1. Introduction
Ubiquitin (Ub) is a highly conserved protein of 76 amino acids that is covalently linked to target proteins altering their localization, function, or stability 1-3. Proteins can be modified with a large number of different isoforms of ubiquitin and these different ubiquitins are thought to signal different outcomes. The question of how these different forms of ubiquitin are recognized is central to understanding the specificity of various types of ubiquitination 4-6.
Ubiquitin acts as a signal by being conjugated to proteins through three sequential steps. In the first step, ubiquitin is activated by the ATP-dependent formation of a thiolester bond between the C-terminus of ubiquitin and the active site cysteine of an ubiquitin activating enzyme or E1. The second step involves the transfer of the ubiquitin molecule from the E1 to the active site cysteine of an ubiquitin-conjugating enzyme or E2. Finally, the ubiquitin is transferred to a lysine residue of the target protein in a reaction catalyzed by an ubiquitin ligase or E3. This last step occurs in a substrate-specific manner and it is highly regulated 7-9.
Several rounds of ubiquitination can occur on ubiquitin itself, leading to the formation of a polyubiquitin chain. Any of seven lysines, or the amino terminus, of ubiquitin can be used to polymerize ubiquitin and so there are a huge number of differently linked polyubiquitin signals that can be formed. Chains can be linked by the same lysine on each ubiquitin (K29, K48, K63, etc.) to yield homogeneous chains, or utilize different lysines on some ubiquitins to yield heterogeneous chains. In the latter case, the lysine used can vary from ubiquitin to ubiquitin, or chains can be formed that are branched at a single ubiquitin by linking two ubiquitins to two different lysines at the branch point. It is commonly assumed that different polyubiquitin chains are associated with different cellular fates. Receptors are thought to recognize the different ubiquitin modifications (mono- and polyubiquitin) attached to the target proteins and to mediate the different signaling outcomes 4,10. These receptors have ubiquitin binding domains that interact with ubiquitin or polyubiquitin, and may also have domains that can also interact with the modified target proteins or other macromolecules.
Like most posttranslational modifications, ubiquitination is reversible 11 and its removal is carried out by enzymes collectively known as deubiquitinating enzymes (DUBs) 12. DUBs are proteases that have been implicated in a wide variety biological processes 12,13. They are responsible for the removal of ubiquitin or polyubiquitin from target proteins, the processing of ubiquitin precursors, and the disassembly of unanchored polyubiquitin (a polyubiquitin chain not attached to another protein) that is either synthesized de novo, or released by the action of other DUBs. Thus, like the cellular targeting receptors they recognize the different forms of ubiquitin and polyubiquitin. For instance, the tumor suppressor CYLD acts exclusively on K63-linked chains 14, yeast OTU1 prefers long K48-linked chains 15, and USP5cleaves both linkages 16. Nearly 100 DUBs in five different protein families are encoded by the human genome. Several DUBs have been shown to bind or process polyubiquitin or polyubiquitinated substrates in vivo, and many DUBs have been shown to cleave polyubiquitin in vitro.
This review will discuss the specificity of ubiquitin and polyubiquitin binding by DUBs. The DUBs discussed will be limited to those where binding and specificity have been directly demonstrated, either through structure determination or direct binding and catalytic studies. It will focus on the current body of knowledge regarding structure of ubiquitin binding domains of DUBs and the mechanisms by which these DUBs recognize and selectively disassemble different polyubiquitin chains. In addition to clarifying the mechanisms of chain recognition by DUBs, the conclusions gleaned from these proteins may well serve as a model for the recognition of these chains by other receptors.
2. The polyubiquitin modification
Ubiquitin adopts a β-grasp fold that consists of a central five stranded β-sheet wrapping one α-helix and a short 310-helix 17. The C terminus of ubiquitin, involved in the formation of the isopeptide bond with target proteins or between ubiquitins, protrudes from the body of ubiquitin. The form of ubiquitin that becomes attached to the target protein (linkage and length) appears to ultimately determine the signaling outcome 10,18-20. Monoubiquitination, the conjugation of one ubiquitin to a target protein, acts as a signal that has been implicated in histone regulation, DNA repair, endocytic trafficking and virus budding 18,21,22. Polyubiquitination, the conjugation of polyubiquitin to a target protein, results from the subsequent conjugation of ubiquitin monomers to any of the seven lysine residues of ubiquitin (K6, K11, K27, K29, K33, K48 or K63) or to M1 of ubiquitin 4,5,10, 3,23,24, 25. Conjugation of differently linked isoforms of polyubiquitin to proteins are thought to target the modified proteins to different fates 4,5,10. The various signaling outcomes probably result from the recognition by receptors of the different three-dimensional structures adopted by the different polyubiquitin isoforms. Ultimately these structures depend on which lysine residue is utilized in the polyubiquitin chain formation 26-31.
The best understood isoforms of polyubiquitin are the K48- and K63-linked chains. K48-linked polyubiquitin is usually, although not always 32, involved in proteasomal degradation, while K63-linked chains act as non-proteolytic signals in intracellular processes including endocytosis, activation of kinases in the NF-κB (Nuclear Factor Kappa-light-chain-enhancer of activated B cells) pathway, DNA repair, autophagy, and ribosome function 20,33-37. Structural studies aimed at understanding the differences between various chains have largely focused on K48 and K63-linked polyubiquitin since they can be synthesized in vitro in large quantities. The structure of these two types of chains reveals that they adopt different conformations in solution.
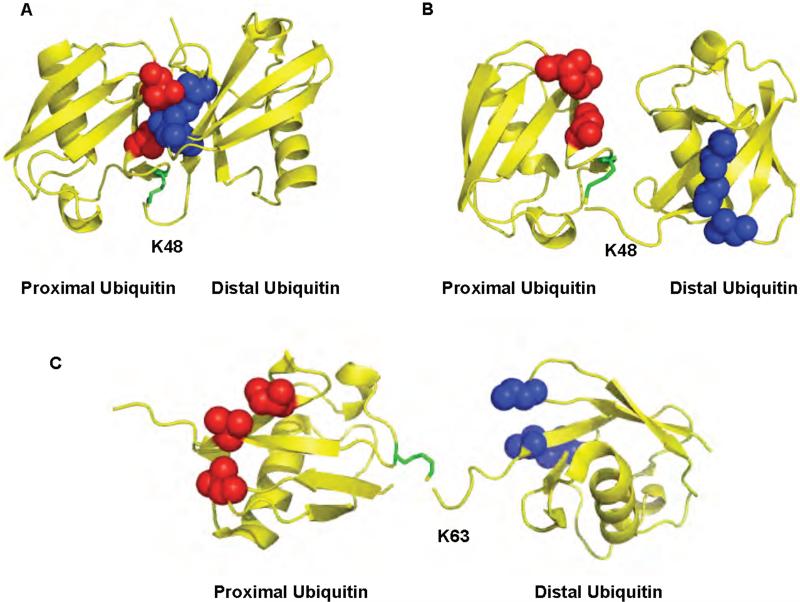
NMR and X-ray crystallographic studies show that K48-linked chains can adopt at least two different conformations, demonstrating that these chains are flexible 26,27,29,31,38. In the “closed” conformation the L8-I44-V70 hydrophobic patch, implicated in the binding to most ubiquitin binding domains (UBDs) 39,40 including those present on the proteasome 41, forms intra-chain contacts between adjacent ubiquitin units causing the patch to be sequestered at the ubiquitin/ubiquitin interface in the chains (Figure 1A). In the “open” conformation, the hydrophobic patch of ubiquitin is solvent exposed. This would allow the hydrophobic patch to directly participate in the interaction with UBDs (Figure 1B). Although, K48-linked polyubiquitin can adopt two distinct conformations in solution, little is known about the predominant form adopted when the chains are bound to polyubiquitin binding proteins. NMR studies show that a UBD, the ubiquitin associated domain (UBA) of Rad23, binds preferentially K48-linked polyubiquitin by interacting with surfaces on both ubiquitins that surround the isopeptide bond, thereby stabilizing the open conformation 30,42. The closed conformation has been proposed to occur only at the distal end of the chain since K48 of the distal ubiquitin in the closed conformation is not accessible for isopeptide linkage to another ubiquitin. Thus, the steric constraints of the observed structure prevent the closed conformation from being accommodated in the interior ubiquitins of a chain 29,38.
Figure 1.
The structures of K48 and K63-linked diubiquitin. A. Closed conformation of K48-linked diubiquitin. B. Open conformation of K48-linked diubiquitin. C. Extended conformation of K63-linked diubiquitin. The ubiquitin moieties in the chain are colored in yellow. The hydrophobic patch in the distal and proximal ubiquitins is shown in blue and red respectively. K48 or K63 of ubiquitin that participated in the isopeptide bond is colored in green. The protein data bank codes are 1AAR, 1TBE, and 2JF5 for A, B, and C, respectively
K63-linked chains predominantly form an extended conformation that, like the open conformation observed in K48-linked polyubiquitin, has the I44 hydrophobic patch exposed to the solvent (Figure 1C) 28. In this conformation there is no direct contact between the hydrophobic surfaces on adjacent ubiquitin monomers in diubiquitin. However, it is possible that longer K63-linked chains could adopt a more compact conformation through hydrophobic contacts between non-adjacent ubiquitins in the chain 10.
In contrast to our detailed understanding of the role and three-dimensional structures of K48 and K63-linked polyubiquitin, much less is known about other polyubiquitin isoforms. For instance, both K29 and K11-linked polyubiquitin have been implicated in proteasome-dependent protein degradation 43,44, but no structures are available to compare to those of the K48- or K63-linked chains.
The above conclusions are derived from studies on homogenous polyubiquitin (ubiquitin chains linked through only a single lysine residue). However, heterogeneous polyubiquitin chains linked through multiple lysines can also be synthesized in vitro 45,46, and have been detected in vivo using mass spectrometric analyses 24. Heterogeneous chains contain mixed linkages using different lysines to link monomers in a chain. Heterogeneous chains can also be branched, using two different lysines of a single ubiquitin at the branch point to attach two distal ubiquitins. It is not known how frequently these types of chains are formed in vivo, nor what physiological roles they might serve 46. In the ubiquitin fusion degradation (UFD) pathway, it is thought that heterogeneous chains linked through K29 and K48 are utilized to target proteins for proteasomal degradation 43. These early studies did not demonstrate conclusively that the chains attached to the target protein were heterogeneous, but instead relied on mutagenesis of the lysine residues of ubiquitin to show both K29 and K48 were required. Indeed, a more recent study, which employed mass spectrometry analysis, showed that some protein substrates modified with branched heterogeneous chains are resistant to proteasomal degradation, and that branched polyubiquitin is poorly disassembled by the proteasome-associated DUBs in vitro 46.
How are the different forms of polyubiquitin recognized by receptors and DUBs? Studies aimed at understanding recognition have focused predominantly on monoubiquitin derivatives and K48- or K63-linked polyubiquitin. Although some DUBs exhibit little polyubiquitin isoform selectivity 47, some have been shown to be specific for one chain isoform over another 14,48-51. The chain specificity of DUBs can be intrinsic to their catalytic core domains 14 or can be mediated by additional domains, including UBDs. At least 16 UBDs have been described, including: UBA, UIM, MIU, DUIM, CUE, GAT, NZF, A20 ZnF, UBP ZnF, UBZ, Ubc, UEV, UBM, GLUE, Jab1/MPN and PFU. Multiple surfaces on ubiquitin interact with these UBDs, but most often a hydrophobic patch consisting of L8, I44, and V70. The affinities of thes isolated UBDs with monoubiquitin-are commonly weak, with Kd>100 microM. Affinity can be increased avidity, commonly by polymerizing ubiquitin or by utilizing multiple UBDs in a single enzyme or receptor. The observed ubiquitin binding affinity for varies widely for DUBs. Yeast OTU1 shows no binding up to 2 mM ubiquitin, UCH-L3 binds ubiquitin with an affinity of 100-500 μM and USP5 binds with a Kd of 50 nM. Polyubiquitin affinity is usually higher and USP5 binds K48-linked tetraubiquitin with a dissociation constant of less than 1 nM.
