Abstract
Objective To describe the development of young boys with fragile X syndrome (FXS). Methods Fifty-five boys (aged 8–48 months at study entry) with the full mutation FXS received multiple developmental assessments. Results As expected, the boys’ rate of development was significantly lower than chronological age expectations. No evidence of slowing in the rate of development was found. Autistic behavior was negatively associated with development, but maternal IQ was not. Developmental delays were evident in some domains as early as 9 months; however, initial detection of delays is complicated by measures and criteria used. Developmental age scores at 31 months of age were related to scores obtained at 61 months of age only in the global composite and visual reception domain. Conclusions Developmental delays are evident in some infants with FXS as young as 9 months of age. Pediatric psychologists need to be informed about the developmental profiles in young children with FXS to accurately diagnose, treat, and support these children and their families.
Keywords: cognitive development, fmr1, fragile X
Introduction
Developmental delays affect 12–17% of the general pediatric population (Glascoe, 2000). However, only 20–30% of the children with disabilities are identified prior to school entry, which suggests that early identification needs to be improved (AAP, 2006; Sand et al., 2005). Fragile X syndrome (FXS) is the most common inherited cause of delay affecting 1 in 4,000 males and 1 in 8,000 females (Crawford, Acuna, & Sherman, 2001). FXS can be accurately diagnosed prenatally or at birth by genetic testing but the average age of diagnosis is 32 months due to a number of barriers (Bailey, Skinner, & Sparkman, 2003) including a lack of information regarding the phenotype of FXS during the early years. Earlier identification would provide access to early intervention, help tailor specific health or educational treatments, identify recurrence risk in siblings, and provide family support to optimize outcomes (Bailey et al., 2003; Srour, Mazer, & Shevell, 2006).
The full mutation of FXS results from an expansion of ≥200 CGG repeats on the X-linked FMR1 gene. Reduction in fragile X mental retardation protein (FMRP) is associated with increased clinical involvement (Bailey, Hatton, Skinner, & Mesibov, 2001; Hatton et al., 2006). Due to random X chromosomal inactivation, females are more variably affected; approximately 50% display cognitive deficits and the remainder manifest mild to no cognitive or behavioral effects. Most males with the full mutation have a moderate intellectual disability, attention problems, and elevated risk for other co-occurring conditions (Bailey, Raspa, Olmsted, & Holiday, 2008), but these features are not evident at birth. A decline in IQ standard scores (not loss of skill) has been documented (Bailey, Hatton, & Skinner, 1998; Skinner et al., 2005), but the age at which the decline is first evident is unclear.
A co-morbid diagnosis of autism occurs in at least 30% of children with FXS (Rogers, Wehner, & Hagerman, 2001) with recent evidence that autistic behavior increases over time (Hatton et al., 2006). A diagnosis of autism or the presence of elevated autistic behavior, regardless of meeting diagnostic criteria, is associated with poor developmental outcome (Hatton et al., 2006; Rogers et al., 2001). Likewise, maternal IQ and education have been examined as predicting developmental outcome in children with FXS, given that that mothers of boys with FXS could have the full mutation themselves or could be affected by subtle learning difficulties documented in females with the premutation (Minquez et al., 2008). While preliminary, this work has shown that maternal education is related to academic achievement (Roberts et al., 2005), and parental IQ is associated with performance IQs in school-aged boys with FXS and full scale IQs in girls with FXS (Dyer-Friedman et al., 2002). In contrast, maternal education does not appear related to the nonverbal intelligence in school-aged children with FXS (Skinner et al., 2005).
Research on young children with FXS is sparse. Since most children are not identified until nearly 3 years of age, it has been difficult to find an adequate sample of very young children. Some studies report average or borderline development (Freund, Peebles, Aylward, & Reiss, 1995; Hagerman et al., 1994) while others report moderate delays during the early childhood years (Bailey et al., 1998; Roberts, Hatton, & Bailey, 2001). Only three published studies include infants 12 months of age or younger. A longitudinal study of 26 boys with FXS (four were 12 months at entry) found that global developmental delays were evident as early as 12 months with language skills most delayed (Roberts et al., 2001). Skills increased with age, and developmental scores at early ages were correlated with scores at older ages. A longitudinal study using developmental screening measures with 13 boys with FXS at 9, 12, and 18 months of age reported that scores on the Denver II identified 91% of the boys as delayed at 9 months of age and 100% as delayed at both 12 and 18 months of age (Mirrett, Bailey, Roberts, & Hatton, 2004). In a retrospective video analysis of sensory-motor features of 12-month-old infants with FXS (n = 11) when compared to age and developmental level matched controls with autism without FXS (n = 11), nonspecific developmental delay (n = 10), and typically developing children (n = 11), infants with FXS were distinguishable by their lack of object play and increased leg stereotypes (Baranek et al., 2005). Sensory-motor features strongly predicted early developmental milestones (e.g., level of object play predicted age of walking).
