Abstract
Objective and methods A model specifying body mass index (BMI) as mediating the relationship between lifestyle factors (aerobic fitness determined by peak oxygen consumption; physical activity by 7-day physical activity recall; diet by 24 hr dietary recall), and lipid profile were tested in a sample of 205 adolescents (73% boys), who were on average at risk of overweight, aerobically unfit, and from ethnic minority groups. Results In this well-fitting model, consuming a diet low in fat and cholesterol, and being aerobically fit predicted lower BMI, which together resulted in increases in high-density lipoprotein cholesterol and decreases in triglycerides and low-density lipoprotein cholesterol. Being physically active, predicted greater aerobic fitness. Conclusions In addition to furthering understanding of the interrelationships among predisposing, major, and conditional coronary heart disease risk factors in adolescents, these data suggest that improving diet and aerobic fitness will reduce BMI and result in a better lipid profile.
Keywords: adolescents, body mass index, coronary heart disease, lifestyle factors, lipid profile
Predisposing coronary heart disease (CHD) risk factors promote the development of major and conditional CHD risk factors, which may be direct causes of CHD (Grundy et al., 2000). Among youth, an increase in predisposing CHD risk factors such as being overweight and following unhealthy lifestyle practices (i.e., being physical inactive, having unhealthy dietary habits) is evident (Eisenmann, 2003). According to the 1999–2002 National Health and Nutrition Examination Survey (NHANES), about 16% of youth ages 6–19 were overweight, which is defined as having a body mass index (BMI; kg/m2) value at or above the 95th percentile of the gender-specific BMI growth charts (Centers for Disease Control and Prevention [CDC], 2005a) and which represents a 45% increase from the 1988 to 1994 NHANES estimates. With respect to physical activity, 55.4% of American youths aged 14–18 engage in moderate physical activity and 35.3% do vigorous physical activity less than twice a week according to the 1999 Youth Risk Behavior Survey (Eisenmann, Bartee, & Wang, 2002). Regarding diet, <40% of youth meet the Dietary Guidelines for Americans for saturated fat, 80% do not eat five servings of fruits and vegetables per day, and 61% do not meet recommendations for fiber intake (CDC, 2005b). In sum, youth on average are not meeting national recommendations for weight and lifestyle practices.
Major CHD risk factors [e.g., elevations of low-density lipoprotein cholesterol (LDL-C) level; low high-density lipoprotein cholesterol (HDL-C) level] and conditional risk factors (elevations of triglycerides, small LDL-C particles) tend to cluster in youth and adults, resulting in an increased risk for coronary events such as a myocardial infarction in later adulthood (Grundy et al., 2000). BMI has been consistently associated with the clustering of these CHD risk factors. In addition to waist circumference and subscapular skinfold thickness, BMI predicted greater risk factor clustering in adults from the Framingham Heart Study and Framingham Offspring Study (Kannel, Wilson, Nam, & D’Agostino, 2002), and in youth from the Bogalusa Heart Study (Katzmarzyk et al., 2004), the Cardiovascular Risk in Young Finns Study (Raitakari, Porkka, Rasanen, & Viikari, 1994), and a mid-Western American sample (Daniels, Morrison, Sprecher, Khoury, & Kimball, 1999).
Poor lifestyle factors are thought to account for an increase in BMI, indirectly affecting the clustering of CHD risk factors. Lifestyle factors such as eating unhealthy foods and physical inactivity may contribute to the development of overweight status in adolescents (Goran, 2001; Hill & Melanson, 1999). High fat convenience foods such as French fries and inexpensive high calorie foods such as muffins and potato chips are thought to contribute to the increased prevalence of overweight status in youth (Yensel, Preud’Homme, & Curry, 2004), and are now readily available at schools due to “pouring rights” with food companies regarding vending machines and to outsourcing of food production to fast food chains (Van Staveren & Dale, 2004). Furthermore, there are few opportunities and little encouragement for youth to be physically active to counterbalance the excess calories and fat consumed from foods and beverages (Van Staveren & Dale, 2004). Fewer students are attending daily physical education classes, and walking or biking to school (Lowry et al., 2005). The decreasing trend in physical activity parallels a decline in aerobic fitness among youth from industrialized countries over the past few decades (Corbin & Pangrazi, 1992; Eisenmann, 2003; Westerstahl, Barnekow-Bergkvist, Hedberg, & Jansson, 2003). Therefore, youth today are largely physically inactive and aerobically unfit. Together, over-consumption of high fat and high calorie foods and beverages, physical inactivity, and low aerobic fitness may contribute to the development of overweight status in youth.
Lifestyle factors appear to influence the accumulation of fat, which in turn relates to the development of major and conditional CHD risk factors. Once thought to only be an inactive energy storage area in which excess calories were stored as fat, it is now known that adipose tissue also functions as an endocrine gland (Kahn & Flier, 2000). Fat cells secrete free fatty acids, which may stimulate hepatic triglyceride and very low density lipoprotein cholesterol (VLDL-C) production in adults (Bacha, Saad, Gungor, & Arslanian, 2004; Cruz, Bergman, & Goran, 2002; Kahn & Flier, 2000; Weiss et al., 2003). There is evidence to suggest a similar relationship in youth. Excess fat has often been associated with elevated LDL-C and triglyceride levels, and reduced HDL-C values (Eisenmann, Womack, Reeves, Pivarnik, & Malina, 2001; Freedman, Serdula, Srinivasan, & Berenson, 1999; Tolfrey, Campbell, & Jones, 1999). In addition to its effect on BMI, a diet low in total dietary fat, dietary cholesterol, and saturated fat reduced LDL-C levels according to dietary intervention studies in adults such as the Dietary Approaches to Stop Hypertension (DASH) trial, (Obarzanek et al., 2001) and in youth, i.e., the Dietary Intervention Study in Children (DISC) study (Lauer et al., 2000).
