Abstract
Allogeneic bone marrow chimerism induces robust systemic tolerance to donor alloantigens. Achievement of chimerism requires avoidance of marrow rejection by pre-existing CD4 and CD8 T cells, either of which can reject fully MHC-mismatched marrow. Both barriers are overcome with a minimal regimen involving anti-CD154 and low dose (3 Gy) total body irradiation, allowing achievement of mixed chimerism and tolerance in mice. CD4 cells are required to prevent marrow rejection by CD8 cells via a novel pathway, wherein recipient CD4 cells interacting with recipient class II MHC tolerize directly alloreactive CD8 cells. We demonstrate a critical role for recipient MHC class II, B cells and dendritic cells in a pathway culminating in deletional tolerance of peripheral alloreactive CD8 cells.
Keywords: transplantation, tolerance, CD8 T cells, B cells, MHC class II
Introduction
Specific immune tolerance obviates the need for chronic immunosuppressive therapy with its attendant toxicities and prevents chronic rejection of allografts. Mixed chimerism achieves robust tolerance, permitting acceptance of organ and tissue allografts without long-term immunosuppression (1-3). If used to induce tolerance, chimerism must be achieved with minimal host conditioning without toxicity or graft-versus-host disease. Immune barriers posed by both CD4 and CD8 T cells, each of which can reject fully MHC-mismatched marrow grafts, must be overcome (4). These barriers are overcome using anti-CD154 monoclonal antibody (mAb) and low dose (3 Gy) total body irradiation (TBI) with fully MHC-mismatched allogeneic bone marrow transplantation (BMT), which achieves durable mixed chimerism in mice (5). Engrafted donor hematopoietic stem cells (HSCs) continually supply donor antigens to the thymus, permitting life-long central tolerance (6).
Tolerization of pre-existing peripheral donor-reactive CD4 and CD8 T cells in this model is characterized by rapid deletion (7,8). Whereas we could not find any evidence for a role for regulatory cells in CD4 tolerance induction (7,9), CD8 T cell tolerance is clearly and critically dependent on the presence of a CD4 T cell population, which however is CD25 negative, IL-2-independent and therefore distinct from “natural T regulatory cells” (Tregs). While it might be speculated that the critical CD4 cells are an induced type of Tregs, their requirement is most evident in the first few days posttransplant, and CD4 cells are no longer required by 2 weeks post-BMT. This is also the time by which peripheral donor-reactive CD8 cells have been specifically deleted, obviating any further need for regulation (8). These results implicate CD4 cells in the pathway leading to rapid deletional tolerance of pre-existing donor-reactive CD8 T cells. We have hypothesized that this pathway involves conditioning of donor-derived antigen-presenting cells (APCs) by CD4 cells, making APCs tolerogenic for alloreactive CD8 cells encountering donor antigen on these APCs. However, no absolute requirement for any specific donor class II+ APC population has been demonstrable (Fehr et al, manuscript submitted). We therefore investigated the possibility that interactions of recipient CD4 cells with recipient APCs might be involved in the pathway leading to recipient CD8 T cell tolerance. The present studies provide evidence for such interactions and suggest a novel pathway whereby tolerance of directly alloreactive CD8 cells requires interactions between recipient CD4 cells and recipient class II+ APCs. Recipient class II MHC, recipient B cells and recipient DCs are involved in the tolerization of peripheral alloreactive CD8 cells.
Materials and methods
Mice
Studies were performed under an institutionally-approved protocol in accordance with the NIH Guide. Female C57BL/6 (B6: H-2b, CD45.2), CD45.1 congenic B6, B10.A (H-2a), B10.RIII (H-2r), CIITa-deficient (H-2b), B cell-deficient μMT (H-2b) and diphtheria toxin receptor (DTR)-transgenic (H-2b) mice were purchased from Frederick Cancer Research Center (Frederick, MD) or from The Jackson Laboratories (Bar Harbor, ME). MHC class II-deficient (I-Aβ-/-, H-2b), β2m/I-Aβ double-deficient (β2m-/-/I-Aβ-/-, H-2b) and KbDb double-deficient mice were purchased from Taconic (Germantown, NY). CIITa/KbDb triple-deficient mice were bred in our facility. All mice were housed in a specific pathogen-free microisolator environment.
Conditioning and BMT
Non-myeloablative protocol
Eight-to twelve-week-old mice received 3 Gy total body irradiation (TBI) from a 137Cesium irradiator on Day -1. Anti-mouse CD154 mAb (MR1; 2 mg; produced at National Cell Culture Center [NCCC], Minneapolis, MN) was administered i.p. on Day 0, prior to i.v. injection of 20×106 fully MHC-mismatched B10.A BM cells. For the experiments shown in Fig. 3, 5 and 6, donor BM was depleted of T cells using magnetic beads coated with anti-CD4 and anti-CD8 antibodies and the Super-MACS® system (Miltenyi) with CS columns.