These results suggest that developmental delays may be detectable in very young children with FXS as early as the first year of life. However, further research is needed to determine when developmental delays are evident and the form in which they are expressed. Such information could help pediatric professionals refer children for FXS testing (Visootsak, Warren, Anido, & Graham, 2005). Furthermore, given the recent policy statement by the American Academy of Pediatrics that developmental screening tests be administered at the 9-, 18-, and 30-month well-child visits (Council on Children with Disabilities, 2006), it is important to examine if development in infants with FXS is clearly delayed by 9 month of age. If not, these infants are at risk for not being identified until the subsequent screening interval at 18 or 30 months. Additionally, in the absence of the diagnosis of FXS, which allows eligibility for early intervention based on an established condition, children must demonstrate a significant delay to meet criteria for early intervention services (IDEA, 2004 632[5][A]). Individual states have latitude in the determination of eligibility criteria and great variability across states currently exists. However, two primary means for defining a developmental delay include (a) a 25% delay in at least one area of development, and (b) 2 SD(s) below the mean on a norm-referenced instrument.
While existing work provides important preliminary information about the phenotype of FXS during the first years of life, it has included small samples focused on a narrow age range with few assessments with children under 2 years of age, and failed to include predictors of development. Furthermore, there has been a reliance on screening measures that have elevated false-positive rates of developmental delay and are limited in providing detailed developmental information upon which to base phenotypic-specific profiles. Needed are studies with larger samples using comprehensive measures of development in a prospective longitudinal design.
The primary aim of this study is to describe the trajectories and examine predictors of development for boys with FXS during the first 5 years of life. We hypothesized that young children with FXS would demonstrate stable developmental gains over the first 5 years of life, that language skills would be less well developed than other domains, and that development would be negatively affected by increased autistic behaviors and lowered maternal IQ. Secondary aims of this study are to identify at what age development is clearly delayed and to determine the extent to which early developmental scores are associated with later measures. We hypothesize that development will be clearly delayed by 12 months of age. Furthermore, we predict that early developmental scores will be moderately related to later developmental scores.
Method
Data were drawn from two independent, yet related, longitudinal studies of early development, using a comprehensive measure, the Mullen Scales of Early Learning (MSEL; Mullen, 1995). The first study (Mirrett et al., 2004) included 13 males with FXS assessed at 9, 12, and 18 months of age. The second study focused on family adaptation (Bailey, Sideris, Roberts, & Hatton, 2008) and included 45 males with FXS whose initial age was between 12 and 38 months, with three assessments completed at 18-month intervals. Eleven boys participated in both studies. Combining the data across these two studies increased the sample size, allowing for more confidence in the findings. The number of assessments also increased from a maximum of three (per study) up to six (combining studies) and allowed us to examine development from 9 to 68 months in 10 participants. Although participants entered and exited the two studies at different ages and were assessed at different intervals, we used hierarchical linear modeling, an analytic technique that takes into consideration variability in both the number and timing of assessments and allows for the modeling of growth curves over time.
Participants
Participants were 55 boys, aged from 8 to 68 months, with full mutation (>200 CGG repeats) FXS as verified by genetic report. The children entered the study at various ages: ten entered between 8 and 9 months of age, seven entered between 10 and 12 months of age, 6 entered between 16 and 21 months of age, ten entered between 22 and 30 months of age, eighteen entered between 31 and 38 months of age, and four entered at between 40 and 48 months of age. All participants had two or more developmental assessments as part of their participation in the longitudinal studies (six children had two assessments, 49 children had three or more). Data included a total of 189 assessments (M = 3.44 per child, range of 2–7). Fifty-one assessments were conducted with children 24 months and younger. Because of the difficulty in finding very young children, participants had been drawn from around the United States and were recruited through our existing and completed studies (Bailey et al., 2008/∼FXS Registry), FXS family support groups (http://www.fragilex.org/html/links.htm), and an FXS parent list serve. The majority (N = 48/55) were European Americans, and the median household income was $40,344 (SD = 25,933, range = 10,000–100,000). All mothers were the biological parent of the participant and a carrier of FXS (one had the full mutation). The study was approved by the University of North Carolina Institutional Review Board, and parents provided written consent.
Measures
Development
The MSEL was selected as the primary measure of development because of the broad age range covered (birth = 68 months), excellent reliability and validity with high correlations (r = 0.70) with the Bayley's Mental Development Index. The MSEL includes fine motor (FM), receptive language (RL), expressive language (EL), and visual reception (VR) domains allowing for a differentiated view of development. Age equivalent scores are generated for each domain. Although an early learning composite standard score can be generated based on the raw scores of the four domains, 88% (166/189) of the composite standard scores of our sample fell at or near the floor of 49, severely limiting our ability to detect meaningful differences using that metric. We therefore created a global developmental age score by averaging the age equivalents across the four MSEL domains, as reported in other studies (Humphrey, Williams, Pinto, & Bolton, 2004; Shanahan, Roberts, Hatton, Reznick, & Goldsmith, 2008). The global developmental age and domain developmental ages were the dependent variables in this study.
