Abstract
Objective
To compare the patterns of grey matter loss in subjects with amnestic Mild Cognitive Impairment (aMCI) who progress to Alzheimer's disease within a fixed clinical follow-up time versus those who remain stable.
Methods
Twenty-one aMCI subjects were identified from the Mayo Clinic Alzheimer's research program that remained clinically stable for their entire observed clinical course (aMCI-S), where the minimum required follow-up time from MRI to last follow-up assessment was three years. These subjects were age and gender-matched to 42 aMCI subjects who progressed to AD within 18 months of the MRI (aMCI-P). Each subject was then age and gender-matched to a control subject. Voxel-based morphometry (VBM) was used to assess patterns of grey matter atrophy in the aMCI-P and aMCI-S groups compared to the control group, and compared to each other.
Results
The aMCI-P group showed bilateral loss affecting the medial and inferior temporal lobe, temporoparietal association neocortex and frontal lobes, compared to controls. The aMCI-S group showed no regions of grey matter loss when compared to controls. When the aMCI-P and aMCI-S groups were compared directly, the aMCI-P group showed greater loss in the medial and inferior temporal lobes, the temporoparietal neocortex, posterior cingulate, precuneus, anterior cingulate, and frontal lobes than the aMCI-S group.
Conclusions
The regions of loss observed in aMCI-P are typical of subjects with AD. The lack of grey matter loss in the aMCI-S subjects is consistent with the notion that patterns of atrophy on MRI at baseline map well onto the subsequent clinical course.
INTRODUCTION
Amnestic mild cognitive impairment (aMCI) usually represents the prodromal phase of Alzheimer's disease (AD), with subjects showing memory impairment but with normal activities of daily living1, 2. A high proportion of subjects with aMCI progress to a clinical and pathological diagnosis of AD2, 3, although some fail to progress even after a long clinical follow-up2, 4. There is potential clinical utility in predicting which aMCI subjects will progress to a diagnosis of AD. MRI studies have shown that atrophy of the medial temporal lobe and rates of brain loss can predict which subjects with aMCI will progress to AD5–13. However, these studies employed techniques that only assessed a limited number of brain structures. Voxel-based morphometry (VBM)14 is an automated technique that assesses patterns of regional atrophy throughout the whole brain. Previous studies that have applied VBM to assess patterns of atrophy in aMCI progressors versus stables have failed to find many differences between the two groups15, 16, most likely because of the small number of subjects and short clinical follow-up time.
This study used VBM as well as hippocampal measurements to assess differences in the patterns of grey matter atrophy in a large number of subjects with aMCI that progress to AD versus those that remain stable. In order to select subjects that are truly clinically stable, and avoid the problem whereby subjects progress shortly after the follow-up cutoff point, subjects labeled stable were required to retain the clinical diagnosis of aMCI throughout their entire available clinical history, with a minimum follow-up of three years.
METHODS
Subjects
All subjects were identified from The Mayo Clinic Alzheimer's Disease Research Centre (ADRC) and Alzheimer's Disease Patient Registry (ADPR) data base. Informed consent was obtained for participation in the studies, which were approved by the Mayo Institutional Review Board. All subjects recruited into the ADRC and ADPR were followed prospectively and underwent annual neurological, neuropsychological and neuroimaging assessments. Patients were diagnosed as having aMCI if they fulfilled the following criteria: 1) memory complaint, preferably corroborated by an informant; 2) memory impairment for age; 3) essentially normal general cognitive function; 4) generally preserved activities of daily living; 5) not demented1, 2. Typically memory measures in subjects diagnosed as aMCI fall in the −1.0 to −1.5 SD below the means for age and education appropriate individuals in our community17. The memory measures used for assessment include the Wechsler Memory Scale Revised Logical Memory and Visual Reproductions subtests18, the Auditory Verbal Learning Test (AVLT)19, and the Free and Cued Selective Reminding Test20. The most salient measures are those involving delayed recall. For the other domains, subtests from the Wechsler Adult Intelligence Scale Revised are used such as Digit Span, Digit Symbol Substitution, Block Design, Picture Completion, Object Assembly, as well as other measures including the Boston Naming Test21, category fluency, and Trailmaking A and B. Measures of global function are used such as the Clinical Dementia Rating (CDR)22 and Mini-Mental State Examination (MMSE)23. Typically, aMCI subjects fall less than −1 SD below the appropriate means on these non-memory measures1. These well established criteria have been used by our institution for many years and are essentially the same as those adopted by the National Institute on Aging (NIA) Alzheimer's Disease Centers Program and the Alzheimers Disease Neuroimaging Initiative (ADNI) (http://www.adni-info.org/images/stories/Documentation/adni_protocol_03.02.2005_ss.pdf). In all cases the diagnosis was made on clinical grounds without reference to MRI. Patients were reevaluated annually and the decision of whether subjects had progressed to clinically probable AD was made at a consensus committee meeting as previously described 24. The diagnosis of probable AD was made according to NINCDS-ADRDA criteria 25.