Processes underlying the development of major and conditional CHD risk factor clustering in youth are not fully understood. To date, pediatric investigations of the relationship among predisposing CHD risk factors and lipid profile offer mixed results possibly due to the limitations of study design and/or the analytical strategies employed (Boreham et al., 2001; Eisenmann et al., 2001; Katzmarzyk, Malina & Bouchard, 1999; Tolfrey et al., 1999). These investigations also often recruited youth of varying age ranges, making it difficult to compare results, since age and sexual maturation influence the lipid profile of youth (Berenson, Srinivasan, Cresanta, Foster & Webber, 1981). An approach is needed that would facilitate further understanding of theses mechanisms. One such statistical strategy is structural equation modeling (SEM), which simultaneously tests several causal relationships. Furthermore, SEM incorporates confirmatory factor analysis to develop an aggregate construct (latent variable), which incorporates multiple measures of a construct, while reducing measurement error specific to each measure.
This study assessed the associations among predisposing CHD risk factors [i.e., aerobic fitness, physical activity, an atherogenic (high fat, high cholesterol) diet, BMI], and major (i.e., elevated LDL-C; low HDL-C) and conditional (i.e., elevated triglycerides) CHD risk factors in late pubertal adolescents using SEM. The proposed model tested whether BMI mediates the relationship between lifestyle factors, and HDL-C, LDL-C, and triglycerides. In this analysis, an atherogenic diet was modeled as a latent variable. Furthermore, we hypothesized that: (a) physical activity would increase aerobic fitness; (b) low aerobic fitness and an atherogenic diet would increase BMI; (c) BMI would increase lipid mobilization leading to increases in triglycerides and LDL-C, and decreases in HDL-C; and (d) an atherogenic diet would raise LDL-C levels.
Methods
Participants
Participants were 205 adolescents (150 boys; 55 girls) participating in a larger investigation examining cardiovascular risk factors in adolescents. Approximately half of the participants had a positive parental history of hypertension, and had elevated blood pressure defined as systolic blood pressure and/or diastolic blood pressure at or above the 90th percentile for age, gender, and height. Three-fourths of the samples were boys, and the majority were from self-reported racial and ethnic minority groups (27.8% Black, 41.5% Hispanic). Regarding weight status, 45.4% of the participants were overweight (BMI ≥ 95th percentile) and 18.5% were at risk of overweight (85th percentile ≤ BMI < 95th percentile) (CDC, 2002). On average, boys (M = 3.2, SD = 0.5) and girls (M = 3.6, SD = 0.4) were in late puberty as defined by the Pubertal Development Scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988). Participants did not meet exclusion criteria for the larger investigation, which included blood pressure >160/100 mmHg, age 18 years or older, residing in the United States for <4 years, taking antihypertensive medication, and having a history of asthma, heart murmurs, diabetes, severe allergies, renal disease, secondary hypertension, seizure disorder, developmental disabilities, cancer, bronchial conditions, ventricular arrhythmias, heart disease, amputation or related birth defect, illiteracy, major psychological disorder, spinal cord injury, or arthritis. Additional exclusion criteria assessed at the baseline appointments included an abnormal electrocardiogram or echocardiogram. Table I includes means, standard deviations, and sample sizes for age, casual blood pressure, parental education, lipid profile, BMI, physical activity, aerobic fitness, and diet indicators.
Table I.
Descriptive Statistics of Participant Characteristics and Observed Variables
| Participant Characteristics | n | M | SD |
|---|---|---|---|
| Age (years) | 205 | 16.2 | 0.8 |
| BMI (kg/m2) | 205 | 28.8 | 7.9 |
| Casual SBP (mmHg) | 203 | 128 | 13 |
| Casual DBP (mmHg) | 203 | 72 | 11 |
| Mother's education (years) | 185 | 12.9 | 2.4 |
| Father's education (years) | 170 | 13.5 | 3.1 |
| Total cholesterol (mg/dl) | 192 | 158.8 | 32.7 |
| LDL-C (mg/dl) | 191 | 97.1 | 29.4 |
| HDL-C (mg/dl) | 191 | 42.5 | 7.9 |
| Triglycerides (mg/dl) | 192 | 93.6 | 54.7 |
| Energy expenditure (kcal/kg/day) | 203 | 39.2 | 6.8 |
| Measured VO2 (ml/kg/min) | 159 | 36.2 | 11.1 |
| Calories (kcal) | 191 | 2,493.1 | 1,004.6 |
| Total dietary fat (g) | 191 | 84.6 | 47.4 |
| Saturated fat (g) | 191 | 29.4 | 19.3 |
| Polyunsaturated fat (g) | 191 | 12.9 | 10.5 |
| Dietary cholesterol (mg) | 190 | 301.8 | 198.5 |
| Fiber (g) | 191 | 14.4 | 22.2 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; PDS, Pubertal Development Scale (Petersen et al., 1988); LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Measured VO2, measured volume of oxygen uptake. Missing data were handled with the full information maximum likelihood estimation method.
Procedures
School Screening and Home Visit
Participants were initially identified from an annual hypertension school screening for tenth grade students attending local public high schools, which involved an initial blood pressure screening day followed by a blood pressure recheck screening day for students identified with elevated blood pressure on the initial day using the aforementioned criteria.
Parents or legal guardians of students with elevated blood pressure were sent a letter notifying them of their adolescent's blood pressure, and an invitation letter to schedule a home visit at their home to obtain a reassessment of their adolescent's blood pressure. Home visits were conducted in the primary language of the parent and adolescent (Spanish or English) by staff fluent in the language. Home visit parental consent forms and questionnaires were available in either English or Spanish. For the home visit, staff obtained parental written consent and student written assent. Staff then assessed the student's casual blood pressure. After sitting quietly for 5 min, three mercury sphygmomanometer readings from the right arm were taken at 2 min intervals using an appropriately sized cuff, and the last two readings were averaged. The student's parent completed self-report forms regarding parental education and parental history of hypertension, and the student reported his/her race and/or ethnicity and pubertal status.