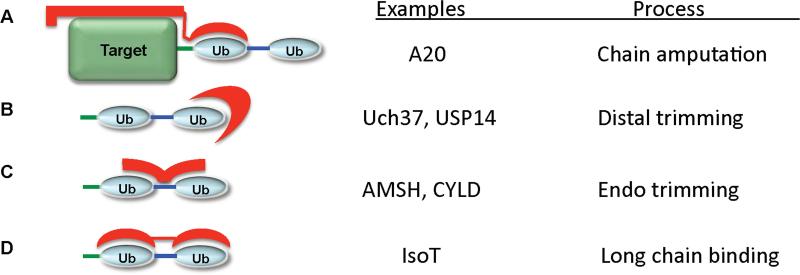
This discussion suggests at least four mechanisms for recognition of polyubiquitin. First, DUBs could interact with both the target protein and the proximal end of polyubiquitin (Figure 2A). A second mechanism would involve the binding of the distal ubiquitin in the chain by the DUB (Figure 2B). This specificity could be enforced by binding to a surface of ubiquitin that is only exposed in the closed conformation or to a surface that would be blocked by a more distal ubiquitin (Figure 2B). A third mechanism would involve the recognition of surfaces on both ubiquitins that surround the isopeptide bond (Figure 2C). These surfaces will vary depending on the type of linkage utilized. Finally, a fourth mechanism by which a DUB can recognize polyubiquitin involves binding to multiple ubiquitin binding domains (Figure 2D). In this mechanism, the flexibility of the polyubiquitin receptor itself would be necessary if the DUB were to bind different isoforms of polyubiquitin. Thus, the constraints on the relative orientation of the ubiquitin binding domains could be a major determinant of specificity.
Figure 2.
Possible mechanisms of polyubiquitin recognition by DUBs. A. DUBs can interact with both the target protein and polyubiquitin. B. DUBs can recognize of the distal ubiquitin the chain. C. DUBs could bind simultaneously two ubiquitins by interacting with surfaces on both ubiquitins that surround the isopeptide bond. D. Finally, a DUB can recognize polyubiquitin through the use of multiple ubiquitin binding domains. Polyubiquitin is shown in blue, the target protein in green, and the polyubiquitin receptor in red.
The consequences of these different modes of interaction could determine the enzymatic specificity. For instance, Model 2A would be ideal for the specific amputation of an intact polyubiquitin chain. Model 2B could result in distal chain trimming, while model 2C could lead to endocleavage of polyubiquitin chains or specific cleavage at the branch points of heterogeneously linked chains. Finally, model 2D would be ideal for recognizing longer polyubiquitin chains. In the following sections we will discuss examples of how ubiquitin and polyubiquitin recognition is achieved by the different DUB families.
3. DUB Families
DUBs constitute one of the larger classes of enzymes in the ubiquitin system. A recent bioinformatics study suggested that humans have approximately 100 different DUBs 13. The catalytic core domains of DUBs are responsible for the recognition and proper active site positioning of ubiquitin containing the scissile bond. Therefore these active sites contain ubiquitin-interacting surfaces. In addition to the catalytic domains, DUBs have N- and C-terminal extensions that modulate their substrate specificity, or cellular localization 12. Some extensions include UBDs, ubiquitin-like domains, and other protein-protein interaction domains 13.
Eukaryotic genomes encode five families of DUBs; four are cysteine proteases and one is a zinc dependent metalloprotease 12,13. The cysteine protease families include the ubiquitin specific processing proteases (USP, or UBP in yeast), the ubiquitin C-terminal hydrolases (UCH), ovarian tumor related proteases (OTU), and the Josephin/Machado-Joseph disease proteases (MJD). The zinc metalloprotease DUBs contain a JAB1/MPN/Mov34 metalloenzyme (JAMM) domain.
Despite the low sequence similarity between the cysteine protease DUB families, and their different overall structure, the catalytic cores that contain the active site residues closely resemble the classical cysteine protease papain 12. This core contains the papain-like C-H-strand and loop 12,15,16,50,52-58. The active site C and H are located on the α-helix and the β-strand respectively. Often, the D/N of the catalytic triad is located in the loop; however, its precise location is variable. The structure of at least one member of each type of DUB family has been solved, in some cases in the presence of monoubiquitin-based inhibitor and in one case in the presence of a polyubiquitin chain isoform. The catalytic core domains of most DUBs recognize monoubiquitin, and in at least one case, polyubiquitin 15,48,53,56,59. The structure of the catalytic core domains (both unbound and bound to ubiquitin based inhibitors) reveals that most must undergo active site rearrangements to productively bind substrate and catalyze its hydrolysis. In the following section we will review the structures of each type of DUB domain and how these structures provide insights into ubiquitin recognition and catalysis.
3.1. The UCH family
The ubiquitin C-terminal hydrolase (UCH) family of DUBs was the first to be identified 60,61. In humans, there are four UCH domain-containing DUBs while in yeast only one, Yuh1 62-64. Early studies suggested that UCH family DUBs preferentially cleave small or flexible leaving groups and, given this substrate preference, this family of DUBs was proposed to act predominantly in the recycling of ubiquitin that has become inappropriately conjugated to intracellular nucleophiles 65. However, the in vivo substrates for UCHs have not been clearly established 65,66, and some UCH family DUBs can disassemble polyubiquitin- and ubiquitin-protein conjugates. For example, UCH37 disassembles polyubiquitin chains 67, UCH-L3 has been implicated in reversing the ubiquitination of the epithelial sodium channel 68, and the Drosophila UCH can act on ubiquitinated protein 69,70. Another proposed role for this class of DUBs is the co-translational processing of ubiquitin precursors. Ubiquitin is always synthesized as a fusion protein that must be processed and the pro-proteins consist of either a single copy of ubiquitin with a C-terminal ribosomal protein or a polyubiquitin precursors that has an additional amino acid following the last ubiquitin monomer 71-73.
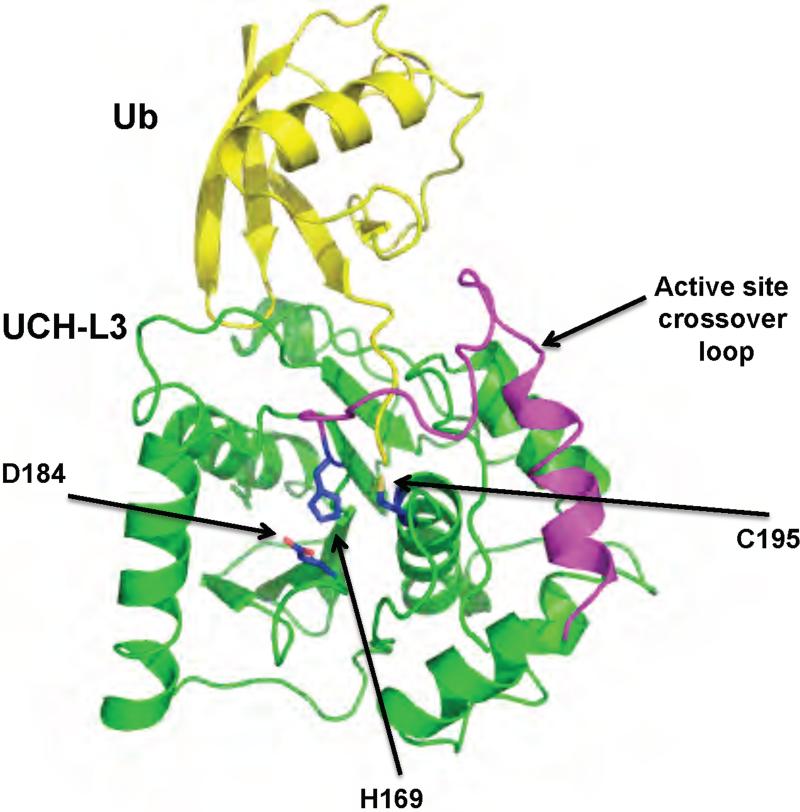
The structures of two human UCH domains DUBs (UCH-L1 and UCH-L3) and that of yeast Yuh1 have been solved, the latter two in the presence of an ubiquitin-based inhibitor (Figure 3) 55,56,63,74. The UCH domain consists of a six- or seven-stranded antiparallel β-sheet surrounded by eight α-helices 63. The catalytic triad is located at the bottom of a pocket in the surface of the protein. This pocket is wide enough to accommodate the diglycine motif of the ubiquitin C terminus but too narrow to accommodate residues with larger side chains. UCH-L3 and Yuh1 were co-crystallized in the presence of the inhibitors ubiquitin vinyl methyl ester (Ub-VME), and ubiquitin aldehyde (Ubal) respectively 55,63. Ub-VME is an irreversible inhibitor that forms a thiolester bond with the active site cysteine 75. Ubal is a reversible inhibitor that forms a thiolhemiacetal with the active site cysteine mimicking the tetrahedral reaction intermediate 75. In both co-crystals contacts between the UCH domains and ubiquitin are made with the C terminus and the first N-terminal loop of ubiquitin.
Figure 3.
Structure of a UCH family DUB bound to Ub-VME, UCH-L3. Ub-VME is colored in yellow, and UCH-L3 in green. The active site loop is colored in magenta. The active site residues are shown in blue. The protein data bank identification code is 1XD3.
The structures of UCH-L3 and the Ub-VME adduct reveal that the active site undergoes significant conformational changes upon binding to ubiquitin. In the unliganded structure, a loop that must cross over the active site could not be resolved, suggesting that it is disordered 56. In the UCH-L3•Ub-VME complex, the disordered loop becomes stabilized into a α-helix followed by an S-shaped loop that crosses over the active site (Figure 3) 55. This conformation is also seen in Yuh1 despite the low sequence identity (approximately 30%), suggesting that other UCHs may undergo a similar conformational change.
The structure of the unliganded UCH-L1, a DUB implicated in Parkinson's disease and neuronal function, indicates that the active sites cysteine and histidine are not in a productive conformation (unlike in the unliganded UCH-L3 structure) 74. The residues are 8.2Å apart suggesting that a conformational change must occur upon substrate binding to place these two residues in close proximity for catalysis. An additional constraint on catalysis is that in the unliganded UCH-L1 the active site loop covers the active site. For an ubiquitinated substrate to enter the active site the leaving group must be less than 10Å in diameter (the distance between the loop and the active site cleft) or the loop must be displaced as is observed in UCH-L3.
Thus, purified UCH isozymes bind to mono-ubiquitin and rapidly cleave small 70 or disordered domains by model 2A where large folded domains are not well tolerated. There is no evidence that purified UCH isozymes can cleave polyubiquitin at a significant rate, probably because the jutadistal ubiquitin is tightly folded and prevents access of the scissile bond to the active site cleft. UCH37 can cleave polyubiquitin when bound to the proteasome, but that reaction may require the additional action of proteasome components to partially unfold the polyubiquitin chain. Other purified UCHs can cleave ubiquitin fusion proteins very slowly and may require the partial unfolding of the leaving group by conformational flexibility or the action of other accessory proteins. The identification and structural studies of a complex between UCH-L1 and an in vivo substrate should clarify the specificity and the mechanism of activation of this class of enzymes.
3.2. The USP/UBP family
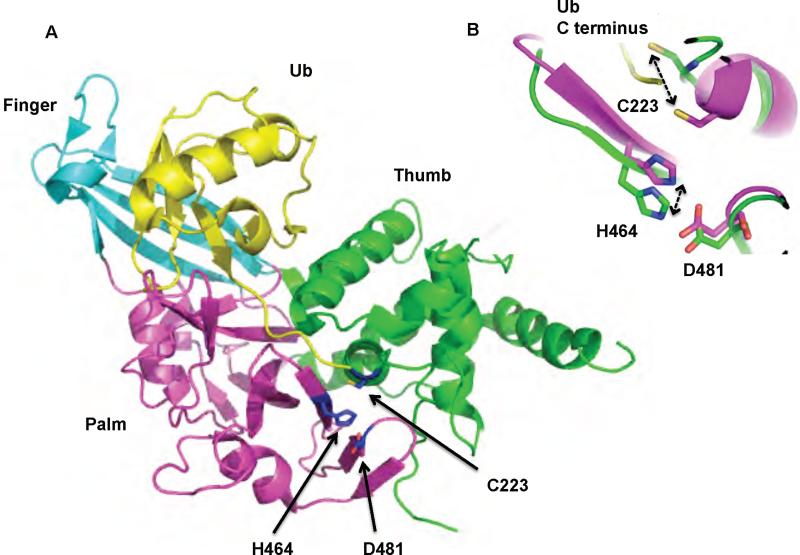
The USP family is the largest and most diverse family of DUBs. There are 16 UBPs in yeast, and more than 50 USPs in humans 12,13,76. The USP catalytic core domain is approximately 350 amino acids long. In contrast to the UCH family, USPs generally cleave larger leaving groups from the C terminus of ubiquitin 13. The USP family contains two well-conserved sequences, the Cys box and His box, which contain the active site residues that form the catalytic triad. Recently the structures of six different USP domains have been described 14,48,53,77,78. Despite the low sequence similarity, the USP domain fold is highly conserved. Three well-defined domains (termed Thumb, Finger and Palm) form a structure that resembles a right hand (Figure 4A).