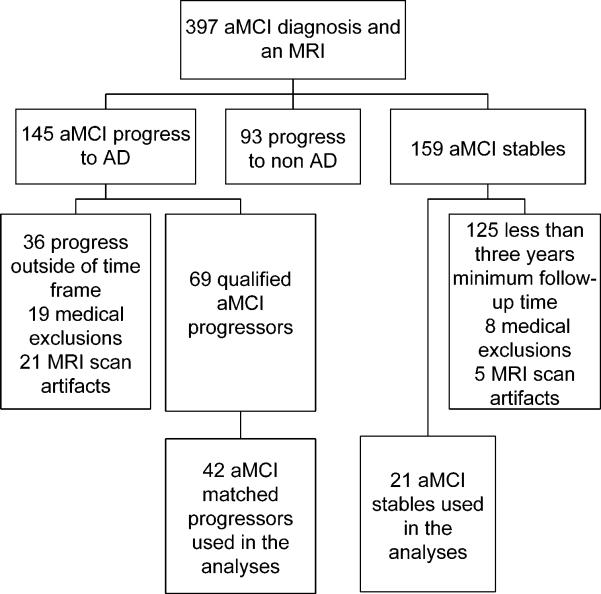
All subjects that had retained the clinical diagnosis of aMCI throughout not only a minimum required three years follow-up from the time of MRI but throughout their entire clinical course were classified as aMCI-stables (aMCI-S). These requirements help to ensure that the aMCI-S subjects were truly stable and did not simply progress shortly after the required three year follow-up. The aMCI-S subjects were then 2:1 matched by age and gender to aMCI subjects that had progressed to a clinical diagnosis of AD within 18 months of the baseline MRI (aMCI-P). Selecting progressors that had progressed to a diagnosis of AD within 18 months ensures separation between the groups on clinically evident grounds. The clinical history and MRI scans were reviewed in all cases. Subjects with structural abnormalities that could produce cognitive impairment, or who had treatments or concurrent illnesses interfering with cognitive function either at baseline or during follow-up were not included in this study. MRI scans were rejected for poor quality. A flow chart depicting the selection process is shown in Figure 1.
Figure 1.
Flow chart illustrating the subject selection process.
Each aMCI subject that was included in the analysis was age and gender matched to a control subject. Control subjects were defined as subjects that were cognitively normal at the time of scan, had at least three years of observation as a cognitively normal, and had no subsequent history of conversion to MCI or any other neurodegenerative disease throughout their entire available follow-up. The date/year that the scans were performed were also matched in an attempt to control for any temporal fluctuations associated with different scanner platform versions. All the control subjects were prospectively recruited via the same mechanism as the aMCI subjects into the Mayo Clinic ADRC, or the ADPR, and were identified from the ADRC/ADPR database. Control subjects were cognitively normal individuals that had been seen in internal medicine for routine physical examinations and asked to enroll in the ADRC and ADPR. All subjects were then evaluated by a neurologist to verify the normal diagnosis. Controls were identified as individuals who a) were independently functioning community dwellers, b) did not have active neurologic or psychiatric conditions, c) had no cognitive complaints, d) had a normal neurological and neurocognitive examination, and e) were not taking any psychoactive medications in doses that would affect cognition.
Image analysis
Imaging parameters
All MRI studies were performed with a standardized imaging protocol. T1-weighted 3D coronal volumetric SPGR images with 124 contiguous partitions, and 1.6mm slice thickness (22×16.5 or 24×18.5cm FOV, 25° flip angle) were used for the VBM analysis and hippocampal tracings, and a T1-weighted sagittal sequence with 5mm contiguous sections was used for the measurement of total intracranial volume (TIV). An axial Fluid Attenuated Inversion Recovery (FLAIR) scan (TR=11,000 ms; TE=147ms; TI=2,250 ms; 3 mm interleaved images of the whole head) was also acquired and was used to perform visual assessments of vascular burden.