Eligible students were invited to participate in the larger investigation. Written informed consent was obtained from the parent or legal guardian, and written assent was obtained from the student. Students with elevated blood pressure who enrolled in the investigation were asked for the name of a friend to potentially recruit a normotensive participant in the program. Among 320 students screened, 294 were eligible. Of the eligible students, 268 consented to participate. A total of 205 completed the baseline assessment and 63 withdrew from the study prior to the baseline assessment. The present study, therefore, included 205 students with both elevated and normal blood pressures. The University of Miami's Institutional Review Board approved the program protocol.
Assessments
Participants completed baseline assessment appointments and received a monetary compensation of $25 for each appointment. As this study is part of a larger investigation, only procedures relevant to this study are detailed subsequently. Casual blood pressure, lipid profile, body composition, 7-day physical activity recall, 24 hr dietary recall, and 12-lead electrocardiogram were completed on the first day. Participants attended two additional appointments to assess aerobic fitness and to complete an echocardiogram.
Casual blood pressure. Blood pressure was measured on the right arm using an appropriately sized cuff after sitting quietly for 5 min. Three mercury sphygmomanometer readings from the right arm were taken at 2 min intervals. The last two readings were averaged.
Lipid profile. A fasting blood sample obtained with an indwelling catheter was analyzed for total cholesterol, triglycerides, HDL-C, and LDL-C. Chemistry assays were performed by automated analyzer utilizing commercially available kits according to manufacturer's instructions. Instrument set-up, run procedures, and maintenance policies were strictly adhered to according to the manufacturer's instructions. Total cholesterol was measured in ethylenediaminetetraaceitic acid (EDTA) plasma after release from its esters by an ester hydrolase using the cholesterol oxidase technique. Intra- and inter-assay coefficient of variations (CV) were 2.5 and 3.5%, respectively. Triglyceride level was measured in EDTA plasma after hydrolysis by lipoprotein lipase by assay of released glycerol, and the inter-assay CV was 5.3%. HDL-cholesterol was measured using Dextran sulfate (50,000 MW) and magnesium chloride, and the inter-assay CV was 2.5%. LDL-C was calculated according to the method of Friedewald. After extrapolation of VLDL-C from the fasting triglyceride divided by five, then:
LDL − C = Total cholesterol − (VLDL − C + HDL − C)
Body composition. Participants emptied their pockets and removed their shoes to assess their height in inches and weight in pounds using a balance beam scale with a height rod. BMI was calculated as weight in kilograms (kg) divided by the height in meters squared (m2).
Seven-day physical activity recall. This measure provides an estimate of kilocalories per kilograms per day based on energy expended during exercise and leisure activities. Though originally developed and tested for an adult cohort (Sallis et al., 1985), this measure is reliable and valid for use with adolescents (r =.81 for 11th graders) (Sallis, Buono, Roby, Micale, & Nelson, 1993). Participants reported the number of hours they spent sleeping and engaging in physical activities for the 7 days prior to the appointment, and the intensity of each activity (moderate, hard, and very hard). The procedure to calculate the total energy expended each day is described by Sallis and colleagues (1985).
24 hr dietary recall. This widely used measure of dietary intake provides a valid and reliable assessment (correlations ranged from .6 to .8) of group mean nutrient intake (Balogh, Kahn, & Random, 1971; Block, 1982; Linusson, Sanjur, & Erickson, 1974; Rockett & Colditz, 1997; Women's Intervention Nutrition Study, 1993). Staff was trained to probe participants’ responses as per study protocol to gather an exhaustive, detailed recall. Participants identified everything they ate and drank the day prior to the appointment. Staff queried participants regarding items identified to ascertain product specifications (e.g., brand name, low fat), and to approximate quantities consumed using standard project measuring devices such as models (e.g., measuring cups) and charts. Diet components were determined using Nutritionist Pro software programs. Variables included were: percentages of total calories from total dietary fat, saturated fat, and polyunsaturated fat (calories from variable divided by total calorie consumption multiplied by 100); grams of dietary cholesterol; milligrams of fiber.
Aerobic fitness. Peak VO2 (volume of oxygen; ml/kg/min) was determined on a motorized treadmill using a modified Balke exercise protocol. Each participant started walking at a speed of 4.5 mph with a 2.5% grade. Every 2 min, the grade increased by 2.5%, while the speed remained constant. At the end of every 2 min, the trained technician asked each participant to rate their level of physical exertion on a 6–20 scale using the Borg Rate of Perceived Exertion (RPE) Scale. Expired gases were continuously sampled using a low resistance mass flow sensor. These gases were analyzed using a Sensor Medics Vmax229 metabolic cart. Test termination criteria included three of the following: a Borg RPE greater than 17; a respiratory quotient (carbon dioxide exhaled divided by oxygen inhaled) greater than 1.1; heart rate within 20 beat of age predicted maximal heart rate; and an instance when an increase in absolute VO2 was less than 0.1 l with an increase in workload.
Pubertal status. Participants self-reported their pubertal development using the PDS. This scale provides a valid and reliable measure of pubertal status (Petersen et al., 1988) and is correlated with Tanner staging criteria. Girls evaluated their physical development based on four items (body hair, breast development, skin change, and growth spurt) on a 4-point Likert scale from 1 (not started) to 4 (seems complete) and one dichotomous item (menarche) scored as 1 (no) and 4 (yes). Boys rated their physical development based on five items (body hair, voice change, skin change, growth spurt, and facial hair) using the same Likert scale. The average of the items indicated the student's stage of development ranging from 1 (prepubertal) to 4 (late to postpubertal).