Figure 4.
Structure of a UBP/USP family DUB, USP7. A. Structure of Usp7 bound to Ubal. Ubal is colored in yellow. The three domains that make up the USP domain are colored in cyan (Finger), green (Thumb), and magenta (Palm). The active site residues are shown in blue. B. Conformational rearrangement of the active site residues upon binding of Ubal to Usp7. Ubal unbound and bound Usp7 are colored in green and magenta respectively. The ubiquitin C terminus is colored in yellow. The protein data bank identification codes for the unbound and bound structure of Usp7 are 1NB8 and 1NBF respectively.
The Thumb is predominantly alpha helical and contains the Cys Box, a motif that includes the active site cysteine. The Palm is composed of beta strands supported by alpha helices and contains the remaining active site residues that form the catalytic triad; a His and an Asp or Asn residue. The junction between the Thumb and the Palm domains forms a cleft that accommodates the C-terminal tail of ubiquitin, and the active site residues involved in catalysis. The K48 side chain of ubiquitin aldehyde bound to the catalytic site is oriented towards the Thumb domain and is only partially solvent exposed. In contrast the side chain of K63 is entirely solved exposed, which would allow the enzymes to tolerate an ubiquitin distal to the ubiquitin bound at the USP domain. If this conformation is catalytically relevant it would suggest that K48 linked chains might be disassembled from the distal end while K63-linked chains may also be cleaved internally. The Finger domain is composed of four β-strands and in USP8 and USP2 it contains a CXXCXnCXXC motif that chelates one zinc 77,78. Although four of the solved structures lack this motif, the overall fold of the Finger domain is maintained. The CXXCXnCXXC motif is lacking in only nine of the 54 putative human USPs, suggesting that the zinc binding ability is dispensable for the integrity of the Finger domain fold 77. The role of the Finger domain is to serve as a scaffold that contacts the globular body of ubiquitin, and the zinc-chelating site does not appear to be involved in catalysis 77. Interestingly, in the USP domain of CYLD the Finger domain is significantly smaller due to the shortening of the β-strands 14. CYLD also differs from the other USP domains by the insertion of a Zn binding domains that closely resembles a B-box. This insertion occurs between β9 with β10 in the Palm domain. The B-box is not required for deubiquitinating activity, but instead appears to be important for the cytoplasmic localization of CYLD 14.
Multiple studies have demonstrated that the catalytic activity of USP DUBs is regulated by substrate- or scaffold-induced conformational changes 48,53,78. The structures of two USP family DUBs (both in the presence and absence of Ubal) reveal at least two different modes of catalytic activation 48,53. The first structure corresponds to USP7 (also known as HAUSP, Herpes associated USP), a DUB that preferentially deubiquitinates MDM2 (Murine Double Minute 2), the ubiquitin ligase for the tumor suppressor p53, as well as p53 itself 53. In the unliganded form of USP7, the catalytic triad is misaligned. The active site cysteine is approximately 10Å away from the active site histidine, too far for catalysis to occur (Figure 4B). Upon binding to the inhibitor, Ubal, a major conformational change occurs in the catalytic core domain causing the active site cysteine and histidine to be positioned within hydrogen bond distance from one another, rendering the enzyme catalytically competent.
The structure of USP14 in the presence and absence of Ubal reveals a different type of activation mechanism 48. USP14 is a proteasome-associated DUB that helps remove polyubiquitin from protein substrates that are being degraded by the proteasome 48 (see section 4.1). Binding of USP14 or its yeast ortholog, UBP6, to the proteasome activates the catalytic activity of the DUB through an unknown mechanism 79. However, comparison of the structures of the free and Ubal-bound USP domain of USP14 suggests a mechanism for the activation 48. Unlike USP7, the catalytic triad of the free USP14 is productively aligned, indicating that the active site is catalytically competent. However, the binding groove that accommodates the C-terminal tail of ubiquitin is blocked by two surface loops that undergo significant conformational changes upon ubiquitin binding. It is proposed that the movement of this loop out of the catalytic cleft could also occur upon binding to the proteasome, resulting in the observed activation of the enzyme 79.
A third type of conformational change is thought to occur in USP8 (also known as UBPY), a DUB that is involved in removing ubiquitin from endocytosed substrates such as the epidermal growth factor 78 (see section 4.4.1). In the structure of the unliganded USP domain of USP8, the tip of the Finger domain is positioned inward towards the Palm resulting in a closed conformation that leaves insufficient room for an ubiquitin molecule. Unlike the USP7, USP14, or UBP6 USP domains, USP8 and USP2 have a unique α-helix juxtaposed to the fingers and this helix may be involved in stabilizing the closed conformation observed in USP8 in the absence of ubiquitin 77,78. Although there is no structure available of a USP8-ubiquitin complex, the closely related USP domain of USP2 has been solved in the presence of ubiquitin 77. In the USP2-ubiquitin complex the Finger domain is displaced outward to adopt the conformation observed in the other USP domains. It is possible that upon target protein binding, the Finger domain of USP8 moves to the position observed in the other USPs, allowing the activation of the enzyme and subsequent binding to ubiquitin.
3.3. The OTU Family
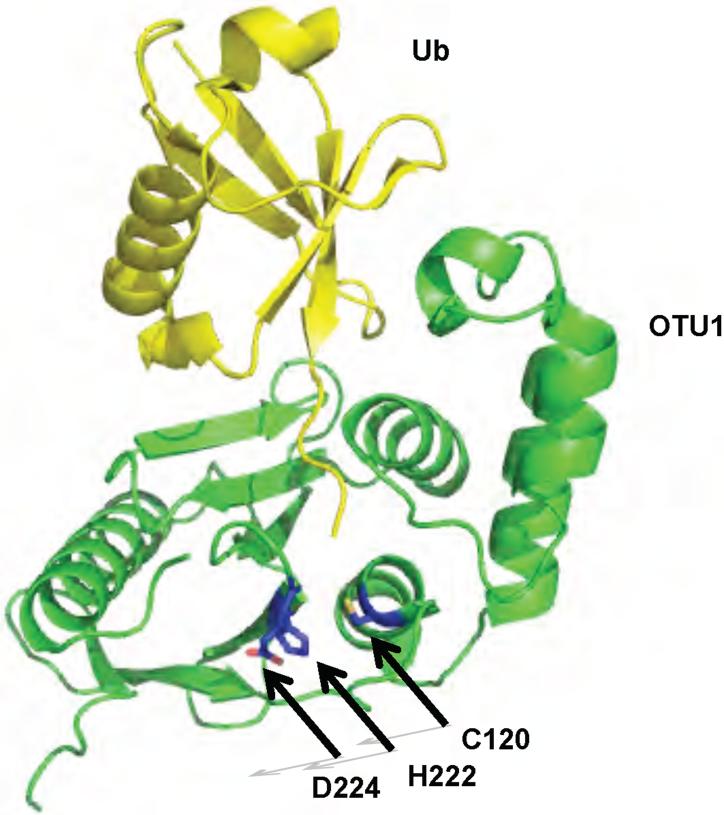
The ovarian tumor gene is a gene that is involved in the development of Drosophila melanogaster ovaries 80,81. A bioinformatics study using the OTU gene and its homologues found a family of genes encoding viral, eukaryotic and pathogenic bacterial cysteine proteases 82. Recent studies have demonstrated that OTU-related proteases have deubiquitinating activity and the crystal structures of four different DUBs containing OTU domains have been recently described 50,52,83-85: Otubain-2, a protein of unknown biological 52 function; Otubain-1, a DUB that regulates the E3 ligase GRAIL (Gene Related to Anergy In Lymphocytes) and which functions in the induction of CD4 T cell anergy; A20, a negative regulator of the NF-κB pathway; and OTU1, a yeast DUB that was shown to interact with the AAA-ATPase (ATPases Associated with diverse cellular Activities) Cdc48 50,52,86-88. In all four OTU domain DUBs the core domain is formed by a five-stranded β-sheet sandwiched between two helical domains (Figure 5). The size of the helical domains varies among OTU DUBs 15,50,89. Only OTU1 has been crystallized in the presence of a monoubiquitin based active site inhibitor, Ub-Br3 15 . The active site cysteine and histidine are properly positioned in all four DUBs. However, the identity of the third residue of the catalytic triad is unclear and has been suggested to be Asp224 in OTU1, Asn226 in human Otubain 2 52, or D70 in A20 50. D70 is located on α-helix 3 and is positioned 4.4 Å away from the putative active site histidine residue, suggesting that if D70 forms part of the catalytic triad, a conformational change must during catalysis occur to position D70 within H-bonding distance of the histidine residue. This change in conformation would be reminiscent of the reorganization of the active site residues of USP7 upon binding of Ubal. In the Otubain-2 crystal structure, the active site residues appear to be positioned in a catalytically productive conformation, however a disordered loop, and six residues preceding the disordered loop are oriented into a position that would clash with the bound ubiquitin 52. In the structure of OTU1 reacted with the active site probe Ub-Br3, this loop becomes ordered into a β-strand that contacts the globular body of ubiqutin 15. This suggests that Otubain-2 may exist in a self-inhibited state, and that upon substrate binding the loop may adopt the conformation observed in the OTU1•Ub-Br3 adduct.
Figure 5.
Structure of yeast OTU1 bound to Ub-Br3. OTU1 is shown in green. Ub-Br3 is shown in yellow. The active site residues are shown in blue. The protein data bank identification code is 3BY4.
3.4. The Josephin/MJD family
Like the OUT family, the Josephin domain family of DUBs was discovered through a bioinformatics approach 90 that identified the Josephin domain in Ataxin-3 and at least 30 other proteins as putative DUBs. Biochemical studies confirmed that Ataxin-3 had DUB activity and mutation of the active site cysteine rendered the protein inactive against a model substrate 91. Furthermore, DUB activity has been detected for other Josephin domain containing proteins 92.
Ataxin-3 is mutated in spinocerebellar ataxia type 3, also known as Machado-Joseph disease 91. This disease is an autosomal dominant neurodegenerative disorder caused by the expansion of a polyglutamine stretch in the Ataxin-3 gene, leading to protein misfolding, aggregation and cellular toxicity. Ataxin-3 binds both K48- and K63-linked chains, however, it selectively hydrolyzes long K63-linked chains (albeit, extremely slowly) 93. The N-terminal UIM domains mediate Ub binding and mutation of these domains abolishes chain specificity. A model is suggested whereby the UIM domains bind to polyubiquitin but only K63 linkages can be positioned for cleavage. However, it must be noted that this cleavage is so slow that its raises the possibility that a cofactor or proper cellular location may enhance the enzymatic activity of Ataxin-3.
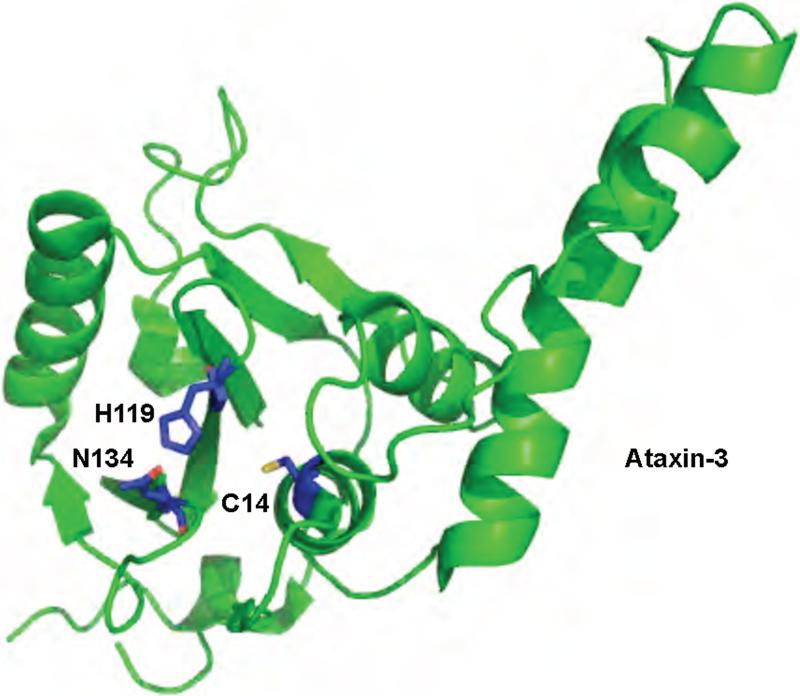
The structure of the Josephin domain of Ataxin-3 has been solved by NMR methods only in its unliganded form (Figure 6) 54,58,94. The overall fold of the Ataxin-3 Josephin domain resembles that of the UCH family of DUBs. Based on the structural similarity to the UCH domain, and on NMR chemical shift mapping, a binding surface for ubiquitin has been proposed 54,58. The Josephin domain is predicted to interact with the C terminus of ubiquitin, the first N-terminal loop of ubiquitin, and the C-terminal half of ubiquitin's α-helix. Residues located in α3, the loop between α3 and α4, and the C-terminal portion of α2 of the Josephin domain are the most perturbed upon ubiquitin binding. This binding mode would predict discrimination against K6 and K29 linked polyubiquitin although this has not been tested. A structure of the Josephin domain with polyubiquitin, ubiquitin, or a ubiquitin-based inhibitor may confirm these interactions.