Voxel-based morphometry
An optimized method of VBM was applied, implemented using SPM2 (http://www.fil.ion.ucl.ac.uk/spm). In order to reduce any potential normalization bias across the disease groups, customized templates and prior probability maps were created from all aMCI subjects and controls in the study. To create the customized template and priors all images were registered to the Montreal Neurological Institute (MNI) template using a 12 degrees of freedom (dof) affine transformation and segmented into grey matter (GM), white matter (WM) and CSF using MNI priors. GM images were normalized to the MNI GM template using a nonlinear discrete cosine transformation (DCT). The normalization parameters were applied to the original whole head and the images were segmented using the MNI prior probability maps. Average images were created of whole head, GM, WM and CSF, and smoothed using 8mm full-width at half-maximum (FWHM) smoothing kernel. All images were then registered to the customized whole brain template using a 12dof affine transformation and segmented using the customized prior probability maps. The GM images were normalized to the custom GM template using a nonlinear DCT. The normalization parameters were then applied to the original whole head and the images were segmented once again using the customized priors. All images were modulated and smoothed with an 8mm FWHM smoothing kernel. In addition, a re-initialization routine was implemented as previously described26. Grey matter differences between aMCI-P and controls, aMCI-S and controls, and between aMCI-P and aMCI-S, were assessed using two-sided t tests after correction for multiple comparisons using the false discovery rate (FDR) (p<0.05). Age and gender were included in the model as nuisance variables.
Region of interest measurements
Hippocampal measurements were performed after several image-preprocessing steps had been performed27. The borders of the left and right hippocampi were traced sequentially from posterior to anterior. In-plane hippocampal anatomic boundaries were defined to include the CA1 through CA4 sectors of the hippocampus proper, the dentate gyrus, and subiculum. The posterior boundary was determined by the oblique coronal anatomic section on which the crura of the fornices were identified in full profile. The inferior boundary of the hippocampus is determined by the grey-white interface formed by the subiculum and underlying parahippocampal gyrus. Test re-test reproducibility expressed as co-efficient of variation (CV) for hippocampal volume measurements has been previously measured as 0.28%28. Total intracranial volume was determined by tracing the margins of the inner table of the skull on contiguous images of the T1-weighted spin echo sagittal MR scan.
Visual rating of vascular burden
The presence of cerebro vascular disease was assessed semi quantitatively on FLAIR scans by an experienced research technician who was blinded to pathological and clinical diagnosis using a synthesis of published criteria29–31. The number of lacunar infarcts was counted in each subject and white matter hyperintensity (WMH) load was graded with a visual analog scale. Each incoming FLAIR study was registered to a common template and compared against a bank of example scans to assign it a WMH load in units of cm3. WMH burden in units of cm3 had been determined quantitatively for each example case using an algorithm developed in our lab32. The technician assigned each new incoming scan a WMH burden on a continuous scale (i.e. visual analog scale) using an electronic slider bar. The intra-class correlation coefficient for inter-rater reliability of this visual analog WMH grading scale was 0.96. The concordance correlation coefficient between quantitative measures and visual WMH grading is 0.93.
Statistics
Kruskal-Wallis tests were used to compare the aMCI-P, aMCI-S, and the control groups on age and years of education. A chi-squared test was used to compare groups on gender and the proportion of apolipoprotein epsilon 4 (APOE e4) carriers. Two-sided two-sample Wilcoxon rank-sum tests were used to compare the aMCI-P to the aMCI-S groups on the cognitive test scores, including the MMSE, CDR sum of boxes (CDR-SOB), and AVLT sum of trials 1 through 5. The cognitively healthy subjects were excluded from tests of differences on cognitive scores since by definition they are cognitively intact. We report medians and use nonparametric methods due to skewness in the numeric clinical variables.
Prior to analysis, hippocampal volumes were converted to age, sex and TIV-adjusted W-scores33 which can be interpreted as the number of standard deviations the subject's hippocampal volume is from the mean volume among cognitively healthy individuals on a zero mean scale. Hippocampal W-scores and WMH load were compared across groups using the Wilcoxon rank-sum tests. Categorical vascular measures were compared between groups using chi-squared tests or Fisher's exact test when cell counts were small. Because the pairwise tests described above each address a clinical question of a priori interest, we do not adjust reported p-values for multiple comparisons34.
To graphically characterize longitudinal change in cognitive scores, we used linear mixed-effects models specifying a random subject-specific intercept35. In these models the time component is expressed as years from the MRI and modeled as a quadratic slope. Age, sex, and education are included as covariates.