Predictors of Development
The relationship of autistic behavior and maternal intelligence to development was examined. The Childhood Autism Rating Scale (CARS; Schopler, Reichler & Renner, 1988), an examiner rating of autistic behavior that represents a continuum of autistic behaviors, was completed through consensus of the two examiners as part of the standard protocol that included the MSEL. The most recent CARS score was used due to documented age effects (Hatton et al., 2006). The mean CARS score was 27.61 (SD = 5.74, range = 18–40). Consistent with our previous work and other reports (Hatton et al., 2006; Kaufmann et al., 2004), a large proportion of boys displayed one or more autistic behaviors and 31% scored in the mild to severe autistic range of the CARS. Maternal intelligence was assessed using the two-subtest standard abbreviated version (Vocabulary and Matrix Reasoning) of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). The mean maternal IQ was 109.26 (SD = 12.96, range = 73–131).
Procedure
Participants were assessed using the standardized protocol designed for each of the two studies. For both studies, the assessments spanned 2 days to maximize child compliance, and two examiners were present to facilitate consistency of administration and scoring for the various measures. The majority of infant assessments were conducted by PhD level clinicians (authors JER and PLM); the remainder was conducted by trained research associates, most of them had or were pursuing graduate degrees.
Data Analysis
Descriptive analyses and correlations were used to describe the sample. Hierarchical linear models (HLM; SAS Institute, 2003) were run to examine the longitudinal data and predictor variables. Regression models, simple correlations, and t-tests were used to examine the relationship between the early and the final developmental scores and to detect the age at which development differed significantly from chronological age expectations. The Benjamini–Hochberg procedure adjusted for multiple comparisons by allowing exact control of the false discovery rate (0.05 for these analyses) without the accompanying loss of power that characterizes methods for controlling the family-wise error such as Bonferroni (Benjamini & Hochberg, 1995).
Results
Developmental Trajectory of Global and Domain Scores: Rate, Stability, and Predictors
A series of HLM models through SAS Proc Mixed were run to address the research questions regarding the developmental trajectories during the first 5 years, the stability of development over time, variation across developmental domains, and predictors of development. In the first set of HLM models, the global and domain scores were predicted as a function of chronological age and included high order functions (quadratic and cubic) for age. These functions were not significant (all p > .05), suggesting that the rate of change in development is stable and a decline in development is not evident in this age range. The linear effect of age was significant (p < .001) for all outcomes, but the slopes were less than one indicating that developmental age scores increased more slowly than chronological age. This finding is explored and tested more fully in the second set of models.
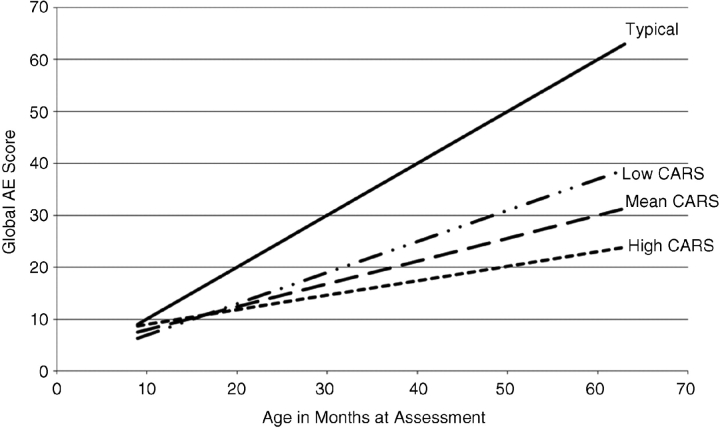
In the second set of models, the global and domain scores were predicted as a function of age, CARS total score and maternal IQ as well as the interactions of age with CARS maternal IQ (Table I). Correlations among the variables were all near zero except maternal IQ and CARS score, which was under 0.3. First, a model was run on the global score. Results indicated that the global score increased over time at a rate of approximately 5 months of developmental gain for every 12-month time period. An interaction with CARS and Age indicates that participants with less autistic behavior display a higher slope/faster rate of development as they age (Figure 1). Maternal IQ did not predict development.
Table I.
Hierarchical Linear Modeling for MSEL Global and Domain Scores
| Parameter estimates (SE) | Lower CL | Upper CL | |
|---|---|---|---|
| MSEL composite | |||
| Chronological age | 0.45 (0.01)*** | 19.88 | 21.81 |
| Mom IQ | −0.02 (0.04) | 0.42 | 0.48 |
| CARS total | −0.60 (0.09)*** | −0.77 | −0.42 |
| Composite age × Mom IQ | 0.00 (0.00) | −0.001 | 0.004 |
| Composite age × CARS total | −0.03 (0.00)*** | −0.03 | −0.02 |
| MSEL developmental domain | |||
| Domain | F = 16.54*** | ||
| Chronological age | 0.45 (0.02)*** | 0.41 | 0.48 |
| Mom IQ | −0.03 (0.04) | −0.11 | 0.06 |
| Cars total | −0.68*** | −0.88 | −0.48 |
| Chronological age × Domain | F = 15.15*** | ||
| Chronological age × Mom IQ | 0.00 (0.00) | −0.001 | 0.004 |
| Chronological age × CARS Total | −0.03*** | −0.04 | −0.02 |
| Mom IQ × Domain | F = 0.47 | ||
| Cars total × Domain | F = 5.66*** | ||
| Mom IQ × CARS total × Domain | F = 0.64 |
MSEL Global, the mean of the four age equivalent scores from the Mullen Scales of Early Learning domains; maternal IQ, Wechsler Abbreviated Scale of Intelligence; CARS, the total score from the Childhood Autism Rating Scale; MSEL domain, the age equivalent score from the receptive language, expressive language, visual reception, and fine motor domains. Parameter estimates are not provided for categorical effects.