Statistical Analysis
We proposed a mediational model to examine whether BMI mediates the relationship between lifestyle factors (aerobic fitness, physical activity, and an atherogenic diet), and lipid profile. The model was tested using SEM with Mplus version 3.1 (Múthen & Múthen, 1998). Normality assumptions were assessed using PROC UNIVARIATE in SAS Version 9.1 for Windows (SAS Institute Inc., 2003). Variables with outliers, kurtosis greater than or equal to four, and skewness greater than or equal to two were identified. Triglycerides, total cholesterol, LDL-C, total dietary fat, and dietary cholesterol had eight legitimate outliers, which were then winsorized. Polyunsaturated fat, saturated fat, and fiber intake were naturally log-transformed. Missing data were handled with the full information maximum likelihood estimation method, which assumes data were missing at random. A diet latent factor was specified using intake of total dietary fat, saturated fat, polyunsaturated fat, dietary cholesterol, and fiber. Also, dietary cholesterol was divided by 1000 and total dietary fat was divided by 100 to achieve a standard metric for the diet variables. Finally, percentage of total calories from diet variables (calories from diet variable divided by total calorie consumption multiplied by 100) for total dietary fat, saturated fat, and polyunsaturated fat were used instead of total grams consumed. Other variables were incorporated as observed variables in the model. Four indices were used to assess overall model fit: (a) chi-square with p-value >.05; (b) the comparative fit index (CFI) greater than .95; (c) the root mean square error of approximation (RMSEA) less than .06; and (d) standardized root-mean-square residual (SRMR) less than.09 (Yu & Bentler, 1999).
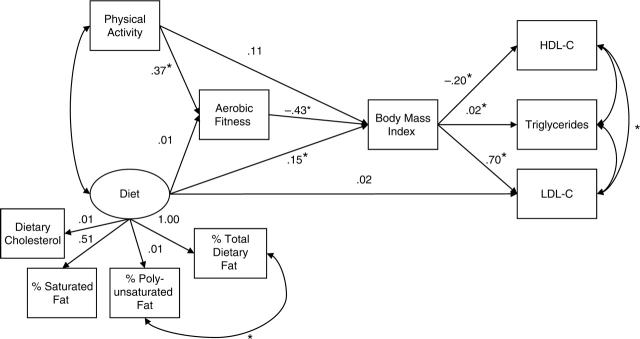
Results
Measurement Model
Correlations among BMI, physical activity, aerobic fitness, diet variables, and lipid profile are displayed in Table II. The unstandardized regression coefficients for the observed indicators of the diet latent variable are found in Fig. 1. First, the measurement model for the latent variable diet was tested. The latent variable diet comprised of total dietary fat, saturated fat, polyunsaturated fat, dietary cholesterol, and fiber did not fit the sample data well {χ2 (5) = 20.99, p <.001; CFI =.96; RMSEA =.13 [90% confidence interval (CI) = 0.08–0.19]; SRMR =.04}. However, by conceptualizing the diet latent variable as one of unhealthy diet indicators by excluding fiber and by correlating residuals of polyunsaturated fat and total dietary fat, the model fit the data [χ2 (1) =.36, p =.55; CFI = 1.00; RMSEA = <.001 (90% CI = 0.00–0.16); SRMR =.01].
Table II.
Correlations Among Lipid Profile, BMI, Physical Activity, Aerobic Fitness, and Diet Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Total cholesterol (mg/dl) | – | .43† | .01 | .94† | .20** | −.01 | −.24** | −.07 | −.03 | −.05 | −.02 | −.08 |
| 2. Triglycerides (mg/dl) | – | −.39† | .18* | .26† | −.02 | −.07 | −.02 | −.02 | −.14 | −.002 | −.05 | |
| 3. HDL-C (mg/dl) | – | −.10 | −.20** | .04 | .08 | −.004 | −.09 | .14 | −.002 | −.007 | ||
| 4. LDL-C (mg/dl) | – | .19** | −.01 | −.27** | −.07 | −.01 | −.04 | −.02 | −.11 | |||
| 5. BMI (kg/m2) | – | −.05 | −.58† | .05 | .10 | −.06 | −.005 | −.05 | ||||
| 6. Energy expenditure (kcal/kg/day) | – | .24** | .10 | .08 | .12 | .09 | .17 | |||||
| 7. Measured VO2 (ml/kg/min) | – | .21** | .13 | .23** | .20* | .22** | ||||||
| 8. Total dietary fat (g) | – | .56† | .60† | .87† | .36† | |||||||
| 9. Dietary cholesterol (mg) | – | .41† | .59† | .22** | ||||||||
| 10. Polyunsaturated fat (g) | – | .48† | .32† | |||||||||
| 11. Saturated fat (g) | – | .25† | ||||||||||
| 12. Fiber (g) | – |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Measured VO2, measured volume of oxygen uptake.
*p <.05; **p <.01; †p < .001.
Figure 1.
Unstandardized parameter estimates of the final SEM: BMI mediates the effect of lifestyle factors on CHD risk factors in adolescents. Model fit statistics were: χ2 (27) = 33.78, p =.17; CFI =.99; RMSEA =.04 (90% CI = 0.00–0.07); SRMR =.05. Oval represents latent variable; squares represent observed values. HDL-C = high-density lipoprotein cholesterol (mg/dl); LDL-C = low-density lipoprotein cholesterol (mg/dl); % total dietary fat = percentage of calories from total dietary fat intake; % polyunsaturated fat = percentage of calories from polyunsaturated fat intake; % saturated fat = percentage of calories from saturated fat intake. An asterisk indicates p < .05.
Structural Model
The structural model displayed in Fig. 1 was tested and found to fit the sample data well [χ2 (27) = 33.78, p =.17; CFI =.98; RMSEA =.04 (90% CI = 0.00–0.07); SRMR =.05]. Of the 11 indirect paths examined, four achieved statistical significance (all p's <.05). BMI mediated the relationship between aerobic fitness and HDL-C, LDL-C, and triglycerides (unstandardized coefficients = .09, −.30, and −.008, respectively). HDL-C is expected to increase by .09 units given an increase of one unit in aerobic fitness via its effect on BMI. Similarly, LDL-C is expected to decrease by .30 units given an increase of one unit in aerobic fitness via its effect on BMI. Triglycerides are expected to decrease by .008 units given an increase in one unit in aerobic fitness via its effect on BMI. The indirect effect analyses of diet on lipid profile by way of BMI did not reach significance in spite of significant direct paths from diet to BMI and from BMI to lipid profile (see subsequently). It, however, is recognized that tests of indirect effects tend to have less power than tests of direct effects, as their standard errors tend to be positively biased (Shrout & Bolger, 2002). A secondary finding was that aerobic fitness mediated the relationship between physical activity and BMI (unstandardized coefficient = −.16). BMI is expected to decrease by .16 units given an increase of one unit in physical activity via its effect on aerobic fitness.