Figure 6.
Structure of a Josephin domain family DUB, Ataxin-3. Ataxin-3 is shown in green. The active site residues are shown in blue. The protein data bank identification code is 1YZB.
3.5. The JAMM family
The JAMM domain is found in both prokaryotes and eukaryotes, although bacteria are assumed to lack ubiquitin and an ubiquitin-like conjugation system. Three eukaryotic JAMM domains have DUB activity 49,95-97, suggesting that JAMM domains may have evolved to have deubiquitinating activity only in higher organisms. These three JAMM domain DUBs are: Rpn11 in yeast (POH1 in humans) 95-97, a subunit of the proteasome that cleaves ubiquitin chains from substrate proteins that are being degraded by the proteasome; CSN5, a subunit of the COP9 signalosome 98 which cleaves Nedd8 (Neural Precursor Cell Expressed, Developmentally Down-regulated 8, a ubiquitin-like protein) conjugates; and AMSH (associated molecule with the SH3 domain of STAM) a deubiquitinating enzyme involved in endocytosis 49.
The crystal structure of the JAMM domain of a protein from Archaeoglobolus fulgidus, AF2198, was the first structure of a JAMM domain to be determined 99,100 and the structure of three other JAMM-motif proteins were recently solved: Prp8 (pre-mRNA processing factor 8) 101,102; Mov34 (a metalloprotein subunit of the proteasome) 103; and AMSH-LP (AMSH-like protein 59). Prp8 and Mov34 proteins do not bind zinc and are not expected to have DUB activity. However, the JAMM motif of Prp8 has ubiquitin binding activity suggesting that a subset of these domains may act as ubiquitin binding domains 104,105. AMSH-LP (Associated Molecule with the SH3 of STAM Like Protein) is a DUB involved in endocytosis that binds both ubiquitin subunits of di-ubiquitin using the JAMM domain and two AMSH-family specific sequences (see below).
The overall structure of the JAMM domain core resembles that of cytidine deaminase 100. The catalytic zinc residue is chelated by a histidine and an aspartic acid residue located in β3 and α2 of the domain respectively. Due to the structural similarity with cytidine deaminase, the JAMM domain has been proposed to use a similar mechanism in the hydrolysis of the isopeptide bond in ubiquitin or ubiquitin-like protein conjugates. In cytidine deaminase, the zinc ion activates a water molecule to form a hydroxide ion that makes a nucleophilic attack on the C4 carbon in the pyrimidine ring 106. In the JAMM domain DUBs, the nucleophilic attack would occur in the carbonyl carbon of the isopeptide bond. In both cases the result is the formation of a tetrahedral intermediate that subsequently collapses releasing ammonia in the deamination reaction or the target protein in the isopeptide hydrolysis reaction. Both proteins also contain a glutamic acid residue that can function as a proton donor or acceptor during the deamination or isopeptide hydrolysis reaction. Consistent with this hypothesis, mutation of the homologous glutamic acid residue in CSN5 causes a defect in activity 97.
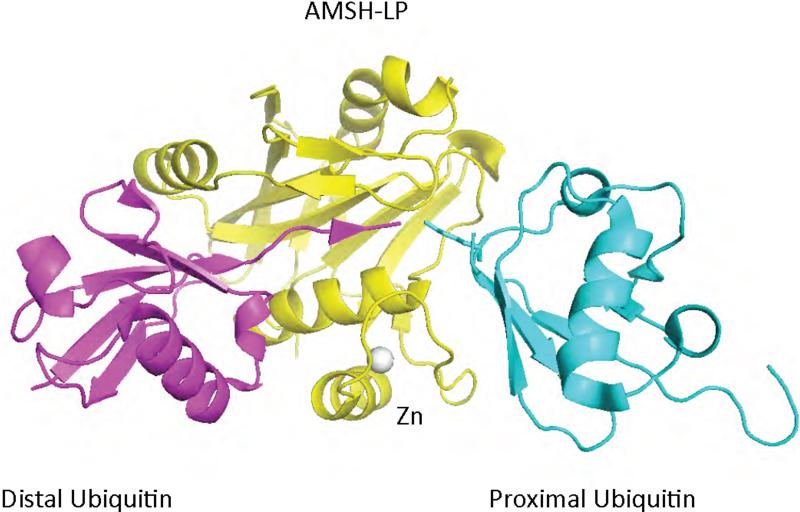
The core JAMM domain of AMSH-LP resembles the structure of the JAMM domain from AF2198, and coordinates an active site zinc ion through two His residues, one Asp, and a water molecule, which is hydrogen bonded to a Glu residue 59 (Figure 7. However, the JAMM domain of AMSH-LP differs from AF2198 by the presence of two amino acid insertions, Ins-1 and Ins-2, and an additional zinc-binding motif. The second zinc ion is chelated by two His and one Cys residues from Ins-2 and a His residue from the core domain. The structure of the JAMM domain of an AMSH-LP has also been solved in complex with K63-linked polyubiquitin 59 (see below) providing for the first time a molecular description of the catalytic core domain of a DUB bound to polyubiquitin (Figure 7).
Figure 7.
Structure of a JAMM domain (yellow) from the human AMSH-like protein complexed to K63-linked di-ubiquitin 59 (cyan and magenta). The structure consists of the JAMM domain core surrounding the zinc atom (grey) and two AMSH-specific inserts that interact with the proximal (cyan) and distal (magenta) domains of diubiquitin. The protein data bank identification code is 2ZNV.
4. Mechanisms of polyubiquitin recognition and disassembly by DUBs
Recent studies aimed at understanding how DUBs cleave and recognize polyubiquitin chains have shown that these enzymes can process polyubiquitin through various mechanisms. DUBs have been shown to cleave polyubiquitin chains from the proximal end, the distal end and the interior of the polyubiquitin chain through an endocleavage activity. In the following section we will discuss mechanisms utilized by DUBs that disassemble polyubiquitin.
4.1. Disassembly of polyubiquitin by proteasome associated DUBs
The proteasome is a multi-protein complex formed by two subcomplexes: the 20S core particle and the 19S regulatory particle 107-109. The 20S core particle contains the proteolytic sites and consists of four stacked rings, each formed by seven α subunits 110. The outer rings are composed of seven different α-subunits, which are implicated in binding the 19S regulatory particle. The two inner rings each consist of seven different β-subunits, three of which (from each set) contain the protease active sites 111. Deubiquitination of substrates is necessary for optimal rates of protein degradation and is carried out by the 19S regulatory particle 79,95-97,112-115. The 19S regulatory particle includes 19 different subunits, 10 of which form a base that binds the α-subunits of the 20S proteasome. Six proteins of the 10-protein-base are AAA-ATPases that are thought to help unfold and translocated the substrate into the lumen of the 20S protease. The remaining protein subunits of the 19S regulatory particle form the lid. Some of the subunits of the lid and base are implicated in binding to the polyubiquitin signal 116-120. The removal of the polyubiquitin signal from target proteins during protein degradation is carried out by DUBs that are a core component of, or associated with, the 19S regulatory particle 79,121,122. Failure to remove the polyubiquitin chain from the substrate protein is thought to impede passage of substrates through the entrance pore of the 20S proteasome. Perhaps for this reason deubiquitination has also been shown to be required for efficient proteolysis. Most eukaryotes contain three proteasome associated deubiquitinating enzymes: POH1 95-97,123, Usp14 48,124-126, and Uch37 112-115. The budding yeast S. cerevisiae, only has two of these DUBs, Rpn11 95,96 and Ubp6 79,127, the homologues of POH1 and Usp14 respectively. POH1/Rpn11 belongs to the JAMM domain family of DUBs, is a core component of lid of the 19S regulatory particle and is associated with the proteasome in stoichiometric amounts., POH1/Rpn11 is essential for viability while the other two proteasomal associated DUBs are dispensable. Mutations in the metal chelating residues of the JAMM domain of POH1 in Drosophila 128 or human cells 129, and Rpn11 in yeast are lethal 95,96. One group has reported that a mutation at the putative active site in yeast was not lethal but lead to defects in substrate degradation 130. Furthermore, knockdown of POH1 in human cells results in accumulation of polyubiquitin conjugates and defects in protein degradation, in part due to a defect in proteasome assembly 123. The deubiquitinating activity of POH1/Rpn11 in the proteasome is dependent on ATP hydrolysis, suggesting that deubiquitination may be coupled to protein unfolding during degradation 95,96. POH1/Rpn11 deubiquitinating activity can only be detected when bound to the 26S proteasome 96, the 19S regulatory particle 95, or the lid subcomplex 130. Together these data suggest that other proteasomal subunits associated with the lid and POH1/Rpn11 may modulate the DUB activity. POH1/Rpn11 appears to remove polyubiquitin from substrate proteins en bloc, cleaving the isopeptide bond linking the polyubiquitin chain to the substrate protein 95. These, and other, data are consistent with a model in which POH1/Rpn11 activity is coupled to protein unfolding as the protein is being degraded by the 20S proteasome. A defect in POH1/Rpn11 deubiquitinating activity would impede translocation of the protein due to steric hindrance by the polyubiquitin chain.
Like POH1, UCH37 is a stoichiometric component on the proteasome that associates with the proteasome through the proteasomal subunit Adrm1, the human homologue of Rpn13 113-115,123. Adrm1 interacts with the C-terminus of UCH37 via a C-terminal domain, which is absent in S. cerevisiae Rpn13. This interaction not only recruits UCH37 to the proteasome, but it also activates its catalytic activity. However, while the interaction of UCH37 with Adrm1 activates the hydrolysis of the in vitro substrate Ub-AMC, it does not activate the hydrolysis of diubiquitin 113. When bound to the 19S regulatory particle, UCH37 exhibits approximately 100-fold increased activity for a diubiquitin-based substrate, suggesting proper localization is required for activation. UCH37 has been shown to cleave polyubiquitin from the distal end 67 and a recent study has provided insights into how this cleavage may occur when bound to Adrm1 120. Adrm1 also acts as a polyubiquitin receptor that interacts with polyubiquitin through a novel ubiquitin binding domain, the Pru domain 119,120. The Pru domain is located in the N-terminus of Adrm1 and mediates both the binding to the Rpn2/S1 subunit of the proteasome and to polyubiquitin. Notably, the Pru domain of Adrm1 interacts with the proximal ubiquitin in diubiquitin 120 and this interaction may allow the positioning of the distal ubiquitin in close proximity to UCH37 to facilitate polyubiquitin hydrolysis from the distal end of the chain. Since UCH37 cleaves polyubiquitin chains from the distal end, it has been proposed to have an editing function to prevent the degradation of poorly modified or inappropriate substrates 67.
Unlike POH1/Rpn11 and UCH37, USP14/Ubp6 does not stably associate with the 26S proteasome; instead its association is reversible and salt-sensitive 79,123. Usp14/Ubp6 contains an N-terminal ubiquitin like domain (UBL) that mediates its interaction with the Rpn1 and Rpn2 subunits of the base of the proteasome 79. Like UCH37, USP14/Ubp6 activity is significantly increased upon incorporation into the proteasome 48,79. Ubp6 is not an essential gene in yeast, but its absence causes an ubiquitin-depletion phenotype 79,131. Ubp6 appears to delay the breakdown of proteins by the proteasome since deletion of Ubp6 causes an enhancement in the rate of hydrolysis by the proteasome 127. Surprisingly, this inhibitory effect does not depend on its catalytic activity, suggesting a non-catalytic function of Ubp6 in the regulation of proteasomal protein degradation 127. Ubp6 was also shown to work in cooperation with a proteasome associated E3, Hul5, to modulate the length of the polyubiquitin chains attached to target proteins 121. Increased Hul5 activity leads to retention of the polyubiquitinated protein, while deubiquitination by Ubp6 has the opposite effect. In agreement with this function, Usp14, like UCH37, appears to cleave monoubiquitin from the substrate or polyubiquitin chains from the distal end of the chain 48. The cooperation of these two activities may be regulated upon changes in the cellular environment, such as lack of nutrients, or throughout the cell cycle.