***p < .001.
Figure 1.
Global Age Equivalent by Chronological Age and CARS Scores. Note: Mean CARS is 27.61, Low CARS is 1 SD below the mean 21.87, and High CARS is 1 SD above the mean 33.35.
A model was run on the domain scores and included the effects of the predictors. To test this, a HLM was fit that included a variable indicating domain (VR, FM, RL, and EL) as a predictor so differential effects across domains could be tested. This model also allows separate parameter estimates to be computed for each of the domains. Similar to the previous model with the global score, the domain scores each increased over time, but at different rates across domains. The rate of growth in fine motor was significantly slower than all other domains (all p < .001), and the rate of growth on the receptive communication domain was faster than in the expressive communication domain (p = .005). An interaction with CARS and age was evident for all domains; however, CARS scores had more effect on the FM domain than any of the other three domains (all p < .01). Maternal IQ did not predict development in any of the domains.
Early Developmental Scores Predictive of Later Developmental Outcome
To examine the relationship of early to late scores on the MSEL, we ran a series of correlations and multiple regression models on the full sample and then on a restricted sample (Table II). The restricted sample included only children who had assessment intervals of 12 months or greater, to control for the significant variability in time between assessments and due to our interest in examining stability of scores over as long a period of time as was feasible with the data. Furthermore, we restricted the data to early assessments at 12 months of age and older to reduce the significant variability in age at early assessment and to reduce error associated with the instability of developmental assessments during infancy. In this restricted sample (N = 38), the average age at early assessment was 31 months (SD = 7.39, range = 16–45), the average age at final assessment was 61 months (SD = 7.73, range = 46–77), and the average assessment interval was 30 months (SD = 9.05, range = 12–48). Results focus on the restricted sample with reference to results with the full sample.
Table II.
Mullen Scales of Early Learning Developmental Age Equivalents for Participants over Time
| Chronological age cluster in months (range)a Sample size | 9 (8–10) n = 11 | 12 (11–12) n = 17 | 18 (16–21) n = 19 | 24 (22–30) n = 17 | 36 (31–39) n = 41 | 48 (40–53) n = 42 | 60 (54–68) n = 44 |
|---|---|---|---|---|---|---|---|
| Globalb | |||||||
| M | 7.11 | 9.04 | 11.76 | 15.62 | 19.48 | 25.22 | 31.11 |
| SD | 1.37 | 2.37 | 3.16 | 5.38 | 5.38 | 5.65 | 10.06 |
| 25% delay | 55% | 53% | 83% | 94% | 94% | 98% | 100% |
| Visual reception | |||||||
| M | 7.18 | 8.94 | 12.89 | 16.76 | 20.93 | 27.10 | 28.73 |
| SD | .87 | 1.95 | 3.65 | 4.34 | 5.70 | 6.43 | 7.78 |
| 25% delay | 27% | 53% | 72% | 82% | 94% | 98% | 100% |
| Fine motor | |||||||
| M | 8.09 | 10.53 | 14.05 | 17.47 | 19.56 | 24.67 | 25.18 |
| SD | 1.87 | 1.70 | 2.09 | 2.65 | 3.42 | 6.27 | 8.49 |
| 25% delay | 18% | 29% | 39% | 94% | 100% | 100% | 98% |
| Receptive language | |||||||
| M | 7.00 | 8.94 | 10.68 | 14.53 | 19.27 | 27.21 | 28.64 |
| SD | 1.41 | 1.56 | 2.60 | 4.47 | 6.98 | 8.83 | 9.82 |
| 25% delay | 36% | 59% | 89% | 94% | 91% | 93% | 91% |
| Expressive language | 24.68 | ||||||
| M | 6.18 | 7.76 | 9.42 | 13.71 | 18.17 | 24.55 | |
| SD | 1.60 | 1.48 | 2.63 | 3.67 | 7.72 | 9.72 | 11.07 |
| 25% delay | 73% | 82% | 94% | 100% | 94% | 93% | 95% |
aAge clusters reflect a range of ages given that participants were assessed across different ages. bMSEL Global is the mean of the four age equivalent scores from the Mullen Scales of Early Learning domains.