The unstandardized regression coefficients for the direct effects are displayed in Fig. 1. Aerobic fitness was predicted by physical activity, such that aerobic fitness is expected to increase by .37 units given an increase of one unit in physical activity. The diet latent variable and aerobic fitness predicted BMI. BMI is expected to increase by .15 units given an increase of one unit in the diet latent variable, controlling for aerobic fitness. BMI is expected to decrease by .43 units given an increase in one unit of aerobic fitness, controlling for the diet latent variable. BMI predicted HDL-C, LDL-C, and triglycerides. HDL-C is expected to decrease by .20 units given an increase of one unit in BMI, LDL-C is expected to increase by .70 units given an increase of one unit in BMI, and triglycerides are expected to increase by .02 units given an increase of one unit in BMI. All other direct paths did not reach significance. The final model accounted for 4.9% of the variation in aerobic fitness, 38.0% in BMI, 6.8% in triglycerides, 3.9% in HDL-C, and 3.5% in LDL-C.
Discussion
This study contributes to understanding the relationships among predisposing CHD risk factors (i.e., being overweight, lifestyle factors such as physical inactivity and an atherogenic diet), and major (e.g., elevated LDL-C; low HDL-C) and conditional (e.g., elevated triglycerides, small LDL-C particles) CHD risk factors in adolescents. To our knowledge, this is the first study to test a mediational model to explore this network of relationships in adolescents. This investigation examined whether BMI mediated the relationship between lifestyle factors on the one hand (an atherogenic diet, aerobic fitness, physical activity), and LDL-C, HDL-C, and triglycerides on the other in a predominantly at risk of overweight, inactive, ethnic minority, and adolescent sample.
Our model supported the notion that BMI mediates the relationship between two lifestyle factors (an atherogenic diet, aerobic fitness), and HDL-C, LDL-C, and triglycerides in adolescents. This finding suggested that consuming a diet high in total dietary fat, saturated fat, polyunsaturated fat, and dietary cholesterol, and having a low aerobic fitness level increases BMI, which then decreases HDL-C and increases triglycerides and LDL-C. This result is consistent with previous investigations in youth (Boreham et al., 2001; Katzmarzyk et al., 1999; Sallis et al., 1988; Tolfrey et al., 1999). Furthermore, this investigation integrates the hypothesized relationships into a single model, providing a concise and comprehensive understanding of how lifestyle factors affect causal and conditional CHD risk factors via BMI in adolescents. Past research examined these relationships using multiple linear regression and/or correlational analyses in a piecemeal fashion, testing one hypothesized causal path or mediation at a time and often yielding mixed results. The current investigation improves upon this work by testing several causal paths simultaneously as well as utilizing multiple variables to arrive at a latent construct of interest without measurement error.
A secondary finding was noted. The model was consistent with the notion that aerobic fitness mediated the relationship between physical activity and BMI, such that physical activity increases aerobic fitness, which then decreases BMI. Therefore, physical activity did not predict BMI directly above and beyond diet and aerobic fitness. This result was reported by others (Katzmarzyk et al., 1999; Sallis & Owen, 1999) and supported the assertion that physical activity, accrued over time, increases aerobic fitness. However, the assessment of physical activity by self-report may have increased the measurement error of this complex behavior (Sallis & Owen, 1999) and minimized its effect on BMI relative to aerobic fitness, which was assessed objectively.
An unanticipated result was that the diet latent factor comprised of total dietary fat, saturated fat, polyunsaturated fat, and dietary cholesterol did not predict LDL-C. Previous dietary intervention studies in children and adults, the DISC and DASH trial, respectively, found that consuming a diet low in total dietary fat, saturated fat, and dietary cholesterol lowers LDL-C (Lauer et al., 2000; Obarzanek et al., 2001). Four differences may account for this discrepancy. First, results from the DISC trial were found in preadolescent children with consistently elevated levels of LDL-C. In contrast, the current sample was comprised of postpubescent adolescents whose levels of LDL-C were on average in the normal range (Table I). In fact, further analyses on the DISC data suggested sexual maturation may play a larger role than diet on LDL-C (Kwiterovich et al., 1997). Our data was consistent with this notion as our sample was comprised of sexually mature adolescents. Second, methodology may have played a role. Children from the DISC trial completed three 24 hr dietary recalls at each time point, while adolescents from the current study completed one 24 hr dietary recall. Therefore, dietary information from the DISC trial may have captured fewer days when participants’ diets were atypical. Third, the DISC trial was a longitudinal intervention, while the current study does not describe an intervention, but is based on a cross-sectional design. Fourth, the present findings suggest that diet's effect on LDL-C is mediated by BMI. This result is consistent with the literature, which finds either an indirect effect or a weak direct effect of an atherogenic diet on lipid profile (Morley et al., 1998; Raitakari et al., 1994; Tolfrey et al., 1999).
Risk Factor Clustering and CHD
The clustering of CHD risk factors such as hypertension, glucose intolerance, hyperinsulinemia, increased triglyceride levels, decreased HDL-C levels, and being overweight has been observed in adults from the Framingham Offspring Study (Meigs et al., 1997), and in youth from the Young Finns Study (Raitakari et al., 1994). To understand the mechanisms underlying the clustering of CHD risk factors, theoretical models implicated a positive feedback loop involving insulin resistance, compensatory hyperinsulinemia, and sympathetic nervous system activation as the primary mediating factors linking environmental influences (emotional stressors, poor dietary habits, a sedentary lifestyle, smoking) and genetic predisposition (race, sex, and age) with CHD outcomes (Goran, 2001; Hurwitz & Schneiderman, 1998; Reaven, 1988). Environmental factors along with genetic influences were thought to combine to promote the CHD pathogenesis through their effects on being overweight, insulin resistance, and increased sympathetic drive.
