroquinolones, by themselves, do not possess a significant antifungal growth inhibitory activity. However, fluoroquinolones have the capacity to bind to fungal topoisomerase.10 Thus, fluoroquinolones may inhibit fungal DNA replication and thereby exhibit an antifungal effect. Since this effect is only apparent when fluoroquinolones are combined with antifungal agents, it might be possible that antifungal agents may alter fungal cell membrane permeability and thereby increase intracellular concentrations of fluoroquinolones. This could explain the synergistic interactions between antifungal agents and fluoroquinolones. Furthermore, fluoroquinolones may also enhance the activity of antifungal agents resulting in synergistic interactions by: (i) increasing intracellular levels of antifungal agents, as fluoroquinolones are effluxed via ATP-binding cassette (ABC) transporters,11,12 (ii) co-operating with amphotericin B molecules in forming pores in the fungal membrane,11,13 as fluoroquinolones and amphotericin B are also amphoteric molecules,13 and (iii) increasing the penetration of antifungal agents or increasing the sensitivity of glucan synthase to echinocandins. These hypotheses warrant further study.
The comparative pharmacodynamic interaction analysis revealed stronger synergistic interactions with ciprofloxacin than levofloxacin and moxifloxacin against A. fumigatus. Physicochemical differences between ciprofloxacin, levofloxacin and moxifloxacin (e.g. structure, lipophilicity and membrane permeability)3 may lead to differences in antimicrobial activity and in pharmacodynamic interactions with the antifungal agents. Notably, ciprofloxacin has the smallest molecular weight and is less lipophilic, more water soluble and less permeable through kidney cell lines than levofloxacin and moxifloxacin.14 Thus, ciprofloxacin could be concentrated in the fungal cell membrane in higher amounts than the other two fluoroquinolones.
The genus-specific differences between Candida and Aspergillus in the interactions between fluoroquinolones and antifungal agents reported here may be related to different affinities for fungal topoisomerase or cell membrane transporter molecules. For example, both ciprofloxacin and levofloxacin have in common a 7-piperazine substitution on the 4-quinolone molecule that may explain their common synergistic activity with antifungal agents against A. fumigatus. Whereas, for C. albicans, the greater interactive activity of levofloxacin and moxifloxacin over that of ciprofloxacin may be related to the 8-isoxane and 8-methoxy substitutions, respectively, in comparison with no 8-substitutions for ciprofloxacin. Formal structure-activity studies would be necessary to substantiate these possibilities.
It is of note that antagonistic effects were observed for the combinations of levofloxacin/moxifloxacin + fluconazole against C. albicans. Antagonistic effects were also found for the combination of ciprofloxacin + fluconazole against one out of three C. albicans isolates. However, since this phenomenon was not noticed for the other two strains, the statistical analysis resulted in indifference. In agreement with these findings, Petrou and Rogers15 also found antagonism between ciprofloxacin and norfloxacin, and different azoles (ketoconazole, miconazole and itraconazole) at 10 mg/L quinolones. The mechanism of the antagonistic interaction between quinolones and azoles at high concentrations of quinolones is not known. Previous studies suggest that fluoroquinolones can be substrates of ABC.11,16 Azoles are also substrates of MDR and CDR efflux pumps, which belong to the ABC superfamily.17 Thus a potential interaction between fluoroquinolones and triazoles may occur on the ABC transporter function, which could result in increased efflux of azoles from fungal cells, resulting in antagonism. Further studies are required to reveal the mechanisms that explain the antagonistic interactions between moxifloxacin/levofloxacin + fluconazole against C. albicans.
The combination of amphotericin B with the quinolones was synergistic in the present study. However, in a previous study, we observed antagonism at a 0.01 proportion of amphotericin B in the mixture and at ciprofloxacin concentrations of 44–64 mg/L. Petrou and Rogers15 also found the same phenomenon at 100 mg/L ciprofloxacin concentrations using the agar dilution method. In the present study, amphotericin B proportions ranged between 0.04 and 4; therefore this antagonism was not observed.
The interaction between antifungal agents and quinolones may not be purely pharmacodynamic as moxifloxacin alone prolonged the survival of mice infected with C. albicans and A. fumigatus.18,19 Measurement of different cytokines indicated that perhaps this was most likely due to an immunomodulating activity conferred by moxifloxacin. This suggests that even if fluoroquinolones do not always have pharmacodynamic synergistic interaction with antifungal agents against C. albicans and A. fumigatus, they can exhibit protective effects by immunomodulatory mechanisms.
In conclusion, the present study demonstrated significant in vitro interactions between fluoroquinolones and antifungal agents against C. albicans and A. fumigatus. As immunosuppressed patients frequently require both antifungal and antibacterial therapy, the knowledge of the pharmacodynamic interactions between these agents is important. The choice of the best combination among the fluoroquinolones and an antifungal agent could potentially improve the outcome of a patient with concurrent bacterial and fungal infections.
Funding
This study was supported by the intramural research programme of the National Cancer Institute, Bethesda, MD, USA, and by the Hippokration Hospital, Aristotle University of Thessaloniki, Greece.
Transparency declarations
None to declare.