Results from the correlation matrix of the restricted sample (Table III) indicate that the early global score was moderately related to the final global score, r = 0.50, p = < .01 and the final scores of all four domains (all p < .05). Likewise, the early scores from all four domains were related to the final global score (all p < .05). Within the domains, the early VR and RL scores were moderately related to all four final domain scores, the early FM score was moderately related to the final VR, FM and RL domains, and the early EL score was moderately related to the final VR, FM and EL domains. These results closely mirror the correlations with the full and unrestricted sample with two minor changes. The relationship of early to final scores in the FM domain was strengthened (p < .05) in the restricted sample while the relationship of early scores in EL to final scores in RL was reduced (p > .05).
Table III.
Inter-correlation Matrix of Early to Final Developmental Age Scores (N = 38)
| Early global | Final global | Early VR | Final VR | Early FM | Final FM | Early EL | Final EL | Early RL | Final RL | |
|---|---|---|---|---|---|---|---|---|---|---|
| Early global | 1.00 | |||||||||
| Final global | 0.50** | 1.00 | ||||||||
| Early VR | 0.93*** | 0.56*** | 1.00 | |||||||
| Final VR | 0.47** | 0.95*** | 0.56*** | 1.00 | ||||||
| Early FM | 0.79*** | 0.42** | 0.76*** | 0.43** | 1.00 | |||||
| Final FM | 0.52*** | 0.90*** | 0.59*** | 0.88*** | 0.46** | 1.00 | ||||
| Early EL | 0.91*** | 0.39* | 0.76*** | 0.32* | 0.55*** | 0.38* | 1.00 | |||
| Final EL | 0.45** | 0.88*** | 0.45** | 0.74*** | 0.30 | 0.70*** | 0.44** | 1.00 | ||
| Early RL | 0.93** | 0.44** | 0.80*** | 0.41* | 0.66*** | 0.46** | 0.83*** | 0.39* | 1.00 | |
| Final RL | 0.44** | 0.95*** | 0.51** | 0.91*** | 0.37* | 0.78*** | 0.31 | 0.80*** | 0.39* | 1.00 |
Global, the mean of the four age equivalent scores from the Mullen Scales of Early Learning domains; VR, visual reception; FM, fine motor, EL, expressive language; RL, receptive language.
*p < .05; **p < .01; and ***p < .001.
Next, a series of linear regression models were run separately for the global score and each domain in the restricted sample to examine the effects of age and CARS. Results indicate that chronological age at the final assessment and the CARS score strongly predicted the global and all domain final scores (Table IV). Of note, the relationship between the early and the final developmental age scores was not dependent on the chronological age of first assessment (e.g., assessments at early ages were not poorer predictors of final assessments than early assessments at older ages). Consistent with the correlations, the early global score predicts the final global score (F = 5.96, p = .020), and the early VR domain score predicts the final VR score (F = 8.55, p = .006). However, the early FM, EL, and RL scores were not related to their final scores suggesting that age and CARS scores are the primary predictors of the relationship of early to late scores in these three domains. Regression models with the restricted sample are consistent with analyses with the full and unrestricted sample in that the early VR domain scores predicted the final VR score and the early global score predicted the final global score, although it was reduced to a trend level (p = .098).
Table IV.
Summary of Linear Regression Models of Early to Final Developmental Age Scores (N = 38) [Beta Coefficients and Confident Intervals (CI) from Individual MSEL Global and Domain Score Models]
| Parameter | Global B | CI | VR B | CI | FM B | CI | EL B | CI | RL B | CI |
|---|---|---|---|---|---|---|---|---|---|---|
| Initial score | 0.60* | (0.24 to 1.02) | 0.65** | (0.26 to 1.08) | −0.42 | (−0.09 to 0.96) | 0.54 | (−0.03 to 1.04) | 0.24 | (−0.22 to 0.79) |
| Initial chronological age | −0.25 | (−0.94 to 0.30) | 0.00 | (−0.82 to 0.55) | −0.44 | (−0.20 to 1.09) | −0.39 | (−1.26 to 0.21) | −0.50 | (−1.29 to 0.36) |
| Final chronological age | 0.53*** | (0.44 to 0.73) | 0.49*** | (0.42 to 0.76) | 0.46*** | (0.31 to 0.60) | 0.45* | (0.31 to 0.79) | 0.65*** | (0.46 to 0.90) |
| CARS total | −1.08*** | (−1.32 to −0.76) | −1.02*** | (−1.28 to 0.64) | −0.89*** | (−1.14 to −0.61) | −1.09*** | (−1.49 to −0.65) | −1.39*** | (−1.72 to −0.94) |
| Initial score × Initial age | −0.01 | (−0.04 to 0.04) | −0.03 | (−0.06 to 0.02) | 0.03 | (−0.06 to 0.01) | −0.001 | (−0.05 to 0.07) | −0.02 | (−0.04 to 0.07) |
| Model R2 | 0.81 | 0.78 | 0.76 | 0.62 | 0.73 |
Global, the mean of the four age equivalent scores from the Mullen Scales of Early Learning domains; VR, visual reception; FM, fine motor; EL, expressive language; RL, receptive language; CARS, the total score from the Childhood Autism Rating Scale. Each column represents a separate regression model.
*p < .05, **p < .01, and ***p < .001.