The current study supported the notion of BMI as a mediator relating lifestyle factors with major and conditional CHD risk factors. These results corroborated findings implicating weight proxies as promoting the development of CHD risk factor clustering in youth (Daniels et al., 1999; Katzmarzyk et al., 2004), which is similar to results reported in adults (Kannel et al., 2002). In regards to body size distribution, this investigation specifically tested the role of overall body size (indexed by BMI) as a mediating factor, rather than central body size (e.g., waist circumference). Despite the recent emphasis on being overweight and having a large central body size, our results suggested that overall body size continues to play a deleterious role in the promotion of CHD risk in adolescents. Three reasons may explain why overall body size remains an important factor in youth. First, changes in body size are known to occur during sexual maturation (Deurenberg, Pieters, & Hautvast, 1990; Freedman et al., 1999). Second, youth typically have smaller central body size than adults (de Ridder et al., 1992; Goran, Kaskoun, & Shuman, 1995). Therefore, the role of central body size is only beginning to emerge in adolescents (Sangi & Mueller, 1991). Third, there may be gender differences associated with central body size (men may tend to have greater central body size than women) as reflected in waist circumference cutoff points that comprise the metabolic syndrome criteria (National Cholesterol Education Program, 2002), which were not examined here.
Implications and Further Research
The current findings have implications for the primary prevention of CHD in adolescents. Based on our findings, primary prevention efforts in adolescents should begin with lifestyle interventions focusing on both increasing aerobic fitness by way of increasing physical activity and improving diet. Interventions designed to reduce BMI would likely obtain maximal benefits in reducing the incidence or prevalence of CHD risk factors in adolescents. In adults, there is evidence for the efficacy of lifestyle interventions on CHD outcomes as secondary prevention measures (Diabetes Prevention Program Research Group, 2002; Ornish et al., 1998; Tuomilethto et al., 2001). Lifestyle interventions in adolescents with a primary prevention focus have increased healthy lifestyle habits such as the amount of time spent doing moderate-to-vigorous physical activity during school (Luepker et al., 1996; McKenzie, Sallis, Faucette, Kolody, & Roby, 1993; Pate et al., 2005), and the nutrient content of school lunches (Hoelscher et al., 2003; Luepker et al., 1996). The effects of interventions in adolescents, however, did not extend to biological factors (major and conditional CHD risk factors) such as BMI or lipid profile as they do in adults. Future studies should emphasize lifestyle interventions that increase physical activity and improve diet, enabling youth to reduce their BMI. As indicated in the present study, this may improve major and conditional CHD risk factors. Together, more research on primary prevention efforts focusing on lifestyle interventions with adolescents, and the promotion of changes in schools and homes based on primary prevention research such as the Alliance for a Healthier Generation (2006), may improve the future of adolescent's cardiovascular health.
Limitations
We believe this is the first study to examine BMI as a mediator of lifestyle factors on major and conditional CHD risk factors in adolescents using SEM. Because this study used cross-sectional data, causal assertions are not possible. A longitudinal study design would overcome this limitation. Also, data from both genders and all ethnic and racial groups were collapsed. As previous studies have found different relationships among the variables studied in boys and girls (Boreham et al., 2001; Tolfrey et al., 1999), future studies would benefit from larger samples where gender differences could be explored. Future investigations examining the influence of gender and ethnicity will contribute to understanding the development of CHD precursors in different populations. Furthermore, the generalizability of this study is limited as the sample was comprised predominantly of at risk of overweight Black and Hispanic youth. Finally, relatively little variance in aerobic fitness and lipid and lipoprotein variables was accounted for by the variables in the model. Future studies could explore additional factors that may explain more of the variance in the variables examined.
Efforts were made to utilize the best measures available for the lifestyle factors investigated. Despite these efforts, measurement error may still have affected the assessment of diet indicators and of physical activity collected via self-report measures possibly attenuating the relationship among the variables. Future studies may aim to further reduce measurement error in assessing lifestyle factors by using objective measures as well as further utilizing the measurement model aspect of SEM. This feature requires multiple assessments or multiple measures of the construct of interest.
This investigation supported the notion that BMI mediates the relationship between lifestyle factors (an atherogenic diet, low aerobic fitness), and major and conditional CHD risk factors (lipid profile) in an adolescent sample. In light of recent reports of increasing CHD risk factors in youth (CDC, 2005a,b; Eisenmann et al., 2002), there is a need to continue understanding the mechanisms underlying CHD development, and to determine efficacious and effective primary prevention interventions for youth. Future research may continue expanding upon the model examined, and investigating interventions targeting modifiable predisposing CHD risk factors.
Acknowledgments
This investigation was supported by grants from the National Institute of Health (P01HL36588, T32HL07426, and 5M01RR016587). We wish to extend our gratitude to all staff, graduate students, and undergraduate students who assisted with recruitment and data collection. In addition, we are indebted to the participants and their families.
Conflicts of interest: None declared.
References
- Alliance for a Healthier Generation. Clinton Foundation, American Heart Association, Robert Wood Johnson Foundation to help schools create a healthier environment for nation's students. 2006 Retrieved May 26, 2006, from http://www.healthiergeneration.org/schools_release.html.
- Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth. Diabetes Care. 2004;27:547–552. doi: 10.2337/diacare.27.2.547. [DOI] [PubMed] [Google Scholar]
- Balogh M, Khan HA, Random JH. Random repeat 24-hour dietary recalls. American Journal of Clinical Nutrition. 1971;24:304–310. doi: 10.1093/ajcn/24.3.304. [DOI] [PubMed] [Google Scholar]
- Berenson GS, Srinivasan SR, Cresanta JL, Foster TA, Webber LS. Dynamic changes of serum lipoproteins in children during adolescence and sexual maturation. American Journal of Epidemiology. 1981;113:157–170. doi: 10.1093/oxfordjournals.aje.a113080. [DOI] [PubMed] [Google Scholar]
- Block G. A review of validations of dietary assessment methods. American Journal of Epidemiology. 1982;115:492–505. doi: 10.1093/oxfordjournals.aje.a113331. [DOI] [PubMed] [Google Scholar]
- Boreham C, Twisk J, Murray L, Savage M, Strain JJ, Cran G. Fitness, fatness, and coronary heart disease risk in adolescents: The Northern Ireland Young Hearts Project. Medicine and Science in Sports and Exercise. 2001;33:270–274. doi: 10.1097/00005768-200102000-00016. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (CDC; 2002) 2000 CDC Growth Charts for the United States: Methods and Development. Vital and Health Statistics Series 11, No. 246. Retrieved May 28, 2007, from http://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf. [PubMed]
- CDC. Prevalence of overweight among children and adolescents: US, 1999–2002. National Center for Health Statistics Health and Stats. 2005a Retrieved February 13, 2005, from http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overwght99.htm.