RESULTS
Subjects
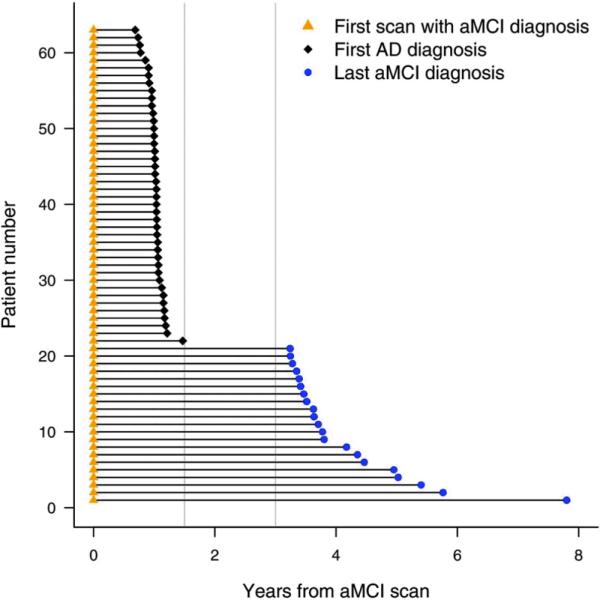
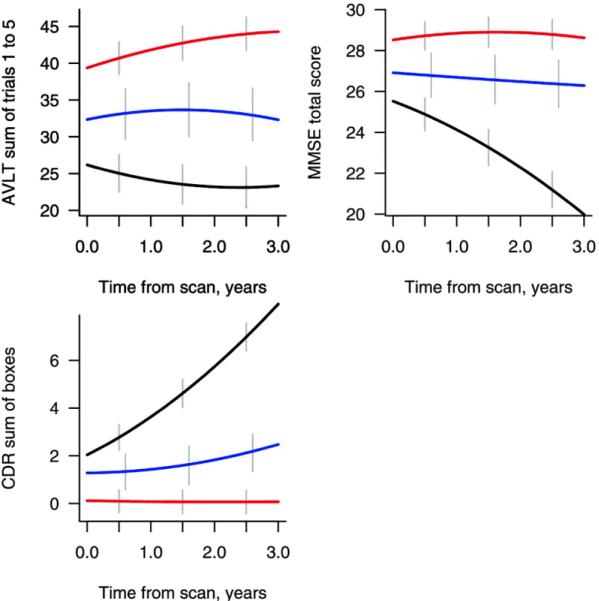
Twenty-one aMCI-S and 42 aMCI-P subjects were studied. Figure 2 shows the time intervals from the baseline MRI to either progression to AD in the aMCI-P subjects, or to the last clinical assessment in the aMCI-S subjects. The median time from the MRI to the diagnosis of AD in the aMCI-P group was 1.0 year (range 0.7–1.5) and the median time of total follow-up in the aMCI-S group was 3.7 years (3.2–7.8) and in the controls was 4.7 years (3.0–12.7). The demographics of the aMCI subjects and the 63 matched controls are shown in Table 1. There was no significant difference across the groups in age at scan, education, or gender ratio. The frequency of APOE e4 carriers was significantly different across groups, with the highest frequency reported in the aMCI-P group. There were also significant differences between the aMCI-P and aMCI-S groups in MMSE, CDR-SOB, and AVLT score with the aMCI-P group showing a lower MMSE, and AVLT score and a higher CDR-SOB score. Figure 3 shows the change in the cognitive scores over three years from the time of the MRI. The aMCI-P group performed progressively worse over time in the MMSE and CDR-SOB, although performed at a relatively constant level on the AVLT. The control and aMCI-S groups showed a stable performance on all tests over time.
Figure 2.
Schematic plot showing the time from baseline MRI to either progress to a diagnosis of AD, or the end of clinical follow-up, for all aMCI subjects. Subjects 1–21 were classified as the aMCI-S subjects that do not progress to AD over their entire follow-up, and subjects 22–63 were classified as the aMCI-P subjects since they progressed within 18 months of the MRI scan. The gray vertical lines indicate the 18 month and three year time-points.
Table 1.
Subject demographics
| aMCI-P n=42 | aMCI-S n=21 | Controls n=63 | P values | |
|---|---|---|---|---|
| No. of females (%) | 24 (57) | 12 (57) | 36 (57) | 1.00 |
| Median (range) age, yrs. | 79 (59, 96) | 79 (61, 97) | 78 (59, 93) | 0.64 |
| Median (range) education, yrs | 14 (7, 20) | 15 (8, 20) | 13 (8, 20) | 0.28 |
| No. of APOE ε4 carriers (%) | 24 (59) | 9 (43) | 12 (19) | <0.001 |
| Median (range) MMSE score | 26 (20, 29) | 27 (24, 30) | 29 (24, 30) | 0.01* |
| Median (range) CDR-SOB | 1.8 (0.5, 4.5) | 0.5 (0.0, 4.5) | 0.0 (0.0, 0.0) | 0.001* |
| Median (range) AVLT sum | 24 (12, 37) | 30 (20,41) | 37 (19, 64) | 0.003* |
The p values for the cognitive scores come from Wilcoxon rank sum test comparing only the aMCI-P and aMCI-S groups
APOE = Apolipoprotein E; MMSE = Mini-Mental Status Examination; CDR-SOB = Clinical Dementia Rating Sum of Boxes; AVLT = Auditory verbal learning test sum of learning over trials 1–5.
Figure 3.
Plots showing the change in MMSE, CDR sum of boxes and AVLT sum of learning over trials 1–5 over three years from the time of the MRI in the aMCI-P (black), aMCI-S (blue) and control subjects (red).