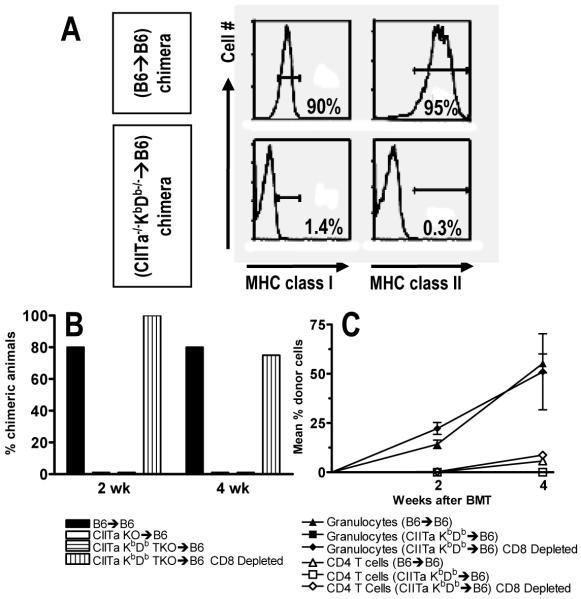
Figure 3. Evidence for a role for host MHC class II-restricted CD4 cells for directly alloreactive CD8 T cell tolerance induction.
Lethally irradiated B6 mice were reconstituted with either WT B6 (B6→B6 chimeras), MHC class II-deficient (CIITa-/-→B6 chimeras) or MHC class I and II-deficient (CIITa-/- KbDb-/-→B6 chimeras) BM. Eight weeks later they received allogeneic B10.A BMT with 3 Gy TBI/anti-CD154, with or without depleting anti-CD8 mAb. (A) MHC class I and II expression on blood B cells (B220+). Representative B6→B6 and CIITa-/-KbDb-/-→B6 chimeras are shown (top and bottom, respectively). (B) Incidence of granulocyte chimerism at indicated times (4-6 animals/group). (C) Mean percent of granulocyte and CD4 cell chimerism (±SEM) over time.
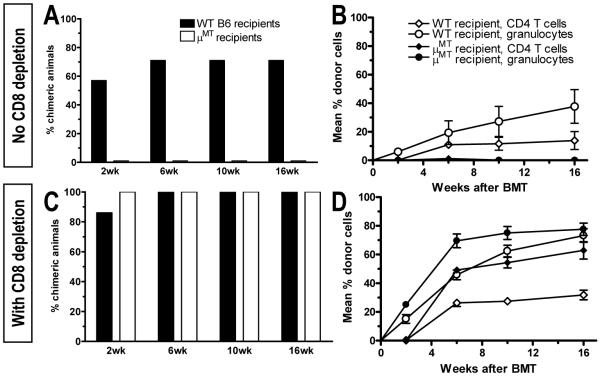
Figure 5. Requirement for recipient B cells for CD8 T cell tolerance induction.
(A) B cell-deficient μMT and WT B6 mice received T cell-depleted B10.A BMT after 3 Gy TBI/anti-CD154. The incidence of WBC granulocyte chimerism is shown at indicated times. One of two similar experiments is shown (7-8 animals/group/experiment). (B) WBC granulocyte and CD4 chimerism (±SEM) for groups in A. (C) Mice treated as in panel A also received depleting anti-CD8 mAb. Incidence of granulocyte chimerism is shown at indicated times. One of two similar experiments is shown (7-8 animals/group/experiment). (D) WBC granulocyte and CD4 chimerism levels (±SEM) for groups in C.
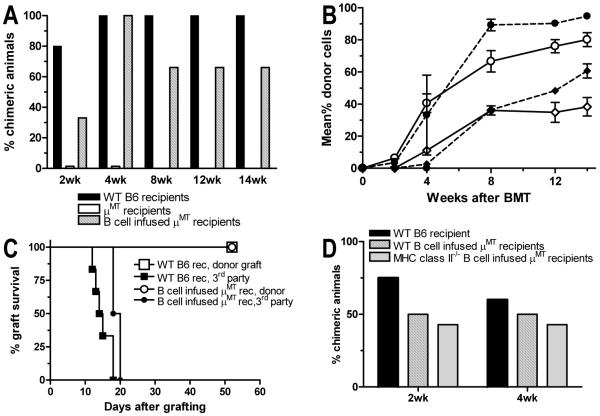
Figure 6. Adoptive transfer of WT or MHC class II-deficient B cells restores chimerism and tolerance.