The unifying theme for all three proteasomal associated DUBs is the requirement for an adapter to interact with their polyubiquitinated substrates. All three interact with polyubiquitinated substrates by virtue of their localization to the proteasome, with the polyubiquitin being presented to the DUB by multiple receptors for polyubiquitin 116-120. In addition all three are active only when interacting with the proteasome scaffold, thereby restricting their DUB activity to proteasomal protein degradation.
4.2. Isopeptidase T (Usp5) recognizes polyubiquitin through its multiple ubiquitin binding domains
Unanchored polyubiquitin can be formed by the release of polyubiquitin from target proteins by DUBs such as POH1/Rpn11 or by de novo synthesis by the conjugation machinery (possibly for subsequent transfer to a target protein) 132,133. The levels of unanchored polyubiquitin are tightly regulated in the cell by deubiquitinating enzymes, probably to prevent inhibition of the proteasome and receptors that recognize polyubiquitinated proteins 132,134,135. Isopeptidase T (IsoT or USP5/UBP14) is a deubiquitinating enzyme that specifically disassembles unanchored polyubiquitin 51. IsoT orthologs have been shown to be responsible for the majority of unanchored polyubiquitin disassembly in four different organisms 132,134-136. Lack of Ubp14, the yeast homologue of IsoT, results in the marked accumulation of polyubiquitin, the sensitivity to the arginine analog canavanine, and defects in the degradation of model proteasome substrates 132. In Dictyostelium deletion causes developmental defects 135 and in Arabidopsis, deletion is lethal 134. Transient knockdown of IsoT in humans cells causes an increase in unanchored chains and defects in the proteolysis of p53. Furthermore, human IsoT can complement the ΔUbp14 phenotype demonstrating that yeast Ubp14 is the functional homologue of human IsoT 132.
IsoT is one of the best biochemically-characterized USP DUBs 16,51,57,137-141. Recombinant IsoT disassembles unanchored polyubiquitin from the proximal end to the distal end of the chain in a non-processive manner 51. Modification of the C-terminus of the proximal ubiquitin in the chain leads to a defect in the rate of disassembly of the polyubiquitin chain indicating the presence of a pocket that recognizes the intact C-terminus of the proximal ubiquitin. Kinetic and thermodynamic studies indicate that IsoT has at least three more ubiquitin binding sites for ubiquitin subunits in linear and K48-linked polyubiquitin 16,51. Correspondingly, IsoT has four putative ubiquitin binding domains, a ZnF UBP domain, a USP/UBP domain, and two UBA domains 16,57. It was recently demonstrated that all four ubiquitin binding domains of IsoT mediate polyubiquitin binding and that each forms a unique site that interacts with one ubiquitin subunit at a time 16. The ZnF UBP domain is responsible for the recognition of the proximal, or first ubiquitin in the chain and structural studies indicate that it possesses a pocket that specifically recognizes the intact C-terminus of the proximal ubiquitin 57. This recognition drives a conformational change that activates the enzymatic cleavage of the (iso)peptide bond to the proximal subunit. The USP/UBP, UBA1 and UBA2 domains recognize the second, third and fourth ubiquitin subunits respectively in both linear and K48-linked polyubiquitin 16. Linear polyubiquitin is predicted to adopt a conformation analogous to K63-linked polyubiquitin and that differs from K48-linked polyubiquitin 10. Interestingly the ZnF UBP and the UBP domain are implicated in the discrimination between linear and K48-linked polyubiquitin based upon the difference in binding affinities for linear and K48-linked diubiquitin 16. However the UBA domains do not significantly discriminate between K48 and linear linkages.
This is an example of polyubiquitin recognition by the mechanism shown in Figure 2D. All four ubiquitin binding domains participate similarly in the interaction between IsoT and two different polyubiquitin isoforms. Each forms analogous sites for binding the ubiquitin subunits of both linear and K48-linked polyubiquitin. Thus, the domains of IsoT and/or polyubiquitin must be flexible enough for similar interactions to take place with the ubiquitin subunits of chains exhibiting very different solution structures. Regions of about 25 amino acids link the different ubiquitin binding domains to each other, raising the possibility that these regions are flexible enough to allow the recognition of multiple polyubiquitin isoforms by the four ubiquitin binding domains of IsoT.
4.3. Processing of polyubiquitinated substrates by DUBs that modulate the NFκB Pathway
NF-κB transcription factors are known to control diverse cellular processes such as inflammation, immunity, and cell survival 142,143. In NF-κB signaling, K63- or K48-linked polyubiquitin can be attached to effectors of the pathway leading to different outcomes. K63-linked polyubiquitination mediates protein-protein interactions that lead to activation of the signaling pathway. In contrast, K48-linked polyubiquitination leads to the degradation of effectors of the pathway. Normally the NF-κB transcription factor is held in the cytoplasm by the inhibitor of NF-κB (IκB). Signaling through the tumor necrosis factor α (TNFα), interleukin 1-β (IL-1β) or Toll-like receptor (TLR) pathways induces the degradation of IκB allowing the NF-κB transcription factor to be translocated to the nucleus and activate the transcription of specific genes 142,144.
Upon receptor binding, several proteins in the pathway are modified with K63-linked polyubiquitin and this modification is a prerequisite for the activation of the subsequent kinase cascade 142. Ligand binding causes the K63-linked polyubiquitination of TRAFs and adapters such as RIP1 (Receptor Interacting Protein 1) leading to the recruitment of a cascade of kinases that ultimately phosphorylate IκB. Phosphorylated IκB is recognized by an ubiquitin ligase complex that conjugates K48-linked polyubiquitin to IκB, resulting in its proteasome-dependent degradation. The degradation of IκB exposes the nuclear localization signal of NF-κB, allowing for its nuclear translocation and the subsequent expression of specific genes. Two different DUBs, CYLD and A20, act as negative regulators of the NF-κB pathway by removing K63-linked polyubiquitin from modified proteins involved in the activation of the pathway 86,142,143,145-148.
4.3.1. The USP domain of CYLD has intrinsic selectivity for K63-linked polyubiquitin
In humans, loss of deubiquitinating enzyme CYLD leads to a disfiguring benign cancer called cylindromatosis 149. CYLD has been shown to deubiquitinate TRAFs (TNF Receptor-Associated Factor) 2 and 6, and NEMO (NF-κB Essential Modulator) thereby suppressing NF-κB signaling. Decreased CYLD expression, or mutations that affect its catalytic activity, lead to sustained NF-κB signaling due to failure in the deubiquitination of TRAF2, TRAF6 and NEMO 146-148. Failure to terminate NF-κB signaling results in prolonged inflammation and immune system dysfunction. In addition to the modulation of the NF-κB signaling, CYLD has also been shown to regulate apoptosis in Drosophila by positively regulating the c-Jun N-terminal kinase pathway 150.
CYLD is a deubiquitinating enzyme belonging to the USP family that preferentially disassembles K63-linked polyubiquitin and this chain specificity is inherent in its catalytic core domain 14. The USP domain of CYLD adopts a fold that contains the Palm and Finger domains observed in other USPs 14. However, the Finger domain is significantly reduced in size due to shortening of the β-sheet that is the central component of the domain. The USP domain of CYLD contains an insertion of a zinc-binding module that resembles a B-box, but that does not appear to influence catalytic activity. Other remarkable differences correspond to the sizes of loops connecting secondary structure elements. One such loop, connecting β12 and β13, is significantly longer in comparison to the equivalent loop in USP7. Truncation of this loop to a size equivalent to that of USP7 leads to reduced activity on K63-linked polyubiquitin without significantly altering the hydrolysis of K48-linked polyubiquitin 14. This experiment suggests that this loop, which is conserved in all CYLD orthologs, is a determinant of CYLD specificity for K63-linked polyubiquitin. Additional biochemical studies by Komander et al. show that CYLD acts as an endodeubiquitinase, thereby hydrolyzing internal isopeptide linkages in K63-linked polyubiquitin chains. In contrast, other USP domain DUBs (such as USP14 and UCH37) appear to cleave polyubiquitin processively from the distal end of the chain 48. This difference in the mechanism of hydrolysis of the chain may be linked to the Finger domain of CYLD. All USP structures contact the side chains of K48 through the Thumb and K63 through the Finger domains. Given that the USP domain of CYLD has a shorter Finger domain with less obstruction of K63 it may be able to utilize a second binding site to interact with a more distal ubiquitin linked through K63, thereby accounting for the endodeubiquitinase activity of CYLD 14. Thus, the basis for the specificity of CYLD mot closely resembles that of Figure 2C.
4.3.2 Recognition of the target protein determines the chain selectivity of A20
Like CYLD, A20 negatively regulates NF-κB signaling 86,145. Mice lacking A20 exhibit severe inflammation, cachexia, and premature death 151. Fibroblasts from these mice are hypersensitive to TNFα and are defective in the downregulation of NF-κB signaling 151. A20 is a remarkable protein that has two enzymatic activities: ubiquitin ligase and deubiquitinating activities 86. A20 can downregulate NF-κB signaling by removing K63-linked polyubiquitin from TRAF6 and RIP1. Through its E3 ligase activity, A20 also mediates K48-linked polyubiquitination of RIP1, causing its degradation and attenuation of NF-κB signaling 86. The E3 ligase activity of A20 resides in its C-terminal domain, which is composed of seven zinc fingers, while its DUB activity resides in its N-terminal OTU domain. Recently, structural and biochemical studies have revealed new insights into the disassembly of polyubiquitin and polyubiquitinated TRAF6 by the OTU domain of A20 50,89. In vitro, A20 has very low activity towards monoubiquitin based substrates and inhibitors, but is more efficient in the disassembly of polyubiquitin. It prefers K48-linked over K63-linked polyubiquitin in vitro, although the opposite was reported in vivo 50,89. Li el al. showed that this contradiction may be explained by the manner in which A20 cleaves polyubiquitinated substrates 89. Li et al demonstrated that A20 can efficiently deubiquitinate K63-linked polyubiquitinated TRAF6 in vitro by releasing free chains via cleavage of the isopeptide bond to TRAF6 in a manner similar as POH1. These data suggest that A20 may recognize specific polyubiquitinated substrates, perhaps through its recruitment to the NF-κB activation complex. This would be an example of polyubiquitin recognition by the model in Figure 2A. Furthermore, Li et al. suggest that A20 deubiquitination of RIP1 may occur prior to A20-dependent K48-linked polyubiquitination. However it remains to be determined whether A20 also cleaves K63-linked polyubiquitin from RIP1 and what prevents it from disassembling K48-linked polyubiquitinated RIP1.
4.4. Polyubiquitin processing by DUBs involved in endocytosis
Ubiquitin also acts as a signal that targets membrane proteins to the lysosome for degradation 18,21. Monoubiquitination and K63-linked polyubiquitination have both been implicated in this process 18,152. Fusion of a single ubiquitin in frame with a cargo protein is sufficient to mediate targeting to the lysosome 153. K63-linked polyubiquitin can enhance the efficiency of this process 152, perhaps by increasing the affinity for ubiquitin binding domains through avidity. More than 50% of the epidermal growth factor receptor (EGFR) 154, a well-studied endocytic cargo, was shown to be modified with K63-linked polyubiquitin, underscoring the role of this modification in endocytic sorting. When a membrane-associated receptor is ubiquitinated, adaptors that recognize the ubiquitinated cargo are recruited. This in turn results in the recruitment of clathrin and epsin proteins, causing internalization of the receptor through plasma membrane involution 155,156. The cargo is released into early endosomes from where it can be either be recycled back to the plasma membrane or enter into the lumen of multivesicular bodies (MVB) that form through involution of the endosomal membrane. These MVBs then fuse with the lysosome (vacuole in yeast) thereby targeting proteins for degradation in the lysosome. Deubiquitinating enzymes have been shown to regulate this process by deubiquitinating the internalized receptor prior to delivery to the lysosome 47,49,157-167. In addition, deubiquitination is also required for recycling of ubiquitin prior to lysosomal degradation 158.