Image Analysis
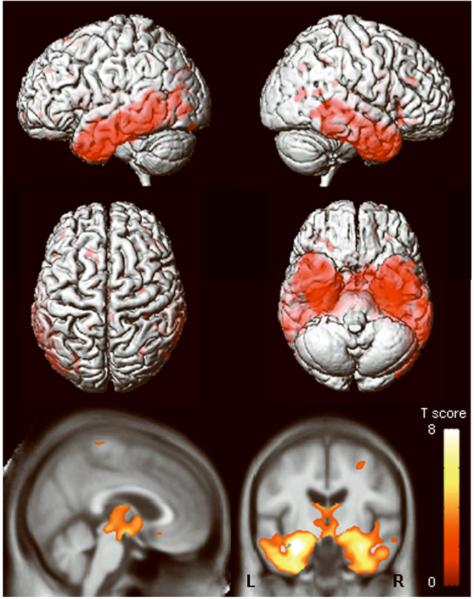
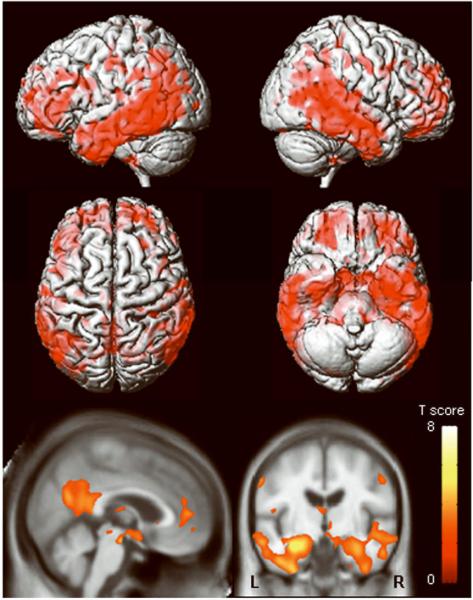
The aMCI-P group showed regions of grey matter loss bilaterally throughout the temporal lobes, including the anterior temporal lobe, hippocampus, amygdala, entorhinal cortex, parahippocampal gyrus, fusiform gyrus, and the inferior and middle temporal gyri, compared to controls (Figure 4). The superior temporal gyrus was only involved in the posterior portions of the temporal lobe. The left temporal lobe showed greater involvement than the right. The parietal lobes were also involved, predominantly in the left hemisphere. Additional grey matter losses were identified in the frontal lobes, basal forebrain, and in the anterior insula. Regions of apparent grey matter loss were also identified around the lateral ventricles. In comparison, no significant regions of grey matter loss were identified in the aMCI-S group when compared to the control group. Reverse comparisons were also performed to assess whether the control group showed greater grey matter loss than the aMCI groups. No regions of greater grey matter loss were identified in the control group compared to either of the aMCI groups.
Figure 4.
Patterns of grey matter loss identified in the aMCI-P group compared to controls (corrected for multiple comparisons, p<0.05). The patterns of cortical atrophy are shown on a 3D surface render (top). In addition the results are shown on a sagittal and coronal slice through the customized template, selected to highlight changes in the cingulate cortex and the medial temporal lobes (bottom). L = left; R = right
A direct comparison was performed between the two aMCI groups in order to highlight the regions that showed greater grey matter loss in the aMCI-P group compared to the aMCI-S group. The aMCI-P group showed a widespread distribution of greater grey matter loss (Figure 5). The temporal lobe showed slightly greater involvement in the left hemisphere, and included the amygdala, hippocampus, entorhinal cortex, parahippocampal gyrus, inferior and middle temporal gyri, and the fusiform gyri. The superior temporal gyrus was only involved in the posterior portions of the temporal lobe. Widespread loss was observed in the frontal lobes, including the orbitofrontal cortex, the inferior and middle temporal gyri, and the medial frontal lobes. Greater grey matter loss was also observed in the basal forebrain, anterior insula, the parietal lobes, the anterior and posterior cingulate, and the precuneus (Figure 5). Only a small amount of grey matter loss was observed around the lateral ventricles.
Figure 5.
Regions that show greater grey matter loss in the aMCI-P group compared to the aMCI-S group (corrected for multiple comparisons, p<0.05). The patterns of cortical atrophy are shown on a 3D surface render (top). In addition the results are shown on a sagittal and coronal slice through the customized template, selected to highlight changes in the cingulate cortex and the medial temporal lobes (bottom). L = left; R = right
Table 2 shows the hippocampal volumes and vascular burden scores. The aMCI-P and aMCI-S groups both had significantly smaller hippocampal volumes than the control group (p=0.001 for both). The aMCI-P group had a greater WMH and lacunar infarct burden than the control group (p=0.001 and p=0.03), although there were no significant differences between the aMCI-S group and controls, or between the aMCI-S and the aMCI-P group, in either measure.