(A) B cell-deficient μMT and WT B6 mice received T cell-depleted B10.A BMT following 3 Gy TBI/anti-CD154. An additional group of B cell-deficient μMT mice also received 20-23×106 purified B cells from WT B6 mice on Day 0. The incidence of WBC granulocyte chimerism is shown at indicated times. One of two similar experiments is shown (3-7 animals/group/experiment). (B) WBC granulocyte and CD4 chimerism levels (±SEM) for the groups in A. Circles: granulocyte chimerism. Diamonds: CD4 T cell chimerism. Open symbols: WT B6 recipients. Closed symbols with continuous line: μMT recipients. Closed symbols with dashed lines: μMT recipients of WT B6 B cells. (C) Chimeras shown in A and B received donor-type B10.A and 3rd party B10.RIII skin grafts 5 weeks after BMT. Graft survival is shown over time. (D) B cell-deficient μMT and WT B6 mice received T cell-depleted B10.A BMT after 3 Gy TBI/anti-CD154 conditioning. Some μMT mice also received 20-23×106 purified B cells from WT B6 mice or CIITa-/- (MHC class II-deficient) mice on Day 0. The incidence of WBC granulocyte chimerism is shown at indicated times (3-7 animals/group/experiment).
Bone marrow reconstitution experiments
Ten-to twelve-week-old B6 mice received 10.25 Gy TBI from a 137Cesium irradiator followed 6-8 hours later by reconstitution with 10×106 T cell-depleted syngeneic BM cells from WT B6, I-Aβ-/-, β2m-/-/I-Aβ-/-, CIITa-/-, CIITa/KbDb triple-deficient or DTR-transgenic mice. For reconstitution with syngeneic MHC class I-deficient marrow, recipient mice were additionally depleted of NK cells by injection of mAb PK136 (0.15mg i.p.) on Day -1 before TBI and BMT.
B cell infusions
Splenic B cells from 8-12-week-old WT or MHC class II-deficient B6 mice were purified using a non-touch B cell isolation kit and Super-MACS® (Miltenyi Biotec Inc., Auburn, CA). Twenty to 23 million highly purified B cells (purity > 97%) were i.v. injected into μMT recipient mice on the day of BMT.
In vivo T cell depletion
To deplete CD8 or CD4 T cells in vivo, anti-CD8 (2.43, 1.44 mg/mouse) or anti-CD4 mAb (GK1.5, 1.76 mg/mouse) produced at NCCC was injected i.p. on Day -1 before BMT.
In vivo DC depletion
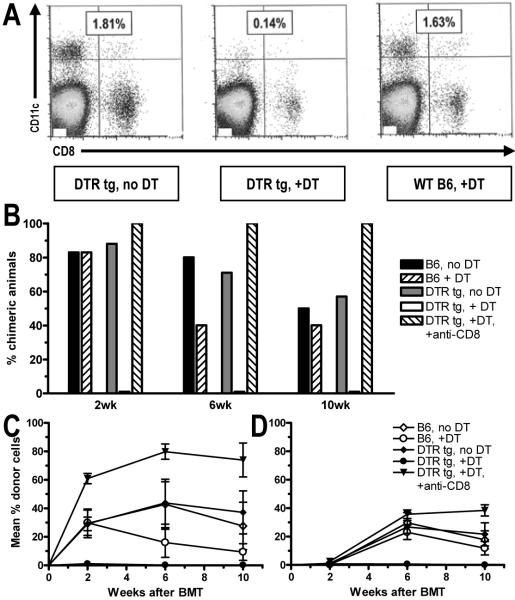
To deplete CD11c+ DCs from reconstituted DTR-transgenic mice, 4ng/g body weight DT was injected i.p. starting on Day -1 before allo-BMT and then 3x/week for 17 days. Efficiency of DC depletion was 90-95% in mice reconstituted with DTR-transgenic marrow, as shown by flow cytometric (FCM) analysis of splenocytes from a non-chimeric mouse from each group on Day 18 (Fig. 7A).
Figure 7. Requirement for recipient DCs for CD8 T cell tolerance induction.