4.4.1. USP8/DOA4 deubiquitinates endocytic intermediates
In yeast, deletion of the deubiquitinating enzyme Doa4 leads to a ubiquitin depletion phenotype that can be partially rescued by blocking MVB maturation, suggesting that Doa4 recycles ubiquitin prior to lysosomal degradation 158. Structurally and functionally, the DUB that most closely resembles Doa4 is USP8 (also called UBPY) 168. Knockdown of USP8 results in the accumulation of ubiquitin at endosomes and decreases the rate of degradation of receptor tyrosine kinases (RTKs) such as the EGFR and the Met receptor, suggesting that it acts primarily in the downregulation of cargo proteins 47. However another group found that knockdown of USP8 results in enhanced downregulation of EGFR 165. In vitro, USP8 can disassemble K48- and K63-linked polyubiquitin suggesting that it could act in both proteasomal and lysosomal dependent protein degradation. Consistent with a role in proteasomal degradation, knockdown of USP8 leads to a depletion of a member of the MVB sorting machinery, STAM (signal transduction adapter protein). Proteasomal inhibition in USP8 knockdown cells results in the accumulation of ubiquitinated STAM, suggesting that USP8 also regulates the protein level of at least one member of the MVB sorting machinery.
The structure of USP8 reveals the presence of a zinc ribbon at the tip of the Finger domain of the USP active site 78. As noted above, this zinc ribbon is conserved in USP2 and other USP domain DUBs 77. In light of the fact that the Finger domain is involved in interactions with K63 of ubiquitin, the zinc-binding module could serve one of two roles. It could act as an additional ubiquitin binding domain that interacts with a second ubiquitin subunit in K63-linked polyubiquitin chains, thereby increasing the affinity for K63-polyubiquitinated cargo proteins 78. Alternatively, protein-protein interactions could position the finger domain in such a way as to occlude access to K63 and lead to a shift in specificity toward K48-linked chains found on STAM. Thus, the binding partners of USP8 could toggle the chain specificity depending on the precise substrate and binding partners involved.
4.4.2. The JAMM domain of AMSH has intrinsic specificity for K63-linked polyubiquitin
In humans, the membrane bound DUB AMSH has also been shown to regulate endocytosis of receptors 49,166,167,169. Knockdown of AMSH results in an increase in the rate of downregulation of EGFR, suggesting that it may act early in the endocytic sorting pathway by recycling receptors before they are committed to degradation by the lysosome 49. Like CYLD, AMSH and a parolog, AMSH-LP, have been shown to specifically disassemble K63 linked polyubiquitin 49,169. The crystal structure of the catalytic core domain of AMSH-LP bound to K63-linked diubiquitin has provided a molecular description of this specificity 59.
The structure of the JAMM domain of AMSH-LP has been solved, both bound (Figure 7) and unbound to K63-linked polyubiquitin 59. AMSH-LP is 55% identical to AMSH and both cleave K63-linked polyubiquitin specifically 170. The structure of AMSH-LP bound to K63-linked diubiquitin demonstrates that the recognition of the proximal ubiquitin in diubiquitin determines AMSH-LP linkage selectivity 59. The proximal ubiquitin is contacted by residues in Ins-2 and the JAMM core domain. Together these two elements form a surface that interacts with the tri-peptide sequence Gln62-Lys63-Glu64 of the proximal ubiquitin, a surface that is unique to K63-linked diubiquitin. Both Ins-1 and the JAMM core domain contact the distal ubiquitin, with hydrophobic and aromatic residue in Ins-1 contacting the hydrophobic patch of the distal ubiquitin . These hydrophobic residues are strictly conserved in POH1/Rnp11, suggesting that this DUB may also recognize ubiquitin through its hydrophobic patch. In addition to interactions with the body of ubiquitin, the core JAMM domain and Ins-1 interact with the C-terminal tail of the distal ubiquitin, and the residues that mediate this interaction are also conserved in POH1/Rnp11. In contrast, the residues implicated in the recognition of the proximal ubiquitin and the zinc coordinating motif in Ins-2 are not conserved in POH1/Rnp11 59. This zinc binding motif was shown to required for hydrolysis of K63-linked diubiquitin by stabilizing the recognition of the proximal ubiquitin 59.
Thus, the structure of the AMSH-LP domain bound to K63-linked polyubiquitin reveals one mode by which DUBs discriminate between polyubiquitin isoforms. This involves recognition of sequences at the linkage site specific for a particular polyubiquitin isoform and represents an example of the model in Figure 2C.
5. Conclusions
Most studies of DUB specificity have focused on the processing of K48-linked, K63-linked and linear polyubiquitin. Very little is known regarding the recognition and processing of other types of polyubiquitin. Specificity must be achieved by taking advantage of the structural differences between different polyubiquitin chains and our understanding will remain incomplete until we learn more about the conformations of free and bound polyubiquitin chains. To date only the structure of a single polyubiquitin specific DUB, AMSH-LP, has been solved bound to polyubiquitin. It remains to be seen how other DUBs, in particular those belonging to the cysteine protease families, can recognize polyubiquitin at the molecular level.
Although new insights into the processing of polyubiquitin or polyubiquitinated substrates by DUBs have emerged in the last decade, studies regarding polyubiquitin recognition by DUBs or other polyubiquitin receptors are still in the early stages. We have discussed several examples and they suggest at least four modes of recognition that can determine cleavage specificity. These include: recognition of the target protein and the proximal ubiquitin leading to chain amputation, recognition of the distal ubiquitin resulting in chain trimming, recognition of specific surfaces on both ubiquitins flanking the isopeptide bond as a means to distinguish different linkages, and the use of multiple ubiquitin binding domains to recognize longer polyubiquitin chains. Each will lend specificity to the process and a given DUB may employ more than one of these recognition modes to achieve unique specificity. Further, we may anticipate a large number of individual mechanisms may be required to distinguish between the very large number of possible polyubiquitin structures.
Many questions still remain regarding how DUBs recognize and discriminate between different types of polyubiquitin at the molecular level. How specific are DUBs? Can all polyubiquitin signals be disassembled? How do localization and protein partners of DUBs regulate specificity? How does transcriptional and proteolytic control of DUBs contribute to specificity? It is likely that a combination of structural, genetic, and physiological studies will be needed to elucidate these questions. Given that the cellular functions of most DUBs still remain unknown, determining which type of ubiquitin modification they process, and how this recognition is achieved may provide new understanding regarding their physiological functions.
References
- 1.Wilkinson KD. Cell. 2004;119:741–5. doi: 10.1016/j.cell.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson KD. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15280–2. doi: 10.1073/pnas.0504842102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hershko A, Ciechanover A. Annu. Rev. Biochem. 1998;67:425–79. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Ye Y. Cell Mol. Life. Sci. 2008;65:2397–406. doi: 10.1007/s00018-008-8090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda F, Dikic I. EMBO Rep. 2008;9:536–42. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welchman RL, Gordon C, Mayer RJ. Nat. Rev. Mol. Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 7.Petroski MD, Deshaies RJ. Nat. Rev. Mol. Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 8.Ardley HC, Robinson PA. Essays Biochem. 2005;41:15–30. doi: 10.1042/EB0410015. [DOI] [PubMed] [Google Scholar]
- 9.Fang S, Weissman AM. Cell Mol. Life. Sci. 2004;61:1546–61. doi: 10.1007/s00018-004-4129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickart CM, Fushman D. Curr. Opin. Chem. Biol. 2004;8:610–6. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson KD. Semin. Cell Dev. Biol. 2000;11:141–8. doi: 10.1006/scdb.2000.0164. [DOI] [PubMed] [Google Scholar]
- 12.Amerik AY, Hochstrasser M. Biochim Biophys Acta. 2004;1695:189–207. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. Cell. 2005;123:773–86. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Komander D, Lord CJ, Scheel H, Swift S, Hofmann K, Ashworth A, Barford D. Mol. Cell. 2008;29:451–64. doi: 10.1016/j.molcel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Messick TE, Russell NS, Iwata AJ, Sarachan KL, Shiekhattar R, Shanks JR, Reyes-Turcu FE, Wilkinson KD, Marmorstein R. J. Biol. Chem. 2008;283:11038–49. doi: 10.1074/jbc.M704398200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyes-Turcu FE, Shanks JR, Komander D, Wilkinson KD. J. Biol. Chem. 2008;283:19581–92. doi: 10.1074/jbc.M800947200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vijay-Kumar S, Bugg CE, Cook WJ. J. Mol. Biol. 1987;194:531–44. doi: 10.1016/0022-2836(87)90679-6. [DOI] [PubMed] [Google Scholar]
- 18.Hicke L. Nat. Rev. Mol. Cell Biol. 2001;2:195–201. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- 19.Pickart CM, Eddins MJ. Biochim Biophys Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Sun L, Chen ZJ. Curr. Opin. Cell Biol. 2004;16:119–26. doi: 10.1016/j.ceb.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Haglund K, Di Fiore PP, Dikic I. Trends Biochem. Sci. 2003;28:598–603. doi: 10.1016/j.tibs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Hicke L, Dunn R. Annu. Rev. Cell Dev. Biol. 2003;19:141–72. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 23.Pickart CM. Trends Biochem. Sci. 2000;25:544–8. doi: 10.1016/s0968-0004(00)01681-9. [DOI] [PubMed] [Google Scholar]
- 24.Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. Nat. Biotechnol. 2003;21:921–6. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 25.Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, Sano S, Tokunaga F, Tanaka K, Iwai K. EMBO J. 2006;25:4877–87. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook WJ, Jeffrey LC, Kasperek E, Pickart CM. J. Mol. Biol. 1994;236:601–9. doi: 10.1006/jmbi.1994.1169. [DOI] [PubMed] [Google Scholar]
- 27.Cook WJ, Jeffrey LC, Carson M, Chen Z, Pickart CM. J. Biol. Chem. 1992;267:16467–71. doi: 10.2210/pdb1aar/pdb. [DOI] [PubMed] [Google Scholar]
- 28.Varadan R, Assfalg M, Haririnia A, Raasi S, Pickart C, Fushman D. J. Biol. Chem. 2004;279:7055–63. doi: 10.1074/jbc.M309184200. [DOI] [PubMed] [Google Scholar]