Table 2.
Hippocampal volumes and vascular ratings
| Groups | P values | |||||
|---|---|---|---|---|---|---|
| aMCI-P n=42 | aMCI-S n=21 | Controls n=63 | aMCI-S vs. aMCI-P | aMCI-S vs. CN | aMCI-P vs. CN | |
| Median (range) Hippocampus W score | −2.76 (−3.08, 0.46) | −2.78 (−2.94, 1.94) | −0.70 (−2.91, 3.09) | 0.52 | 0.001 | <0.001 |
| Median (range) WMH cc | 15 (5,66) | 11 (6,52) | 10 (4,62) | 0.15 | 0.41 | 0.001 |
| Any central grey lacunar infarcts, No. subjects (%) | 30 (73) | 10 (50) | 29 (52) | 0.07 | 0.89 | 0.03 |
WMH = white matter hyperintensity
DISCUSSION
This study shows that aMCI subjects that progress to a diagnosis of AD within 18 months have a greater degree of grey matter loss at baseline than aMCI subjects that do not progress to AD within three years. In fact, the aMCI-S subjects showed no significant regions of grey matter loss on VBM when compared to a group of controls. These results suggest that patterns of atrophy on MRI could be useful to predict subsequent progression to AD in subjects with aMCI if an image analysis method that provided single-subject classification were employed36, 37.
The patterns of atrophy identified in the aMCI-P versus control comparison were typical of those observed in AD33, 38–40. Grey matter loss was observed throughout the medial and inferior temporal lobes, the temporoparietal association neocortex and the frontal lobes on VBM, and hippocampal atrophy was observed using volumetric measurements. These structures have been previously identified in subjects with MCI using both VBM15, 16, 41–44 and ROI techniques5, 45, 46, and are involved pathologically in AD47. The superior temporal gyrus was relatively spared however, suggesting that atrophy of this structure would not help predict progression to AD in subjects with MCI and also perhaps that atrophy of this structure may occur later in the disease course than the other temporal regions. Previous studies have similarly shown less severe involvement of the superior temporal gyrus compared to the medial and inferior temporal lobes in subjects with MCI and AD48, 49. In addition, a previous longitudinal study has shown that atrophy of the superior temporal gyrus does not occur until moderate stages of AD50. The patterns of loss were more widespread than those observed in some previous VBM studies51 of aMCI, most likely because the subjects in this study were all within 18 months of progression to AD. The aMCI-P group also had a greater WMH burden and had a larger proportion of subjects with lacunar infarcts on MRI than the control group. This is consistent with previous studies that have shown that subjects are more likely to be demented if they have a combination of vascular and AD pathology at autopsy52. White matter hyperintensity burden has also been shown to be higher in subjects with AD than those with MCI, and higher in subjects with MCI than controls53.
Interestingly, no significant regions of grey matter loss were identified on VBM in the aMCI-S subjects when compared to controls. These subjects were selected based on the fact that they did not progress to AD during a minimum required follow-up of three years, but also that they did not progress to AD during their subsequent clinical follow-up if this was longer than three years. The median follow-up time was actually three years and eight months. The aim of these inclusion criteria were to try to select subjects that are truly clinically stable, and exclude subjects that progress shortly after the follow-up cut-off point. Indeed, the aMCI-S subjects did not decline cognitively over the three years from the MRI scan. The fact that we observed no significant grey matter loss in this group on VBM raises the possibility that a number of these subjects do indeed not have a progressive neurodegenerative disorder. These subjects may instead be presenting with non-progressive memory impairment. In line with this theory is the fact that the frequency of the AD risk factor APOE e4 was slightly lower (44%) than the aMCI-P group (65%) and the frequency typically observed in AD54. However, the frequency in the aMCI-S group was higher than that observed in the control group (18%) which suggests that a number of these subjects may still have prodromal AD, but of a less rapidly progressive nature. The fact that the volumetric measurements demonstrated hippocampal atrophy in the aMCI-S subjects also fits with this suggestion and reflects the fact that these subjects did have memory impairments. The curious observation that no hippocampal atrophy was observed in the aMCI-S to control VBM comparison may be due to the fact that the aMCI-S subjects were anatomically heterogeneous with some having hippocampal atrophy and others not. It is possible that subjects that have shown memory impairment for so long but still show normal activities of daily living, and therefore do not fulfill criteria for AD, are in some way protected against cognitive decline. Recent studies have shown that high levels of education and occupational attainment help retain cognitive function in old age55. Indeed the aMCI-S subjects had slightly more years of education than the aMCI-P group and performed better on the CDR-SOB and MMSE. Only one previous VBM study has reported patterns of grey matter loss in aMCI-S subjects compared to controls16. In contrast to our study they identified widespread regions of grey matter loss in the frontal lobes, fusiform gyrus and inferior temporal gyri, suggesting that a high proportion of their subjects would ultimately progress to AD. The average clinical follow-up time in that study was however only 28 months compared to the 44 months of follow-up in our study.