Lethally irradiated congenic CD45.1 B6 mice were reconstituted with either WT B6 (B6) or DTR-transgenic (DTR tg) B6 BM. Eight weeks later they received fully MHC-mismatched B10.A BMT with 3 Gy TBI/anti-CD154. Some animals received diphtheria toxin to deplete DCs (see Methods), with or without depleting anti-CD8 mAb. (A) One mouse from each group was euthanized on Day 18. The spleen was harvested, digested with collagenase and then stained with CD11c-PE and, anti-CD8β-APC and analyzed by FCM. The percentage of CD11c+ DCs among total lymphocytes is shown. Efficiency of DC depletion was 90-95%. The incidence of B cell chimerism (B) and percent donor B and CD4 cell chimerism (±SEM) (C and D) is shown. One of two experiments is presented (7-8 animals/group/experiment). The repeat experiment included T cell-depletion of the allogeneic BM, with similar results.
Flow cytometric analysis of multilineage chimerism among WBCs
Chimerism analysis
Multilineage chimerism was assessed by three-color FCM analysis using fluorescein isothiocyanate (FITC)-conjugated anti-Dd mAb 34-2-12 for H-2a or anti-Db mAb KH95 for H-2b (Becton-Dickinson[BD]/PharMingen, San Diego, CA) with phycoerythrin (PE)-or allophycocyanin (APC)-conjugated anti-CD4, -CD8, -B220 (BD/PharMingen), and -Mac1 (Caltag, San Francisco, CA) mAbs. Negative control mAbs included HOPC1-FITC (prepared in our laboratory) and rat anti-mouse IgG2a-PE or -APC (BD/Pharmingen). Propidium iodide (PI) staining was used to exclude dead cells. A lineage was defined as chimeric when ≥5% cells were donor MHC class I positive.
Bone marrow reconstitution after lethal TBI
Lethally irradiated congenic B6 CD45.1 mice were reconstituted with WT, transgenic or knockout B6 CD45.2 BM. Peripheral WBCs were stained 7 weeks later for three-color FCM with anti-CD45.2 mAb 104-FITC, anti-CD45.1 mAb A20-biotin (BD/Pharmingen), and PE-or Peridinin chlorophyll protein-Cychrome 5.5 (PerCP-Cy5.5) -conjugated anti-CD4, -CD8, -B220 or -Mac1 mAbs. PI staining was used to exclude dead cells. MHC class I or class II expression was assessed on B220+ or Mac1+ cells using FITC-KH95 anti-Db and 25-9-17 anti-I-Aβ, respectively.
CML assay
CML assay was performed as previously described (10). Where indicated, human recombinant IL-2, 5 IU/ml, was added. After 5 days, cytolytic activity against 51Cr-labelled concanavalin-A-stimulated splenocytes and percent specific lysis (PSL) was calculated as described (10).
Skin Grafting
Full thickness tail skin (0.5-1.0 cm2) from B10.A (donor-specific) or B10.RIII (3rd party) mice was grafted to the lateral thorax and considered rejected when <10% of the graft remained viable.
Results
CD4 recognition of recipient class II MHC is required for CD8 T cell tolerance induction
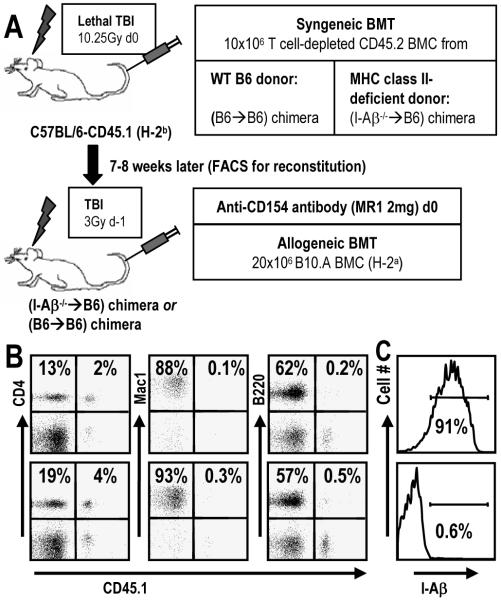
For tolerization of pre-existing peripheral CD8 T cells by fully MHC-mismatched BMT with anti-CD154, CD4 T cells are required (8). We investigated the possibility that CD4 T cell interactions with recipient class II MHC might play a role in this pathway. MHC class II-deficient I-Aβ-/- mice lack CD4 T cells and therefore could not serve this purpose (11). Instead, we generated syngeneic chimeras by transplanting T cell-depleted WT or I-Aβ-/- B6 BM (both CD45.2) to lethally irradiated CD45.1 congenic WT B6 recipients (Fig. 1A). CD4 T cells developed normally due to MHC class II expression on thymic epithelium, but MHC class II+ host APCs were absent (Fig. 1B,C). Eight weeks later, these reconstituted mice received fully MHC-mismatched B10.A BMT with 3 Gy TBI/anti-CD154 (Fig. 2A,B). All B6→B6 reconstituted mice developed stable multilineage chimerism. In contrast, I-Aβ-/-→B6 reconstituted recipients failed to achieve multilineage mixed chimerism unless recipient CD8 cells were depleted (Fig. 2C,D,E). Similar results were obtained when B6 mice were initially reconstituted with bone marrow from class II transactivator-deficient mice (CIITa -/- (12)), which also lack class II MHC expression. Therefore, recognition of recipient class II MHC by recipient CD4 T cells is critical to prevent marrow rejection by CD8 T cells and allow their tolerization by donor marrow and anti-CD154.