- 29.Eddins MJ, Varadan R, Fushman D, Pickart CM, Wolberger C. J. Mol. Biol. 2007;367:204–11. doi: 10.1016/j.jmb.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 30.Varadan R, Assfalg M, Raasi S, Pickart C, Fushman D. Mol. Cell. 2005;18:687–98. doi: 10.1016/j.molcel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Varadan R, Walker O, Pickart C, Fushman D. J. Mol. Biol. 2002;324:637–47. doi: 10.1016/s0022-2836(02)01198-1. [DOI] [PubMed] [Google Scholar]
- 32.Flick K, Raasi S, Zhang H, Yen JL, Kaiser P. Nat. Cell Biol. 2006;8:509–15. doi: 10.1038/ncb1402. [DOI] [PubMed] [Google Scholar]
- 33.Spence J, Sadis S, Haas AL, Finley D. Mol. Cell Biol. 1995;15:1265–73. doi: 10.1128/mcb.15.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spence J, Gali RR, Dittmar G, Sherman F, Karin M, Finley D. Cell. 2000;102:67–76. doi: 10.1016/s0092-8674(00)00011-8. [DOI] [PubMed] [Google Scholar]
- 35.Chiu RK, Brun J, Ramaekers C, Theys J, Weng L, Lambin P, Gray DA, Wouters BG. PLoS Genet. 2006;2:e116. doi: 10.1371/journal.pgen.0020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A. Science. 1989;243:1576–83. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- 37.Flick K, Ouni I, Wohlschlegel JA, Capati C, McDonald WH, Yates JR, Kaiser P. Nat. Cell Biol. 2004;6:634–41. doi: 10.1038/ncb1143. [DOI] [PubMed] [Google Scholar]
- 38.Phillips CL, Thrower J, Pickart CM, Hill CP. Acta Crystallogr. D Biol. Crystallogr. 2001;57:341–4. doi: 10.1107/s090744490001800x. [DOI] [PubMed] [Google Scholar]
- 39.Hurley JH, Lee S, Prag G. Biochem. J. 2006;399:361–72. doi: 10.1042/BJ20061138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hicke L, Schubert HL, Hill CP. Nat. Rev. Mol. Cell Biol. 2005;6:610–21. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
- 41.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trempe JF, Brown NR, Lowe ED, Gordon C, Campbell ID, Noble ME, Endicott JA. EMBO J. 2005;24:3178–89. doi: 10.1038/sj.emboj.7600797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson ES, Ma PC, Ota IM, Varshavsky A. J. Biol. Chem. 1995;270:17442–56. doi: 10.1074/jbc.270.29.17442. [DOI] [PubMed] [Google Scholar]
- 44.Jin L, Williamson A, Banerjee S, Philipp I, Rape M. Cell. 2008;133:653–65. doi: 10.1016/j.cell.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirkpatrick DS, Hathaway NA, Hanna J, Elsasser S, Rush J, Finley D, King RW, Gygi SP. Nat. Cell Biol. 2006;8:700–10. doi: 10.1038/ncb1436. [DOI] [PubMed] [Google Scholar]
- 46.Kim HT, Kim KP, Lledias F, Kisselev AF, Scaglione KM, Skowyra D, Gygi SP, Goldberg AL. J. Biol. Chem. 2007;282:17375–86. doi: 10.1074/jbc.M609659200. [DOI] [PubMed] [Google Scholar]
- 47.Row PE, Prior IA, McCullough J, Clague MJ, Urbe S. J. Biol. Chem. 2006;281:12618–24. doi: 10.1074/jbc.M512615200. [DOI] [PubMed] [Google Scholar]
- 48.Hu M, Li P, Song L, Jeffrey PD, Chenova TA, Wilkinson KD, Cohen RE, Shi Y. EMBO J. 2005;24:3747–56. doi: 10.1038/sj.emboj.7600832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCullough J, Clague MJ, Urbe S. J. Cell Biol. 2004;166:487–92. doi: 10.1083/jcb.200401141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komander D, Barford D. Biochem. J. 2008;409:77–85. doi: 10.1042/BJ20071399. [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson KD, Tashayev VL, O'Connor LB, Larsen CN, Kasperek E, Pickart CM. Biochemistry. 1995;34:14535–46. doi: 10.1021/bi00044a032. [DOI] [PubMed] [Google Scholar]
- 52.Nanao MH, Tcherniuk SO, Chroboczek J, Dideberg O, Dessen A, Balakirev MY. EMBO Rep. 2004;5:783–8. doi: 10.1038/sj.embor.7400201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu M, Li P, Li M, Li W, Yao T, Wu JW, Gu W, Cohen RE, Shi Y. Cell. 2002;111:1041–54. doi: 10.1016/s0092-8674(02)01199-6. [DOI] [PubMed] [Google Scholar]
- 54.Nicastro G, Menon RP, Masino L, Knowles PP, McDonald NQ, Pastore A. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10493–8. doi: 10.1073/pnas.0501732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Misaghi S, Galardy PJ, Meester WJ, Ovaa H, Ploegh HL, Gaudet R. J. Biol. Chem. 2005;280:1512–20. doi: 10.1074/jbc.M410770200. [DOI] [PubMed] [Google Scholar]
- 56.Johnston SC, Larsen CN, Cook WJ, Wilkinson KD, Hill CP. EMBO J. 1997;16:3787–96. doi: 10.1093/emboj/16.13.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reyes-Turcu FE, Horton JR, Mullally JE, Heroux A, Cheng X, Wilkinson KD. Cell. 2006;124:1197–208. doi: 10.1016/j.cell.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 58.Mao Y, Senic-Matuglia F, Di Fiore PP, Polo S, Hodsdon ME, De Camilli P. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12700–5. doi: 10.1073/pnas.0506344102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato Y, Yoshikawa A, Yamagata A, Mimura H, Yamashita M, Ookata K, Nureki O, Iwai K, Komada M, Fukai S. Nature. 2008;455:358–62. doi: 10.1038/nature07254. [DOI] [PubMed] [Google Scholar]
- 60.Pickart CM, Rose IA. Prog. Clin. Biol. Res. 1985;180:215. [PubMed] [Google Scholar]
- 61.Rose IA, Warms JV. Biochemistry. 1983;22:4234–7. doi: 10.1021/bi00287a012. [DOI] [PubMed] [Google Scholar]
- 62.Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, Ishov AM, Tommerup N, Vissing H, Sekido Y, Minna J, Borodovsky A, Schultz DC, Wilkinson KD, Maul GG, Barlev N, Berger SL, Prendergast GC, Rauscher FJ., 3rd Oncogene. 1998;16:1097–112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 63.Johnston SC, Riddle SM, Cohen RE, Hill CP. EMBO J. 1999;18:3877–87. doi: 10.1093/emboj/18.14.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. Science. 1989;246:670–3. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- 65.Larsen CN, Krantz BA, Wilkinson KD. Biochemistry. 1998;37:3358–68. doi: 10.1021/bi972274d. [DOI] [PubMed] [Google Scholar]
- 66.Pickart CM, Rose IA. J. Biol. Chem. 1985;260:7903–10. [PubMed] [Google Scholar]
- 67.Lam YA, Xu W, DeMartino GN, Cohen RE. Nature. 1997;385:737–40. doi: 10.1038/385737a0. [DOI] [PubMed] [Google Scholar]
- 68.Butterworth MB, Edinger RS, Ovaa H, Burg D, Johnson JP, Frizzell RA. J. Biol. Chem. 2007;282:37885–93. doi: 10.1074/jbc.M707989200. [DOI] [PubMed] [Google Scholar]
- 69.Roff M, Thompson J, Rodriguez MS, Jacque JM, Baleux F, Arenzana-Seisdedos F, Hay RT. J. Biol. Chem. 1996;271:7844–50. doi: 10.1074/jbc.271.13.7844. [DOI] [PubMed] [Google Scholar]
- 70.Jin FL, Xu XX, Yu XQ, Ren SX. Protein Expr. Purif. 2008 doi: 10.1016/j.pep.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Finley D, Bartel B, Varshavsky A. Nature. 1989;338:394–401. doi: 10.1038/338394a0. [DOI] [PubMed] [Google Scholar]
- 72.Ozkaynak E, Finley D, Solomon MJ, Varshavsky A. EMBO J. 1987;6:1429–39. doi: 10.1002/j.1460-2075.1987.tb02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiborg O, Pedersen MS, Wind A, Berglund LE, Marcker KA, Vuust J. EMBO J. 1985;4:755–9. doi: 10.1002/j.1460-2075.1985.tb03693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Das C, Hoang QQ, Kreinbring CA, Luchansky SJ, Meray RK, Ray SS, Lansbury PT, Ringe D, Petsko GA. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4675–80. doi: 10.1073/pnas.0510403103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Love KR, Catic A, Schlieker C, Ploegh HL. Nat. Chem Biol. 2007;3:697–705. doi: 10.1038/nchembio.2007.43. [DOI] [PubMed] [Google Scholar]
- 76.Amerik AY, Li SJ, Hochstrasser M. Biol. Chem. 2000;381:981–92. doi: 10.1515/BC.2000.121. [DOI] [PubMed] [Google Scholar]
- 77.Renatus M, Parrado SG, D'Arcy A, Eidhoff U, Gerhartz B, Hassiepen U, Pierrat B, Riedl R, Vinzenz D, Worpenberg S, Kroemer M. Structure. 2006;14:1293–302. doi: 10.1016/j.str.2006.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Avvakumov GV, Walker JR, Xue S, Finerty PJ, Jr., Mackenzie F, Newman EM, Dhe-Paganon S. J. Biol. Chem. 2006;281:38061–70. doi: 10.1074/jbc.M606704200. [DOI] [PubMed] [Google Scholar]
- 79.Leggett DS, Hanna J, Borodovsky A, Crosas B, Schmidt M, Baker RT, Walz T, Ploegh H, Finley D. Mol. Cell. 2002;10:495–507. doi: 10.1016/s1097-2765(02)00638-x. [DOI] [PubMed] [Google Scholar]
- 80.Goodrich JS, Clouse KN, Schupbach T. Development. 2004;131:1949–58. doi: 10.1242/dev.01078. [DOI] [PubMed] [Google Scholar]
- 81.Steinhauer WR, Walsh RC, Kalfayan LJ. Mol. Cell Biol. 1989;9:5726–32. doi: 10.1128/mcb.9.12.5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Makarova KS, Aravind L, Koonin EV. Trends Biochem. Sci. 2000;25:50–2. doi: 10.1016/s0968-0004(99)01530-3. [DOI] [PubMed] [Google Scholar]
- 83.Borodovsky A, Ovaa H, Kolli N, Gan-Erdene T, Wilkinson KD, Ploegh HL, Kessler BM. Chem. Biol. 2002;9:1149–59. doi: 10.1016/s1074-5521(02)00248-x. [DOI] [PubMed] [Google Scholar]
- 84.Balakirev MY, Tcherniuk SO, Jaquinod M, Chroboczek J. EMBO Rep. 2003;4:517–22. doi: 10.1038/sj.embor.embor824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shih SC, Prag G, Francis SA, Sutanto MA, Hurley JH, Hicke L. EMBO J. 2003;22:1273–81. doi: 10.1093/emboj/cdg140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM. Nature. 2004;430:694–9. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 87.Rumpf S, Jentsch S. Mol. Cell. 2006;21:261–9. doi: 10.1016/j.molcel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 88.Soares L, Seroogy C, Skrenta H, Anandasabapathy N, Lovelace P, Chung CD, Engleman E, Fathman CG. Nat. Immunol. 2004;5:45–54. doi: 10.1038/ni1017. [DOI] [PubMed] [Google Scholar]
- 89.Lin SC, Chung JY, Lamothe B, Rajashankar K, Lu M, Lo YC, Lam AY, Darnay BG, Wu H. J. Mol. Biol. 2008;376:526–40. doi: 10.1016/j.jmb.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scheel H, Tomiuk S, Hofmann K. Hum. Mol. Genet. 2003;12:2845–52. doi: 10.1093/hmg/ddg297. [DOI] [PubMed] [Google Scholar]
- 91.Burnett B, Li F, Pittman RN. Hum. Mol. Genet. 2003;12:3195–205. doi: 10.1093/hmg/ddg344. [DOI] [PubMed] [Google Scholar]
- 92.Tzvetkov N, Breuer P. Biol. Chem. 2007;388:973–8. doi: 10.1515/BC.2007.107. [DOI] [PubMed] [Google Scholar]
- 93.Winborn BJ, Travis SM, Todi SV, Scaglione KM, Xu P, Williams AJ, Cohen RE, Peng J, Paulson HL. J. Biol. Chem. 2008;283:26436–43. doi: 10.1074/jbc.M803692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nicastro G, Habeck M, Masino L, Svergun DI, Pastore A. J. Biomol. NMR. 2006;36:267–77. doi: 10.1007/s10858-006-9092-z. [DOI] [PubMed] [Google Scholar]
- 95.Yao T, Cohen RE. Nature. 2002;419:403–7. doi: 10.1038/nature01071. [DOI] [PubMed] [Google Scholar]
- 96.Verma R, Aravind L, Oania R, McDonald WH, Yates JR, 3rd, Koonin EV, Deshaies RJ. Science. 2002;298:611–5. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- 97.Cope GA, Suh GS, Aravind L, Schwarz SE, Zipursky SL, Koonin EV, Deshaies RJ. Science. 2002;298:608–11. doi: 10.1126/science.1075901. [DOI] [PubMed] [Google Scholar]
- 98.Lyapina S, Cope G, Shevchenko A, Serino G, Tsuge T, Zhou C, Wolf DA, Wei N, Deshaies RJ. Science. 2001;292:1382–5. doi: 10.1126/science.1059780. [DOI] [PubMed] [Google Scholar]
- 99.Ambroggio XI, Rees DC, Deshaies RJ. PLoS Biol. 2004;2:E2. doi: 10.1371/journal.pbio.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tran HJ, Allen MD, Lowe J, Bycroft M. Biochemistry. 2003;42:11460–5. doi: 10.1021/bi035033g. [DOI] [PubMed] [Google Scholar]