- CDC. Nutrition and the Health of Young People. Atlanta, GA: 2005b. Retrieved February 13, 2005, from http://www.cdc.gov/healthyyouth/nutrition/pdf/facts.pdf. [Google Scholar]
- Corbin CB, Pangrazi RP. Are American children and youth fit? Research Quarterly for Exercise and Sport. 2002;63:96–106. doi: 10.1080/02701367.1992.10607566. [DOI] [PubMed] [Google Scholar]
- Cruz ML, Bergman RN, Goran MI. Unique effect of visceral fat on insulin sensitivity in obese Hispanic children with a family history of type 2 diabetes. Diabetes Care. 2002;25:1631–1636. doi: 10.2337/diacare.25.9.1631. [DOI] [PubMed] [Google Scholar]
- Daniels SR, Morrison JA, Sprecher DL, Khoury P, Kimball TR. Association of body fat distribution and cardiovascular risk factors in children and adolescents. Circulation. 1999;99:541–545. doi: 10.1161/01.cir.99.4.541. [DOI] [PubMed] [Google Scholar]
- de Ridder CM, de Boer RW, Seidell JC, Nieuwenhoff CM, Jeneson JAL, Bakker CJG, et al. Body fat distribution in pubertal girls quantified by magnetic resonance imaging. International Journal of Obesity. 1992;16:443–449. [PubMed] [Google Scholar]
- Deurenberg P, Pieters JJL, Hautvast GAJ. The assessment of the body fat percentage by skinfold thickness measurements in childhood and young adolescence. British Journal of Nutrition. 1990;63:293–303. doi: 10.1079/bjn19900116. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann JC. Secular trends in variables associated with the metabolic syndrome of North American children and adolescents: A review and synthesis. American Journal of Human Biology. 2003;15:786–794. doi: 10.1002/ajhb.10214. [DOI] [PubMed] [Google Scholar]
- Eisenmann JC, Bartee T, Wang MQ. Physical activity, TV viewing, and weight in U.S. Youth: 1999 Youth Risk Behavior Survey. Obesity Research. 2002;10:379–385. doi: 10.1038/oby.2002.52. [DOI] [PubMed] [Google Scholar]
- Eisenmann JC, Womack CJ, Reeves MJ, Pivarnik JM, Malina RM. Blood lipids of young distance runners: Distribution and inter-relationships among training volume, peak oxygen consumption, and body fatness. European Journal of Applied Physiology. 2001;85:104–112. doi: 10.1007/s004210100445. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: The Bogalusa Heart Study. American Journal of Clinical Nutrition. 1999;69:308–317. doi: 10.1093/ajcn/69.2.308. [DOI] [PubMed] [Google Scholar]
- Goran MI. Metabolic precursors and effects of obesity in children: A decade of progress, 1990–1999. American Journal of Clinical Nutrition. 2001;73:158–171. doi: 10.1093/ajcn/73.2.158. [DOI] [PubMed] [Google Scholar]
- Goran MI, Kaskoun M, Shuman WP. Intra-abdominal adipose tissue in young children. International Journal of Obesity. 1995;19:279–283. [PubMed] [Google Scholar]
- Grundy SM, Bazzarre T, Cleeman J, D’Agostino RB, Hill M, Houston–Miller N, et al. Beyond secondary prevention: Identifying the high-risk patient for primary prevention. Medical office assessment. Circulation. 2000;101:e3–e11. doi: 10.1161/01.cir.101.1.e3. [DOI] [PubMed] [Google Scholar]
- Hill JO, Melanson EL. Overview of the determinants of overweight and obesity: Current evidence and research issues. Medicine and Science in Sports and Exercise. 1999;31:S515–S521. doi: 10.1097/00005768-199911001-00005. [DOI] [PubMed] [Google Scholar]
- Hoelscher DM, Mitchell P, Dwyer J, Elder J, Clesi A, Snyder P. How the CATCH Eat Smart program helps implement the USDA regulations in school cafeterias. Health Education and Behavior. 2003;30:434–446. doi: 10.1177/1090198103253517. [DOI] [PubMed] [Google Scholar]
- Hurwitz BE, Schneiderman N. Cardiovascular reactivity and its relation to cardiovascular disease risk. In: Krantz D, Baum A, editors. Technology and methodology in behavioral medicine. Mahwah, NJ: Lawrence Erlbaum Associates; 1998. pp. 245–273. [Google Scholar]
- Kahn BB, Flier JS. Obesity and insulin resistance. Journal of Clinical Investigation. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Wilson PW, Nam BH, D’Agostino RB. Risk stratification of obesity as a coronary risk factor. American Journal of Cardiology. 2002;90:697–701. doi: 10.1016/s0002-9149(02)02592-4. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Malina RM, Bouchard C. Physical activity, physical fitness, and coronary heart disease risk factors in youth: The Quebec Family Study. Preventive Medicine. 1999;29:555–562. doi: 10.1006/pmed.1999.0592. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS. Body mass index, waist circumference, and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics. 2004;114:e198–e205. doi: 10.1542/peds.114.2.e198. [DOI] [PubMed] [Google Scholar]
- Kwiterovich PO, Jr, Barton BA, McMahon RP, Obarzanek E, Hunsberger S, Simons–Morton D, et al. Effects of diet and sexual maturation on low-density lipoprotein cholesterol during puberty: The dietary intervention study in children. Circulation. 1997;96:2526–2533. doi: 10.1161/01.cir.96.8.2526. [DOI] [PubMed] [Google Scholar]
- Lauer RM, Obarzanek E, Hunsberger SA, Van Horn L, Hartmuller VM, Barton BA, et al. Efficacy and safety of lowering dietary intake of total fat, saturated fat, and cholesterol in children with elevated LDL cholesterol: The dietary intervention study in children. American Journal of Clinical Nutrition. 2000;72 doi: 10.1093/ajcn/72.5.1332s. [DOI] [PubMed] [Google Scholar]
- Linusson EEI, Sanjur D, Erickson EC. Validating the 24-hour recall as a dietary survey tool. Archivos Latinoamericanos de Nutricion. 1974;24:277. [Google Scholar]
- Lowry R, Brener N, Lee S, Epping J, Fulton J, Eaton D. Participation in high school physical education–United States, 1991-2003. Journal of School Health. 2005;75:47–49. [Google Scholar]