Figure 1. Generation of mice lacking MHC class II expression on host hematopoietic cells, but with a normal peripheral CD4 compartment.
(A) Congenic CD45.1 B6 mice were lethally irradiated and reconstituted with MHC class II-deficient CD45.2 BM. Seven to eight weeks later, PBL reconstitution was tested by FCM and syngeneic chimeras then received allogeneic B10.A BMT with 3 Gy TBI and anti-CD154. (B) CD45.1 expression in WBC lineages (CD4 and B cells in the lymphocyte gate, Mac1+ cells in the granulocyte gate), representing residual recipient-derived cells. Representative (B6→B6) and (I-Aβ-/-→B6) chimeras are shown (top and bottom, respectively). A radioresistant population of CD4 and CD8 T cells (10-30%) persisted, whereas B cells, granulocytes and monocytes (not shown) were >99% donor-derived. (C) Histograms of MHC class II (I-Aβ) expression on peripheral B cells for the same two animals shown in panel A.
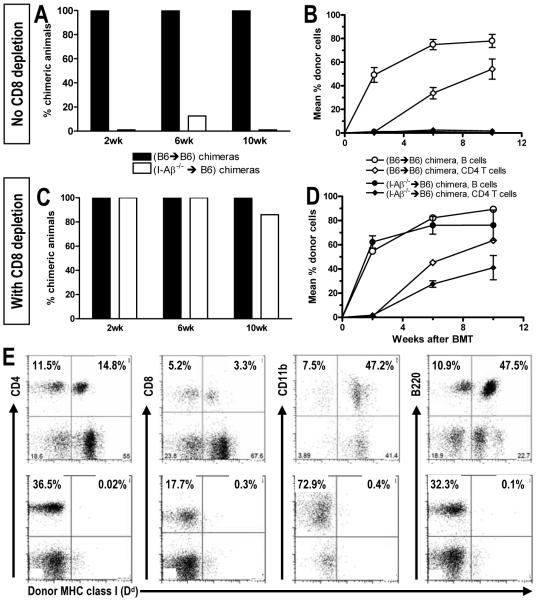
Figure 2. Requirement for CD4 cell interaction with host class II for CD8 tolerance.
BM-reconstituted mice prepared as in Fig. 1 received allogeneic B10.A BMT. (A) The incidence of chimerism in the B cell lineage at various time points. Chimerism was defined as ≥5% donor cells among B220+ B cells. One representative experiment out of three is shown (7-8 animals/group/experiment). (B) Chimerism levels (±SEM) over time for B cells and CD4 T cells in PBL. (C) Mice treated as in panel A also received depleting anti-CD8 on Day -1. CD8 T cells were <0.5% of PBL by 2 weeks post-BMT. The incidence of B cell chimerism is shown at indicated times. One representative experiment of two is shown (6-7 animals/group/experiment). (D) Chimerism levels (±SEM) over time are shown for blood B cells and CD4 T cells for groups in panel C. (E) Representative FACS plots for measuring chimerism are shown 6 weeks post-BMT for a chimeric WT mouse (upper row) and a non-chimeric mouse lacking MHC class II on recipient APCs (lower row) from the experiment shown in panel B. Donor cells are marked with FITC-labelled anti-Dd mAb 34-2-12. The panels showing CD4 and CD8 T cells and B220+ B cells are gated on lymphocytes; the panels showing CD11b+ cells are gated on granulocytes. Percentages indicate the relative frequencies in the total lymphocyte or granulocyte population, respectively. Chimerism was then calculated as the percentage of donor MHC-positive cells versus total cells in a certain lineage (e.g. for CD4 cells: [14.8/(14.8 + 11.5)] * 100 = 56.3%).