Age at Which Development is Delayed
The observed developmental age equivalent of our sample was compared to the expected age (e.g., a typically developing 9-month-old is expected to have a developmental age equivalent of 9 months). The difference between the scores was tested as a one-sample t-test within the mixed model. Results indicate that the mean global, t(118) = 2.26, p = .01, receptive language domain, t(125) = 2.92, p = .002, and expressive language domain, t(113) = 3.44, p ≤ .001 developmental age scores were below expectation when the participants were 9 months old. The mean visual reception domain was below expectation by 10 months of age, t(120) = 2.23, p = .01, and the mean fine motor domain was below expectation by 13 months of age, t(123) = 2.34, p = .01.
In addition to t-tests based on group means, we examined the proportion of infants meeting criteria for developmental delay to allow us to examine individual differences and consider different criteria for detecting delays. Based on the criteria of a 25% delay in their global score, 55% of 9-month-old, 53% of 12-month-old, and 83% of 18-month-old met this criterion with rates of 94% or higher from 24 months of age and older. Based on the criteria of a 2 SD difference from the mean in their composite standard score, 9% of 9-month-olds, 53% of 12-month-olds, and 84% of 18-month-olds met this criterion with rates of 94% or higher from 24 months of age and older.
Discussion
Consistent with our hypotheses, boys with FXS aged 8–68 months exhibited global developmental delays. The rate of development appeared stable over time with no evidence of a decline during this age period. Developmental domain differences were evident, with fine motor skills more delayed than all other domains, and expressive language more delayed than receptive language. As expected, the degree of autistic behavior was a strong predictor of developmental outcome for the global and domain scores, such that children with less autistic behavior displayed a faster rate of development over time. This suggests that children with elevated autistic behavior, regardless of meeting stringent diagnostic criteria for autism, may benefit from recognition and treatment of these autistic or autistic-like behaviors (e.g., gaze aversion and tactile sensitivity).
Maternal IQ, in contrast, did not predict development. This was somewhat unexpected given evidence that parental IQ appears related to child IQ in school-aged boys and girls with FXS (Dyer-Friedman et al., 2002). Yet, the IQ of mothers of preschool-aged children with FXS does appear related to their maternal interaction style, and positive maternal interactions have been associated with higher developmental composite scores in preschool-aged children with FXS (Sterling, Brady, Warren, Fleming, & Marquis, 2006). This suggests that maternal IQ may serve as an indirect or meditational variable that influences other more discreet behaviors, such as maternal responsivity, that have a more primary influence during the first 5 years in children with FXS.
Support for a relationship between the early and the late scores was evident; however, only in select domains. Specifically, early scores at 31 months of age in fine motor, expressive language, and receptive language domains were not related to scores obtained at 61 months of age in these three domains. Age and the degree of autistic behavior were, however, powerful predictors of these scores. Given that deficient communication skills is one of the core features of autism, it is not surprising that the degree of autistic behavior strongly affected the relationship of early to later developmental skills in language domains. We are unclear about the strong relationship between the poor fine motor skills and the elevated autistic behavior. In contrast, early global and visual reception developmental age scores were predictive of later scores independent of age and the degree of autistic behavior. However, it should be noted that there is variability in development across time within and across our sample. For example, the only child whose development was in the average range at 5 years of age (i.e., standard score of 94 on the global composite of the MSEL) displayed significant delays at 9 months of age. Overall, our results suggest that developmental assessments at 31 months of age and older in young boys with FXS have limited predictive capacity so caution should be taken when interpreting developmental assessments in boys with FXS at very young ages.
Present results suggest that development is clearly delayed by 9 months of age in global, receptive language and expressive language scores. This was younger than we predicted and younger than any study using comprehensive developmental measures has reported. It should be noted that this was virtually the youngest age at which delays could be detected in our sample given that the youngest participants in our study were 8 months of age, and there were only 10 infants that were 9 months of age and younger. Thus, delays could be evident earlier than 9 months of age but that is beyond the scope of the current study.
While our data suggest that group mean levels of development appear delayed as early as 9 months of age, examination of individual scores using IDEA criteria imply that eligibility for early intervention services is a complex process largely influenced by the measures and criteria employed. Our current findings show that ∼50% of 9- and 12-month-old boys would be eligible for early intervention by meeting IDEA diagnostic criteria using a 25% delay in composite scores. This suggests that almost half of infant males with FXS would not be eligible for early identification services in their first year of life, and this estimate is likely conservative given that all of the 9- and 12-month-old children in our study had a positive family history so were not likely as severely delayed as children identified based on severity of delay alone. The likely scenario is that these young boys who do not meet strict IDEA criteria initially will be monitored and re-assessed, and our data suggest that the majority will meet criteria by 18 or 24 months of age (i.e., 84% and 94%, respectively). The implications of this scenario are a delay in early intervention services for young children, little support for families, and a delay in the diagnosis of FXS that typically occurs 1 year after a diagnosis of developmental delay, which increases the risk that families may have additional children that could also to be affected with FXS (Bailey et al., 2003).