- Luepker RV, Perry CL, McKinlay SM, Nader PR, Parcel GS, Stone EJ, et al. Outcomes of a field trial to improve children's dietary patterns and physical activity. The child and adolescent trial for cardiovascular health. CATCH collaborative group. Journal of the American Medical Association. 1996;275:768–776. doi: 10.1001/jama.1996.03530340032026. [DOI] [PubMed] [Google Scholar]
- McKenzie TL, Sallis JF, Faucette N, Kolody B, Roby J. Effects of an in-service intervention on the quality and quantity of elementary classroom teachers’ physical education classes. Research Quarterly for Exercise and Sport. 1993;64:178–187. doi: 10.1080/02701367.1993.10608795. [DOI] [PubMed] [Google Scholar]
- Meigs JB, D’Agostino RB, Wilson PW, Cupples LA, Nathan DM, Singer DE. Risk variable clustering in the insulin resistance syndrome. The Framingham Offspring Study. Diabetes. 1997;46:1594–1600. doi: 10.2337/diacare.46.10.1594. [DOI] [PubMed] [Google Scholar]
- Morley R, Baker BA, Greene LC, Livingstone MBE, Harland PSEG, Lucas A. Dietary fibre, exercise and serum lipids and lipoprotein cholesterols in 12 to 15 year olds. Acta Paediatrica. 1998;87:1230–1234. doi: 10.1080/080352598750030889. [DOI] [PubMed] [Google Scholar]
- Múthen LK, Múthen BO. Mplus User's Guide. Los Angeles, CA: Múthen & Múthen; 1998. [Google Scholar]
- National Cholesterol Education Program. (NCEP, 2002) Detection, evaluation, and treatment of high blood cholesterol in adults (ATP III): Final report. Bethesda, MD: National Institutes of Health; [PubMed] [Google Scholar]
- Obarzanek E, Sacks FM, Vollmer WM, Bray GA, Miller ER, III, Lin P, et al. Effects on blood lipids of a blood-pressure lowering diet: the Dietary Approaches to Stop Hypertension (DASH) trial. American Journal of Clinical Nutrition. 2001;74:80–89. doi: 10.1093/ajcn/74.1.80. [DOI] [PubMed] [Google Scholar]
- Ornish D, Sherwitz LW, Billings JH, Gould KL, Merritt TA, Sparler S, et al. Intensive lifestyle changes for reversal of coronary heart disease. Journal of the American Medical Association. 1998;280:2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- Pate RR, Ward DS, Saunders RP, Felton G, Dishman RK, Dowda M. Promotion of physical activity among high-school girls: A randomized controlled trial. American Journal of Public Health. 2005;95:1582–1587. doi: 10.2105/AJPH.2004.045807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer AM. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Raitakari OT, Porkka KVK, Rasanen L, Viikari JSA. Relations of life-style with lipids, blood pressure, and insulin in adolescents and young adults. The cardiovascular risk in young Finns study. Atherosclerosis. 1994;111:237–246. doi: 10.1016/0021-9150(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Rockett HRH, Colditz GA. Assessing diets of children and adolescents. American Journal of Clinical Nutrition. 1997;65:1116S–1122S. doi: 10.1093/ajcn/65.4.1116S. [DOI] [PubMed] [Google Scholar]
- Sangi H, Mueller WH. Which measure of body fat distribution is best for epidemiologic research among adolescents? American Journal of Epidemiology. 1991;133:870–883. doi: 10.1093/oxfordjournals.aje.a115967. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Buono MJ, Roby JJ, Micale FG, Nelson JA. Seven-day recall and other physical activity self-reports in children and adolescents. Medicine and Science in Sports and Exercise. 1993;25:99–108. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, et al. Physical activity assessment methodology in the five-city project. American Journal of Epidemiology. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Owen N. Physical activity and behavioral medicine. Thousand Oaks, CA: Sage; 1999. [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Tolfrey K, Campbell IG, Jones AM. Selected predictor variables and the lipid-lipoprotein profile of prepubertal girls and boys. Medicine and Science in Sports and Exercise. 1999;31:1550–1557. doi: 10.1097/00005768-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Tuomilethto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne–Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine. 2001;344:1343–1392. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Van Staveren T, Dale D. Childhood obesity: Problems and solutions. Journal of Physical Education, Recreation & Dance. 2004;75:44–54. [Google Scholar]
- Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, et al. Prediabetes in obese youth: A syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. The Lancet. 2003;362:951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerstahl M, Barnekow–Bergkvist M, Hedberg G, Jansson E. Secular trends in body dimensions and physical fitness among adolescents in Sweden from 1974 to 1995. Scandinavian Journal of Medicine and Science in Sports. 2003;13:128–137. doi: 10.1034/j.1600-0838.2003.10274.x. [DOI] [PubMed] [Google Scholar]
- Women's Intervention Nutrition Study. (Version March 23, 1993) New York: American Health Foundation; A clinical trial to determine the efficacy of dietary fat reduction, provided in addition to systemic adjuvant therapy, in the management of patients with primary breast cancer. [Google Scholar]
- Yensel CS, Preud’Homme D, Curry DM. Childhood obesity and insulin-resistant syndrome. Journal of Pediatric Nursing. 2004;19:238–246. doi: 10.1016/j.pedn.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Yu L, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: Conventional criteria vs. new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]