CD4 cell interactions with recipient class II are required to tolerize directly alloreactive CD8 cells
Since direct CD8 cell-mediated rejection is likely to eliminate allogeneic cells, we next addressed whether cross-talk from CD4 T cells interacting with recipient APCs may be needed to tolerize directly alloreactive CD8 cells. We generated mice lacking both class I and class II MHC by crossing KbDb double-deficient mice (KbDb-/- (13)) to CIITa-/- (12) mice and used BM from F2 mice that completely lacked class I and class II MHC (i.e. CIITa/KbDb triple-deficient) to reconstitute lethally irradiated WT B6 mice. In CIITa-/- KbDb-/-→B6 mice, normal peripheral CD4 and CD8 T cell reconstitution occurred due to T cell selection on MHC+ thymic epithelium, but neither MHC class I nor class II was detectable on peripheral WBCs (Fig. 3A). After 8 weeks, these mice received 3 Gy TBI, anti-CD154 and T cell-depleted fully MHC-mismatched B10.A BMT. Eighty percent of control B6→B6 reconstituted recipients developed multilineage mixed chimerism, whereas neither CIITa-/-KbDb-/-→B6 nor CIITa-/- →B6 mice achieved allogeneic chimerism unless they were depleted of CD8 cells (Fig. 3B,C). Similar results were obtained using mice reconstituted with I-Aβ/β2m double-deficient BM. These data demonstrate, surprisingly, that recipient CD4 T cell recognition of host MHC class II on a recipient professional APC population is needed in order to tolerize directly alloreactive CD8 T cells.
Early donor-specific CD8 unresponsiveness is overcome by addition of IL-2 only when CD4 T cells are depleted
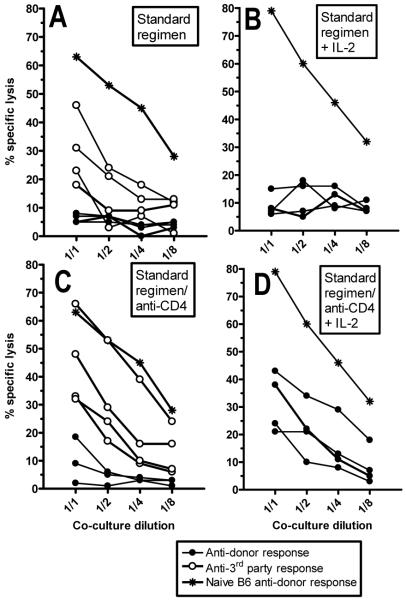
To analyze the mechanism of early CD8 unresponsiveness more precisely, we performed cell-mediated lympholysis (CML) assays in mice receiving 3Gy TBI, anti-CD154 and allo-BMT, with or without in vivo CD4 depletion. Splenocytes harvested on Day 4 post-BMT were stimulated in vitro with donor B10.A or third party B10.RIII cells, with or without exogenous IL-2. Although deletion of donor-reactive CD8 cells is not yet near completion by Day 4 (Haspot, Fehr et al, manuscript submitted), mice receiving BMT without CD4 depletion showed donor-specific unresponsiveness with measurable 3rd party responses (Fig. 4A). Adding IL-2 to the culture did not overcome this donor unresponsiveness (Fig. 4B). When recipient CD4 T cells were depleted in vivo prior to allo-BMT, donor-specific responses were detected in 4 of 4 mice, but only when exogenous IL-2 was also added to the cultures (Fig. 4C,D). Thus, donor-specific CTL tolerance was apparent by 4 days post-BMT, and the most robust tolerance depended on the presence of recipient CD4 cells.
Figure 4. Early donor-specific unresponsiveness is overcome by addition of IL-2 only if recipient CD4 T cells are depleted.
B6 mice received allogeneic B10.A BMT with 3 Gy TBI/anti-CD154 (A,B). One group also received depleting anti-CD4 mAb (C,D). Four days post-BMT, splenocytes were restimulated in vitro with donor B10.A or third party B10.RIII cells, in the absence (A,C) or presence (B,D) of lL-2. After 5 days, cytotoxicity against donor-type or third party target cells was measured. Each line represents one mouse.
Requirement for recipient B cells for CD8 T cell tolerance
We next sought to identify the MHC class II+ recipient APC population(s) needed for CD8 tolerance induction. To examine the role of recipient B cells, we compared B cell-deficient μMT and WT B6 mice as recipients of T cell-depleted MHC-mismatched B10.A BMT. Whereas all but one WT mouse developed durable multilineage chimerism, none of the μMT recipients was chimeric (Fig. 5A,B). However, when CD8 T cells were depleted, both WT and μMT mice achieved robust mixed chimerism (Fig. 5C,D). In a repeat experiment, CD8-depleted WT and μMT chimeras accepted donor-type skin grafts, whereas 3rd party grafts were promptly rejected (data not shown). These data demonstrate that recipient B cells are required in the pathway leading to tolerization of pre-existing donor-reactive CD8 cells.