Implications
Results suggest several diagnostic, treatment, and research implications. First, diagnosis of FXS with a positive family history is primarily determined by family disclosure and a pediatricians’ knowledge of the genetic basis and referral process for genetic testing for FXS. Once diagnosed with FXS, the child is immediately eligible for early intervention due to documentation of an existing condition regardless of the degree of current delay per IDEA guidelines. Implementation of medical management related to FXS, and support to families regarding the challenges in providing care for their child and consideration of family planning options can then be instituted. The current study informs this process by documenting that developmental delays may be subtle or absent in a substantial group of infants diagnosed with FXS, so referring practitioners are encouraged to refer for early intervention services independent of clear developmental delays to provide services to offset the occurrence and severity of delays in the early years.
Diagnosis of developmental delay and subsequent diagnosis of FXS in the absence of a family history during the first 18 months of life appears challenging. Given limited evidence that a FXS phenotype is distinct and recognizable in the first years of life, routine developmental screening for all young children as a standard component of best practice may be the best strategy to detect delays in infants with FXS and other conditions. Yet, even with universal implementation of best practice guidelines by the AAP of developmental screening at 9 months of age, almost half of infants with FXS aged 9–12 months of age might not meet eligibility criteria for services based on comprehensive developmental assessments. Thus, diagnosis of developmental delay is likely to continue to occur in the second year of life with a diagnosis of FXS occurring in the third year of life (Bailey et al., 2003). The timing of both diagnoses has consequences for children who miss out on services/medical management and families who face recurrent risk.
The diagnosis of autism in FXS is also of critical importance for several reasons. First, due to the high rate of co-morbid diagnoses of FXS and autism, FXS should be ruled out in children with autism. Likewise, a differential autism diagnosis should be conducted with all children with FXS to provide prognostic information and guide treatment efforts. Finally, due to the high level of autistic behavior and its impact on development, treatments known to be successful for children with autism should be considered an option for children with FXS regardless of an actual diagnosis of autism.
The research reported in this article has several important limitations. We do not know if the sample is representative of all children with FXS. No participants were younger than 8 months of age, precluding understanding of earlier developmental patterns. We relied on age equivalent scores that have known psychometric limitations, and the age at initial and final assessments varied as did the assessment intervals. Nonetheless, the findings add new information about early developmental patterns in FXS.
Future studies should include females and infants younger than 8 months of age. Measures of more discreet indices of cognition (e.g., visual attention) and psychosocial development (e.g., face processing) are needed in comparison to relevant control groups (e.g., mental age matches) to refine the phenotype of FXS during these early years, to determine if infants with FXS can be differentiated from other nonspecific or specific etiologic groups and to examine stability of the phenotype over time. This is particularly relevant given recent reports of an age-related shift in children with FXS from sensory hypo- to hyper-responsiveness across 9–54 months of age (Baranek et al., 2008) and blunted emotional reactivity in 3-year-old males that is inconsistent with reports of older aged children (Shanahan et al., 2008).
Funding
This research was supported by National Institute of Child Health and Human Development (P30-HD003110-35S1) and the Office of Special Education Programs, U.S. Department of Education (H324C990042).
Conflict of interest: None declared.
REFERENCES
- American Academy of Pediatrics, Council on Children With Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children with Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: An algorithm for developmental surveillance an screening. Pediatrics. 2006;107:1084–1094. [Google Scholar]
- Bailey DB, Jr, Hatton DD, Skinner M. Early developmental trajectories of males with fragile X syndrome. American Journal of Mental Retardation. 1998;103:29–39. doi: 10.1352/0895-8017(1998)103<0029:EDTOMW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism and Developmental Disorders. 2001;31:165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, Holiday D. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics Part A. 2008;146A:2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Sideris J, Roberts J, Hatton D. Child and genetic variables associated with maternal adaptation to fragile X syndrome: A multidimensional analysis. American Journal of Medical Genetics, Part A. 2008;146A:720–729. doi: 10.1002/ajmg.a.32240. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Skinner D, Sparkman KL. Discovering fragile X syndrome: family experiences and perceptions. Pediatrics. 2003;111:407–416. doi: 10.1542/peds.111.2.407. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Danko CD, Skinner ML, Bailey, DB, Hatton DD, Roberts JE, et al. Video analysis of sensory-motor features in infants with fragile X syndrome at 9–12 months of age. Journal of Autism and Developmental Disorders. 2005;35:645–656. doi: 10.1007/s10803-005-0008-7. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Roberts JE, David FL, Sideris J, Mirrett PL, Hatton DD, et al. Developmental trajectories and correlates of sensory processing in young boys with fragile X syndrome. Physical & Occupational Therapy in Pediatrics. 2008;28:79–98. doi: 10.1300/j006v28n01_06. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Council on Children with Disabilities, Section on Developmental Behavioral Pediatrics, Bright Futures Steering Committee, Medical Home Initiatives for Children with Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: An algorithm for developmental surveillance and screening. Pediatrics. 2006;118:405–420. doi: 10.1542/peds.2006-1231. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genetics in Medicine. 2001;3:359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer-Friedman J, Glaser B, Hessl D, Johnston C, Huffman LC, Taylor A, et al. Genetic and environmental influences on the cognitive outcomes of children with fragile X syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:237–244. doi: 10.1097/00004583-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Freund LS, Peebles CD, Aylward E, Reiss AL. Preliminary report on cognitive and adaptive behavior of preschool-aged males with fragile X. Developmental Brain Dysfunction. 1995;8:242–251. [Google Scholar]
- Glascoe FP. Detecting and addressing developmental and behavioral problems in primary care. Pediatric Nursing. 2000;26:251–257. [PubMed] [Google Scholar]
- Hagerman RJ, Hull CE, Safanda JF, Carpenter I, Staley LW, O’Connor RA, et al. High functioning fragile X males: Demonstration of an unmethylated fully expanded FMR-1 mutation associated with protein expression. American Journal of Medical Genetics. 1994;51:298–308. doi: 10.1002/ajmg.1320510404. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey, DB, Roberts J, et al. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics Part A. 2006;140:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Humphrey A, Williams J, Pinto E, Bolton PF. A prospective longitudinal study of early cognitive development in tuberous sclerosis: A clinic based study. European Child and Adolescent Psychiatry. 2004;13:159–165. doi: 10.1007/s00787-004-0383-1. [DOI] [PubMed] [Google Scholar]
- 2004. IDEA: Individuals with Disabilities Education Act. U.S. Department of Education, Office of Special Education Program 632[5][A] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. American Journal of Medical Genetics A. 2004;129:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Minquez M, Ibainez B, Ribate MP, Ramos F, Garcia-Alegria E, Fernandez-Rivas A, et al. American Journal of Medical Genetics, Part B, Neuropsychiatric Genetics. 2008. Risk of cognitive impairment in female premutation carriers of fragile X premutation: Analysis by means of robust segmented linear regression models. [DOI] [PubMed] [Google Scholar]
- Mirrett PL, Bailey, DB, Roberts JE, Hatton DD. Developmental screening and detection of developmental delays in infants and toddlers with fragile X syndrome. Journal of Development and Behavioral Pediatrics. 2004;25:21–27. doi: 10.1097/00004703-200402000-00004. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning: AGS Edition Manual. Circle Pines, MN: AGS; 1995. [Google Scholar]
- Roberts JE, Bailey DB, Jr, Mankowski J, Ford A, Sideris J, Weisenfeld LA, et al. American Journal of Medical Genetics. 2008. Mood and anxiety disorders in females with the FMR1 premutation. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Roberts JE, Hatton DD, Bailey DB. Development and behavior of male toddlers with fragile X syndrome. Journal of Early Intervention. 2001;24:207–223. [Google Scholar]
- Roberts JE, Schaaf JM, Skinner M, Wheeler A, Hooper S, Hatton DD, et al. Academic skills of boys with fragile X syndrome: Profiles and predictors. American Journal on Mental Retardation. 2005;110:107–120. doi: 10.1352/0895-8017(2005)110<107:ASOBWF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Development and Behavioral Pediatrics. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Sand N, Silverstein M, Glascoe FP, Gupta VB, Tonniqes TP, O’Connor KG. Pediatricians’ reported practices regarding developmental screening: do guidelines work? Do they help? Pediatrics. 2005;116:174–179. doi: 10.1542/peds.2004-1809. [DOI] [PubMed] [Google Scholar]
- SAS Institute. Cary, NC: SAS Institute; 2003. SAS System, Version 9. [Google Scholar]
- Schopler E, Reichler R, Renner B. The childhood autism rating scale (CARS) Los Angeles, CA: Western Psychological Servies; 1988. [Google Scholar]
- Shanahan ME, Roberts JE, Hatton DD, Reznick JS, Goldsmith H. Early temperament and negative reactivity in boys with fragile X syndrome. Journal of Intellectual Disability Research. 2008;52:842–854. doi: 10.1111/j.1365-2788.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- Skinner M, Hooper S, Hatton DD, Roberts J, Mirret P, Schaaf J, Sullivan K, Wheeler A, Bailey, DB Mapping nonverbal IQ in young boys with fragile X syndrome. American Journal of Medical Genetics. 2005;132:25–32. doi: 10.1002/ajmg.a.30353. [DOI] [PubMed] [Google Scholar]
- Srour M, Mazer B, Shevell MI. Analysis of clinical features predicting etiologic yield in the assessment of global developmental delay. Pediatrics. 2006;118:139–145. doi: 10.1542/peds.2005-2702. [DOI] [PubMed] [Google Scholar]
- Sterling A, Brady N, Warren S, Fleming K, Marquis J. San Diego: CA; 2006. Mar, What factors influence maternal responsivity in mothers of young children with fragile X syndrome? Poster session presented at the annual meeting of the Gatlinburg Conference on Research and Theory in Mental Retardation and Developmental Disabilities. [Google Scholar]
- Visootsak J, Warren ST, Anido A, Graham, JM Fragile X syndrome: An update and review for the primary pediatrician. Clinical Pediatrics. 2005;44:371–381. doi: 10.1177/000992280504400501. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]