A direct VBM comparison was performed between the two aMCI groups and we found significantly greater grey matter loss in the aMCI-P group compared to the aMCI-S group. Regions that showed greater loss were found in the medial and inferior temporal lobes, the temporoparietal association neocortex, frontal lobes and in the posterior cingulate and precuneus. A number of studies have similarly shown greater atrophy of temporal lobe structures, including the hippocampus5, 56, 57, entorhinal cortex56, 57, and parahippocampal gyrus6, in MCI-P compared to aMCI-S subjects, but no previous studies have shown greater involvement of the temporoparietal cortex and the posterior cingulate. It is notable however that less medial and inferior temporal loss was observed in the aMCI-P group when compared to aMCI-S, than when they were compared to the control group. This fits with the fact that the aMCI-S subjects did show hippocampal atrophy on volumetric measurements, and again supports the earlier suggestion that some of the aMCI-S subjects may ultimately progress to AD. Previous VBM studies that have compared aMCI progressors to stables found very few grey matter differences between the groups15, 16. One previous study identified differences in the hippocampus, parahippocampal gyrus, and lingual and fusiform gyri, whereas another found differences in the frontal lobes, left supramarginal gyrus and the right hippocampus. The reason these studies found fewer differences between the groups was likely because they had smaller numbers of subjects and the clinical follow-up was less than in our study. Therefore a proportion of their “stables” may actually have progressed relatively soon after the follow-up cut-off, but this event was not detected because of short follow up times.
There was however an unusual finding in the patterns of loss across the different group-wise comparisons performed. Greater grey matter loss was observed in the frontal lobes, parietal lobes, posterior cingulate and precuneus, in the comparison between aMCI-P and aMCI-S than in the comparison between aMCI-P and controls. This is counter-intuitive since one would expect the control group to show less atrophy and be more homogeneous than the aMCI-S subjects. Whereas these results suggest the opposite, that the aMCI-S subjects had greater grey matter volumes at baseline than the control subjects. The direct VBM comparison did not show any regions of greater grey matter loss in controls than aMCI-S subjects but this may have due to the harsh statistical threshold applied. It is possible therefore that aMCI subjects that remain stable may have started with a greater grey matter reserve than those that progress to AD, however we can not prove this.
The strengths of this study were the fact that that we had a relatively large number of well matched aMCI-P and aMCI-S subjects with a long period of clinical follow-up. In addition, we had longitudinal cognitive data that showed that the aMCI-S subjects remained stable on tests of cognitive function for at least three years after the MRI, whereas the aMCI-P subjects showed increasing CDR-SOB and decreasing MMSE over time. The fact that the aMCI-P subjects showed a stable performance on the AVLT suggests that this test may not be a good marker of disease progression, although it appears to provide good discrimination between the groups at baseline. However, a limitation to the clinical utility of this study is that although VBM is a useful tool to identify patterns of loss in groups of subjects it does not provide valuable information on a single subject basis. In order for these patterns to be prognostic they will need to be identified in individual subjects36, 37.
ACKNOWLEDGEMENTS
This study was supported by grants P50 AG16574, U01 AG06786, R01 AG11378 from the National Institute on Aging, Bethesda MD, the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer s Disease Research Program of the Mayo Foundation, U.S.A, DSK has been a consultant to GE HealthCare, GlaxoSmithKline and Myriad Pharmaceuticals, has served on a Data Safety Monitoring Board for Neurochem Pharmaceuticals, and is an investigator in a clinical trial sponsored by Elan Pharmaceuticals. RCP has been a consultant to GE Healthcare and is on a Treatment Effects Monitoring Committee for a clinical trial sponsored by Elan Pharmaceuticals. BB is an investigator in a clinical trial sponsored by Myriad Pharmaceuticals. CRJ receives research support in the form of research grants from Pfizer.