To confirm the ability of recipient B cells to promote alloreactive CD8 cell tolerance and to exclude the possibility that the absence of antibodies in μMT mice might be sufficient to preclude donor engraftment, we evaluated the effect of providing WT B cells to μMT recipients of allogeneic marrow with our regimen. Adoptive transfer of 20-23×106 B cells from WT B6 mice at the time of allogeneic BMT allowed the achievement of durable mixed chimerism (Fig. 6A,B) and donor-specific skin graft tolerance (Fig. 6C) in μMT recipients. Infused B cells were identified in the adoptive recipients as host-type B cells and represented 5± 2.9% (SD) of lymphocytes at 1.5 week and 2.9±0.5% of lymphocytes at 4 weeks after BMT. Similar outcomes were achieved in a second experiment (not shown). These results confirm the ability of recipient B cells to promote donor engraftment and make it unlikely that natural antibodies play a critical role in the tolerance pathway, since such antibodies were not present at the time of transplant in μMT mice receiving adoptively transferred B cells.
We next examined the requirement for class II expression on the adoptively transferred B cells to promote CD8 tolerance. As shown in Fig. 6D, adoptive transfer of MHC class II-deficient B cells promoted the achievement of mixed chimerism as effectively as WT B cells in μMT recipients. Thus, recipient B cells promote CD8 T cell tolerance independently of their ability to present antigen on MHC II to recipient CD4 cells.
Requirement for recipient dendritic cells for CD8 T cell tolerance
We then examined the role of recipient DCs in tolerance induction by using diphtheria toxin receptor (DTR)-transgenic mice (14), which express the primate DTR as a transgene under the murine CD11c promoter. Injection with diphtheria toxin (DT) selectively depletes DCs from these mice for 48-72 hrs. Since repeated DT injections induce neurotoxicity in these transgenic mice, we generated CD45 congenic BM chimeras expressing DTR only on hematopoietic cells. These mice tolerated repetitive DT injections well, allowing 90-95% DC depletion (Fig. 7A). B6 mice reconstituted with either DTR tg or B6 BM received DT three times per week on Days -1 through +17 with respect to B10.A BMT with 3Gy TBI and anti-CD154. One group of DTR tg BM-reconstituted mice was also depleted of CD8 T cells. In B6-reconstituted mice, no difference in chimerism was observed between DT-treated and -untreated groups. In contrast, DT treatment in DTR tg BM-reconstituted mice completely prevented chimerism induction (Fig. 7B-D). However, CD8 depletion allowed achievement of robust mixed chimerism in DC-depleted mice. Since CD4 cells in DT-treated DTR tg BM-reconstituted recipients were able to reject allogeneic BM given without anti-CD154 treatment (data not shown), the studies together indicate that recipient CD11c+ DCs are required for tolerance induction of CD8, but not CD4 T cells.
Discussion
The studies presented here demonstrate that host MHC class II recognition by CD4 T cells is required for tolerization of directly alloreactive peripheral CD8 T cells in recipients of allogeneic BMT with anti-CD154. Surprisingly, both recipient B cells and DCs are required in the pathway leading to peripheral CD8 T cell tolerance.
Alloimmune responses are unique in that they involve two pathways of alloantigen recognition, termed direct and indirect (15). Direct recognition denotes recognition of an intact foreign MHC molecule presented on donor cells and is characterized by a high precursor frequency of alloreactive T cells (16,17) and vigorous mixed lymphocyte and cytotoxic responses. The indirect pathway of alloantigen presentation requires uptake and processing of donor antigens by recipient antigen presenting cells (APCs) and presentation of donor peptides on recipient MHC molecules (18,19). The precursor frequency of indirectly alloreactive T cells is about 100-fold lower than that of directly alloreactive T cells (17). The role of indirectly versus directly alloreactive CD4 T cells for tolerance induction using BMT and anti-CD154 and the APC populations involved in this process are unknown. One possible explanation for our results is that indirectly alloreactive CD4 cells promote the tolerance of directly alloreactive CD8 cells. An important role for the MHC class II indirect pathway for successful heart allograft tolerance induction was previously suggested (20,21), but the role of indirect CD4 recognition in tolerizing CD4 versus CD8 cells was not dissected. Since, in contrast to bone marrow rejection, heart allograft rejection in the mouse is highly dependent on CD4 cells, these observations were consistent with a role for indirectly alloreactive CD4 cells in tolerizing directly alloreactive CD4 cells. A study in a skin graft model showed that indirect class II allorecognition promotes tolerance of directly alloreactive CD4 cells with costimulatory blockade (22). In our model, no cross-talk is needed between the two CD4 pathways, since the presence of only directly alloreactive CD4 T cells in MHC class II-deficient recipients allowed achievement of stable mixed chimerism when CD8 T cells were depleted. Surprisingly, however, our studies show that recipients lacking the capacity for indirect presentation not only on MHC class II but also on class I fail to achieve chimerism. Thus, if indirectly alloreactive CD4 T cells are involved, these data suggest the existence of a pathway allowing cross-talk between indirectly alloreactive CD4 cells and directly alloreactive CD8 T cells, as it was recently proposed in a model of fully mismatched tracheal allograft rejection (23).