References
- 1.Agusti C, Rano A, Sibila O, et al. Nosocomial pneumonia in immunosuppressed patients. Infect Dis Clin North Am. 2003;17:785–800. doi: 10.1016/s0891-5520(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 2.Pascoe J, Cullen M. The prevention of febrile neutropenia. Curr Opin Oncol. 2006;18:325–9. doi: 10.1097/01.cco.0000228736.39885.e5. [DOI] [PubMed] [Google Scholar]
- 3.Van Bambeke F, Michot JM, Van Eldere J, et al. Quinolones in 2005: an update. Clin Microbiol Infect. 2005;11:256–80. doi: 10.1111/j.1469-0691.2005.01131.x. [DOI] [PubMed] [Google Scholar]
- 4.Stergiopoulou T, Meletiadis J, Sein T, et al. Isobolographic analysis of pharmacodynamic interactions between antifungal agents and ciprofloxacin against Candida albicans and Aspergillus fumigatus. Antimicrob Agents Chemother. 2008;52:2196–204. doi: 10.1128/AAC.00735-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugar AM, Liu XP, Chen RJ. Effectiveness of quinolone antibiotics in modulating the effects of antifungal drugs. Antimicrob Agents Chemother. 1997;41:2518–21. doi: 10.1128/aac.41.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki E, Maesaki S, Miyazaki Y, et al. Synergistic effect of ofloxacin and fluconazole against azole-resistant Candida albicans. J Infect Chemother. 2000;6:151–4. doi: 10.1007/s101560070014. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima R, Kitamura A, Someya K, et al. In vitro and in vivo antifungal activities of DU-6859a, a fluoroquinolone, in combination with amphotericin B and fluconazole against pathogenic fungi. Antimicrob Agents Chemother. 1995;39:1517–21. doi: 10.1128/aac.39.7.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagenlehner FM, Kees F, Weidner W, et al. Concentrations of moxifloxacin in plasma and urine, and penetration into prostatic fluid and ejaculate, following single oral administration of 400 mg to healthy volunteers. Int J Antimicrob Agents. 2008;31:21–6. doi: 10.1016/j.ijantimicag.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Wagenlehner FM, Kinzig-Schippers M, Sorgel F, et al. Concentrations in plasma, urinary excretion and bactericidal activity of levofloxacin (500 mg) versus ciprofloxacin (500 mg) in healthy volunteers receiving a single oral dose. Int J Antimicrob Agents. 2006;28:551–9. doi: 10.1016/j.ijantimicag.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Shen LL, Baranowski J, Fostel J, et al. DNA topoisomerases from pathogenic fungi: targets for the discovery of antifungal drugs. Antimicrob Agents Chemother. 1992;36:2778–84. doi: 10.1128/aac.36.12.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michot JM, Seral C, Van Bambeke F, et al. Influence of efflux transporters on the accumulation and efflux of four quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob Agents Chemother. 2005;49:2429–37. doi: 10.1128/AAC.49.6.2429-2437.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrison MW, Anderson DE, Campbell DM, et al. Stenotrophomonas maltophilia: emergence of multidrug-resistant strains during therapy and in an in vitro pharmacodynamic chamber model. Antimicrob Agents Chemother. 1996;40:2859–64. doi: 10.1128/aac.40.12.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sternal K, Czub J, Baginski M. Molecular aspects of the interaction between amphotericin B and a phospholipid bilayer: molecular dynamics studies. J Mol Model. 2004;10:223–32. doi: 10.1007/s00894-004-0190-0. [DOI] [PubMed] [Google Scholar]
- 14.Robertson SM, Curtis MA, Schlech BA, et al. Ocular pharmacokinetics of moxifloxacin after topical treatment of animals and humans. Surv Ophthalmol. 2005;50(Suppl 1):S32–45. doi: 10.1016/j.survophthal.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Petrou MA, Rogers TR. In-vitro activity of antifungal agents in combination with four quinolones. Drugs Exp Clin Res. 1988;14:9–18. [PubMed] [Google Scholar]
- 16.Michot JM, Van Bambeke F, Mingeot-Leclercq MP, et al. Active efflux of ciprofloxacin from J774 macrophages through an MRP-like transporter. Antimicrob Agents Chemother. 2004;48:2673–82. doi: 10.1128/AAC.48.7.2673-2682.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S, Perlin DS. Establishing surrogate markers for fluconazole resistance in Candida albicans. Microb Drug Resist. 2005;11:232–8. doi: 10.1089/mdr.2005.11.232. [DOI] [PubMed] [Google Scholar]
- 18.Shalit I, Halperin D, Haite D, et al. Anti-inflammatory effects of moxifloxacin on IL-8, IL-1β and TNF-α secretion and NFκB and MAP-kinase activation in human monocytes stimulated with Aspergillus fumigatus. J Antimicrob Chemother. 2006;57:230–5. doi: 10.1093/jac/dki441. [DOI] [PubMed] [Google Scholar]
- 19.Shalit I, Horev-Azaria L, Fabian I, et al. Immunomodulatory and protective effects of moxifloxacin against Candida albicans-induced bronchopneumonia in mice injected with cyclophosphamide. Antimicrob Agents Chemother. 2002;46:2442–9. doi: 10.1128/AAC.46.8.2442-2449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]