Footnotes
Disclosure: The authors report no relevant conflicts of interest
References
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 3.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 4.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 5.Jack CR, Jr., Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visser PJ, Scheltens P, Verhey FR, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 7.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 8.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 9.Korf ES, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63:94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- 10.de Leon MJ, Golomb J, George AE, et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR Am J Neuroradiol. 1993;14:897–906. [PMC free article] [PubMed] [Google Scholar]
- 11.Jack CR, Jr., Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geroldi C, Rossi R, Calvagna C, et al. Medial temporal atrophy but not memory deficit predicts progression to dementia in patients with mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2006;77:1219–1222. doi: 10.1136/jnnp.2005.082651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeCarli C, Frisoni GB, Clark CM, et al. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol. 2007;64:108–115. doi: 10.1001/archneur.64.1.108. [DOI] [PubMed] [Google Scholar]
- 14.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 15.Chetelat G, Landeau B, Eustache F, et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Bozzali M, Filippi M, Magnani G, et al. The contribution of voxel-based morphometry in staging patients with mild cognitive impairment. Neurology. 2006;67:453–460. doi: 10.1212/01.wnl.0000228243.56665.c2. [DOI] [PubMed] [Google Scholar]
- 17.Ivnik RJ, Malec JF, Smith GE, et al. Mayo's Older Americans Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 through 97. The Clinical Neuropsychologist. 1992;6(Suppl):1–104. [Google Scholar]
- 18.Wechsler D. Wechsler Memory Scale-Revised. Psychological Corporation; New York: 1987. [Google Scholar]
- 19.Rey A. L'examen clinique en psychologie. Universitaires de France; Paris: 1964. [Google Scholar]
- 20.Grober E, Buschke H. Genuine memory deficits in Dementia. Developmental Neuropsychology. 1987;3:13–36. [Google Scholar]
- 21.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd Ed Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- 22.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cogntiive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR., Jr. Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26:600–608. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR, Jr., Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 28.Jack CR, Jr., Petersen RC, Xu Y, et al. Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology. 1998;51:993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheltens P, Barkhof F, Leys D, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- 30.Bryan RN, Manolio TA, Schertz LD, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: the cardiovascular health study. AJNR Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 31.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 32.Jack CR, Jr., O'Brien PC, Rettman DW, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging. 2001;14:668–676. doi: 10.1002/jmri.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jack CR, Jr., Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Brien PC. The appropriateness of analysis of variance and multiple-comparison procedures. Biometrics. 1983;39:787–794. [PubMed] [Google Scholar]
- 35.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Wiley-Interscience; Hoboken, NJ: 2004. [Google Scholar]
- 36.Davatzikos C, Fan Y, Wu X, Shen D, Resnick SM. Detection of prodromal Alzheimer's disease via pattern classification of MRI. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burges CJC. A tutorial on support vector machines for pattern recognition. Data Mining and Knowledge Discovery. 1998;2:121–167. [Google Scholar]
- 38.Baron JC, Chetelat G, Desgranges B, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 39.Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–205. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- 40.Frisoni GB, Testa C, Zorzan A, et al. Detection of grey matter loss in mild Alzheimer's disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2002;73:657–664. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karas GB, Scheltens P, Rombouts SA, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Pennanen C, Testa C, Laakso MP, et al. A voxel based morphometry study on mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2005;76:11–14. doi: 10.1136/jnnp.2004.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitwell JL, Petersen RC, Negash S, et al. Patterns of Atrophy differ among Specific Subtypes of Mild Cognitive Impairment. Arch Neurol. 2007 May 28; doi: 10.1001/archneur.64.8.1130. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bell-McGinty S, Lopez OL, Meltzer CC, et al. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol. 2005;62:1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- 45.Du AT, Schuff N, Amend D, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pennanen C, Kivipelto M, Tuomainen S, et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 47.Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- 48.Busatto GF, Garrido GE, Almeida OP, et al. A voxel-based morphometry study of temporal lobe gray matter reductions in Alzheimer's disease. Neurobiol Aging. 2003;24:221–231. doi: 10.1016/s0197-4580(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 49.Halliday GM, Double KL, Macdonald V, Kril JJ. Identifying severely atrophic cortical subregions in Alzheimer's disease. Neurobiol Aging. 2003;24:797–806. doi: 10.1016/s0197-4580(02)00227-0. [DOI] [PubMed] [Google Scholar]
- 50.Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer's disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci U S A. 2002;99:4703–4707. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirata Y, Matsuda H, Nemoto K, et al. Voxel-based morphometry to discriminate early Alzheimer's disease from controls. Neurosci Lett. 2005;382:269–274. doi: 10.1016/j.neulet.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 52.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 53.Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 55.Staff RT, Murray AD, Deary IJ, Whalley LJ. What provides cerebral reserve? Brain. 2004;127:1191–1199. doi: 10.1093/brain/awh144. [DOI] [PubMed] [Google Scholar]
- 56.deToledo-Morrell L, Stoub TR, Bulgakova M, et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging. 2004;25:1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Tapiola T, Pennanen C, Tapiola M, et al. MRI of hippocampus and entorhinal cortex in mild cognitive impairment: A follow-up study. Neurobiol Aging. 2006 Nov 9; doi: 10.1016/j.neurobiolaging.2006.09.007. [Epub] [DOI] [PubMed] [Google Scholar]