- 101.Pena V, Liu S, Bujnicki JM, Luhrmann R, Wahl MC. Mol. Cell. 2007;25:615–24. doi: 10.1016/j.molcel.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 102.Zhang L, Shen J, Guarnieri MT, Heroux A, Yang K, Zhao R. Protein Sci. 2007;16:1024–31. doi: 10.1110/ps.072872007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sanches M, Alves BS, Zanchin NI, Guimaraes BG. J. Mol. Biol. 2007;370:846–55. doi: 10.1016/j.jmb.2007.04.084. [DOI] [PubMed] [Google Scholar]
- 104.Bellare P, Kutach AK, Rines AK, Guthrie C, Sontheimer EJ. RNA. 2006;12:292–302. doi: 10.1261/rna.2152306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bellare P, Small EC, Huang X, Wohlschlegel JA, Staley JP, Sontheimer EJ. Nat. Struct. Mol. Biol. 2008;15:444–51. doi: 10.1038/nsmb.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Snider MJ, Lazarevic D, Wolfenden R. Biochemistry. 2002;41:3925–30. doi: 10.1021/bi011696r. [DOI] [PubMed] [Google Scholar]
- 107.Coux O, Tanaka K, Goldberg AL. Annu. Rev. Biochem. 1996;65:801–47. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 108.Pickart CM, Cohen RE. Nat. Rev. Mol. Cell Biol. 2004;5:177–87. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- 109.Maupin-Furlow JA, Humbard MA, Kirkland PA, Li W, Reuter CJ, Wright AJ, Zhou G. Curr. Top. Dev. Biol. 2006;75:125–69. doi: 10.1016/S0070-2153(06)75005-0. [DOI] [PubMed] [Google Scholar]
- 110.Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik HD, Huber R. Nature. 1997;386:463–71. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 111.Groll M, Heinemeyer W, Jager S, Ullrich T, Bochtler M, Wolf DH, Huber R. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10976–83. doi: 10.1073/pnas.96.20.10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stone M, Hartmann-Petersen R, Seeger M, Bech-Otschir D, Wallace M, Gordon C. J. Mol. Biol. 2004;344:697–706. doi: 10.1016/j.jmb.2004.09.057. [DOI] [PubMed] [Google Scholar]
- 113.Yao T, Song L, Xu W, DeMartino GN, Florens L, Swanson SK, Washburn MP, Conaway RC, Conaway JW, Cohen RE. Nat. Cell Biol. 2006;8:994–1002. doi: 10.1038/ncb1460. [DOI] [PubMed] [Google Scholar]
- 114.Hamazaki J, Iemura S, Natsume T, Yashiroda H, Tanaka K, Murata S. EMBO J. 2006;25:4524–36. doi: 10.1038/sj.emboj.7601338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qiu XB, Ouyang SY, Li CJ, Miao S, Wang L, Goldberg AL. EMBO J. 2006;25:5742–53. doi: 10.1038/sj.emboj.7601450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lam YA, Lawson TG, Velayutham M, Zweier JL, Pickart CM. Nature. 2002;416:763–7. doi: 10.1038/416763a. [DOI] [PubMed] [Google Scholar]
- 117.Verma R, Oania R, Graumann J, Deshaies RJ. Cell. 2004;118:99–110. doi: 10.1016/j.cell.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 118.Elsasser S, Chandler-Militello D, Muller B, Hanna J, Finley D. J. Biol. Chem. 2004;279:26817–22. doi: 10.1074/jbc.M404020200. [DOI] [PubMed] [Google Scholar]
- 119.Husnjak K, Elsasser S, Zhang N, Chen X, Randles L, Shi Y, Hofmann K, Walters KJ, Finley D, Dikic I. Nature. 2008;453:481–8. doi: 10.1038/nature06926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schreiner P, Chen X, Husnjak K, Randles L, Zhang N, Elsasser S, Finley D, Dikic I, Walters KJ, Groll M. Nature. 2008;453:548–52. doi: 10.1038/nature06924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Crosas B, Hanna J, Kirkpatrick DS, Zhang DP, Tone Y, Hathaway NA, Buecker C, Leggett DS, Schmidt M, King RW, Gygi SP, Finley D. Cell. 2006;127:1401–13. doi: 10.1016/j.cell.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 122.Schmidt M, Hanna J, Elsasser S, Finley D. Biol. Chem. 2005;386:725–37. doi: 10.1515/BC.2005.085. [DOI] [PubMed] [Google Scholar]
- 123.Koulich E, Li X, Demartino GN. Mol. Biol. Cell. 2008;19:1072–82. doi: 10.1091/mbc.E07-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anderson C, Crimmins S, Wilson JA, Korbel GA, Ploegh HL, Wilson SM. J. Neurochem. 2005;95:724–31. doi: 10.1111/j.1471-4159.2005.03409.x. [DOI] [PubMed] [Google Scholar]
- 125.Wilson SM, Bhattacharyya B, Rachel RA, Coppola V, Tessarollo L, Householder DB, Fletcher CF, Miller RJ, Copeland NG, Jenkins NA. Nat. Genet. 2002;32:420–5. doi: 10.1038/ng1006. [DOI] [PubMed] [Google Scholar]
- 126.Borodovsky A, Kessler BM, Casagrande R, Overkleeft HS, Wilkinson KD, Ploegh HL. EMBO J. 2001;20:5187–96. doi: 10.1093/emboj/20.18.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hanna J, Hathaway NA, Tone Y, Crosas B, Elsasser S, Kirkpatrick DS, Leggett DS, Gygi SP, King RW, Finley D. Cell. 2006;127:99–111. doi: 10.1016/j.cell.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 128.Lundgren J, Masson P, Realini CA, Young P. Mol. Cell Biol. 2003;23:5320–30. doi: 10.1128/MCB.23.15.5320-5330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gallery M, Blank JL, Lin Y, Gutierrez JA, Pulido JC, Rappoli D, Badola S, Rolfe M, Macbeth KJ. Mol. Cancer Ther. 2007;6:262–8. doi: 10.1158/1535-7163.MCT-06-0542. [DOI] [PubMed] [Google Scholar]
- 130.Guterman A, Glickman MH. J. Biol. Chem. 2004;279:1729–38. doi: 10.1074/jbc.M307050200. [DOI] [PubMed] [Google Scholar]
- 131.Chernova TA, Allen KD, Wesoloski LM, Shanks JR, Chernoff YO, Wilkinson KD. J. Biol. Chem. 2003;278:52102–15. doi: 10.1074/jbc.M310283200. [DOI] [PubMed] [Google Scholar]
- 132.Amerik A, Swaminathan S, Krantz BA, Wilkinson KD, Hochstrasser M. EMBO J. 1997;16:4826–38. doi: 10.1093/emboj/16.16.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li W, Tu D, Brunger AT, Ye Y. Nature. 2007;446:333–7. doi: 10.1038/nature05542. [DOI] [PubMed] [Google Scholar]
- 134.Doelling JH, Yan N, Kurepa J, Walker J, Vierstra RD. Plant J. 2001;27:393–405. doi: 10.1046/j.1365-313x.2001.01106.x. [DOI] [PubMed] [Google Scholar]
- 135.Lindsey DF, Amerik A, Deery WJ, Bishop JD, Hochstrasser M, Gomer RH. J. Biol. Chem. 1998;273:29178–87. doi: 10.1074/jbc.273.44.29178. [DOI] [PubMed] [Google Scholar]
- 136.Dayal S, Sparks A, Jacob J, Allende-Vega N, Lane DP, Saville MK. J. Biol. Chem. 2008 doi: 10.1074/jbc.M805871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Melandri F, Grenier L, Plamondon L, Huskey WP, Stein RL. Biochemistry. 1996;35:12893–900. doi: 10.1021/bi9612935. [DOI] [PubMed] [Google Scholar]
- 138.Hadari T, Warms JV, Rose IA, Hershko A. J. Biol. Chem. 1992;267:719–27. [PubMed] [Google Scholar]
- 139.Stein RL, Chen Z, Melandri F. Biochemistry. 1995;34:12616–23. doi: 10.1021/bi00039a017. [DOI] [PubMed] [Google Scholar]
- 140.Gabriel JM, Lacombe T, Carobbio S, Paquet N, Bisig R, Cox JA, Jaton JC. Biochemistry. 2002;41:13755–66. doi: 10.1021/bi026096m. [DOI] [PubMed] [Google Scholar]
- 141.Lacombe T, Gabriel JM. FEBS Lett. 2002;531:469–74. doi: 10.1016/s0014-5793(02)03586-x. [DOI] [PubMed] [Google Scholar]
- 142.Adhikari A, Xu M, Chen ZJ. Oncogene. 2007;26:3214–26. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 143.Ravid T, Hochstrasser M. Curr. Biol. 2004;14:R898–900. doi: 10.1016/j.cub.2004.09.074. [DOI] [PubMed] [Google Scholar]
- 144.Scheidereit C. Oncogene. 2006;25:6685–705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- 145.Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A. Nat. Immunol. 2004;5:1052–60. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 146.Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. Nature. 2003;424:801–5. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- 147.Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- 148.Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. Nature. 2003;424:793–6. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- 149.Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, Jones C, Hansen J, Blair E, Hofmann B, Siebert R, Turner G, Evans DG, Schrander-Stumpel C, Beemer FA, van Den Ouweland A, Halley D, Delpech B, Cleveland MG, Leigh I, Leisti J, Rasmussen S. Nat. Genet. 2000;25:160–5. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- 150.Xue L, Igaki T, Kuranaga E, Kanda H, Miura M, Xu T. Dev. Cell. 2007;13:446–54. doi: 10.1016/j.devcel.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 151.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Science. 2000;289:2350–4. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Galan JM, Haguenauer-Tsapis R. EMBO J. 1997;16:5847–54. doi: 10.1093/emboj/16.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Reggiori F, Pelham HR. EMBO J. 2001;20:5176–86. doi: 10.1093/emboj/20.18.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Mol. Cell. 2006;21:737–48. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 155.Urbe S. Essays Biochem. 2005;41:81–98. doi: 10.1042/EB0410081. [DOI] [PubMed] [Google Scholar]
- 156.Williams RL, Urbe S. Nat. Rev. Mol. Cell Biol. 2007;8:355–68. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 157.Row PE, Liu H, Hayes S, Welchman R, Charalabous P, Hofmann K, Clague MJ, Sanderson CM, Urbe S. J. Biol. Chem. 2007;282:30929–37. doi: 10.1074/jbc.M704009200. [DOI] [PubMed] [Google Scholar]
- 158.Amerik AY, Nowak J, Swaminathan S, Hochstrasser M. Mol. Biol. Cell. 2000;11:3365–80. doi: 10.1091/mbc.11.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Luhtala N, Odorizzi G. J. Cell Biol. 2004;166:717–29. doi: 10.1083/jcb.200403139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Amerik A, Sindhi N, Hochstrasser M. J. Cell Biol. 2006;175:825–35. doi: 10.1083/jcb.200605134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nikko E, Andre B. Traffic. 2007;8:566–81. doi: 10.1111/j.1600-0854.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- 162.Richter C, West M, Odorizzi G. EMBO J. 2007;26:2454–64. doi: 10.1038/sj.emboj.7601692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Alwan HA, van Leeuwen JE. J. Biol. Chem. 2007;282:1658–69. doi: 10.1074/jbc.M604711200. [DOI] [PubMed] [Google Scholar]
- 164.Mizuno E, Kobayashi K, Yamamoto A, Kitamura N, Komada M. Traffic. 2006;7:1017–31. doi: 10.1111/j.1600-0854.2006.00452.x. [DOI] [PubMed] [Google Scholar]
- 165.Mizuno E, Iura T, Mukai A, Yoshimori T, Kitamura N, Komada M. Mol. Biol. Cell. 2005;16:5163–74. doi: 10.1091/mbc.E05-06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Agromayor M, Martin-Serrano J. J. Biol. Chem. 2006;281:23083–91. doi: 10.1074/jbc.M513803200. [DOI] [PubMed] [Google Scholar]
- 167.Ma YM, Boucrot E, Villen J, Affar el B, Gygi SP, Gottlinger HG, Kirchhausen T. J. Biol. Chem. 2007;282:9805–12. doi: 10.1074/jbc.M611635200. [DOI] [PubMed] [Google Scholar]
- 168.Clague MJ, Urbe S. Trends Cell. Biol. 2006;16:551–9. doi: 10.1016/j.tcb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 169.McCullough J, Row PE, Lorenzo O, Doherty M, Beynon R, Clague MJ, Urbe S. Curr. Biol. 2006;16:160–5. doi: 10.1016/j.cub.2005.11.073. [DOI] [PubMed] [Google Scholar]
- 170.Nakamura M, Tanaka N, Kitamura N, Komada M. Genes Cells. 2006;11:593–606. doi: 10.1111/j.1365-2443.2006.00963.x. [DOI] [PubMed] [Google Scholar]