An alternative explanation for the requirement for recipient hematopoietic class II in tolerizing directly alloreactive CD8 cells and for other studies implicating the indirect pathway in tolerance models would be that the ability to activate mature CD4 T cells is altered in the absence of peripheral MHC class II expression (24), so that they are incapable of delivering tolerogenic signals to critical host APCs that are needed for CD8 tolerance. In fact, CD4 cells adoptively transferred from WT mice to an MHC class II-deficient environment become hyper-reactive to self, lose regulatory T cell function and reject 3rd party grafts with accelerated kinetics (25). Thus, CD4 cells may be generally hyper-reactive in the absence of peripheral host MHC class II. We do not favor a general lack of “Treg” function as an explanation for our results, because the regulatory CD4 cells required for CD8 cell tolerance in this model do not have characteristics of natural Tregs (8). Furthermore, a general requirement for class II for regulatory function would not help us to understand the results using mice deficient only in B cells or DCs, since in these models MHC class II signal is still delivered by other professional APCs. However, it is possible that tonic interactions of CD4 T cells with MHC class II are needed to make the CD4 cells capable of conditioning host and/or donor APCs in a manner that renders them tolerogenic for CD8 cells.
Our studies demonstrate that recipient B cells and CD11c+ DCs are both necessary to tolerize peripheral alloreactive CD8 cells. To our knowledge, neither cell population has been previously implicated in tolerizing alloreactive CD8 cells in vivo, and the requirement for both cell types is rather surprising. Direct interaction between B cells and DCs has been reported to play an important role for CTL priming (26-28), and such an interaction might also play a role in CTL tolerance. Immature DCs and those differentiated under special conditions in vitro (29,30), which may have low expression of MHC class II and costimulatory molecules (reviewed in (31)), have been shown to suppress immunity in various models. Our findings are consistent with the possibility that recipient DCs present donor-specific peptides to indirectly alloreactive CD4 cells, and that these DCs modify the microenvironment in a manner that leads to tolerance of directly alloreactive CD8 cells encountering antigen on donor APCs. This might occur via secretion of soluble mediators that inhibit T cell responses (32).
Small resting B cells can tolerize naïve T cells recognizing antigens presented by the B cells (33-35). However, the concept that B cells not expressing the donor alloantigens recognized by a CD8 T cell population can tolerize that population is novel. If in our model B cells were merely required as a source of host class II MHC to down-modulate CD4 activation levels, adoptive transfer of B cells at the time of transplant would be unlikely to correct the deficiency rapidly enough to allow achievement of chimerism and tolerance. Moreover, recipient B cells lacking class II MHC expression, when given at the time of allogeneic BMT, promoted chimerism and tolerance in B cell-deficient recipients. This observation confirms that B cells are not simply required as a source of recipient class II and argues for a more specific role for recipient B cells in the induction of CD8 tolerance to the donor.
B cells might promote tolerance by inducing regulatory T cell function and IL-10 production as described in experimental autoimmune encephalitis (36) and in a GVHD model (37). B cells play a role when anti-CD45RB mAb is used for tolerance induction to heart allografts (38). This model is dependent on regulatory T cells (39), but the T cell population tolerized by these B cells was not identified and the role of DCs not investigated. B cells may also produce TGF-β. Indeed, such regulatory B cells have been described in several models of chronic inflammation (40), and TGF-β production by LPS activation has been shown to tolerize resting CD8 T cells (41). B cells might also provide critical tolerogenic signals to CD8 cells, such as PD-L1, which we have recently shown to play an essential role in CD8, but not CD4 cell tolerance in this model (F. Haspot et al, manuscript submitted). Of considerable significance, upregulation of PD-L1 on both DCs and B cells of the recipient requires host CD4 cells in animals rendered tolerant with our regimen (Haspot, Fehr et al, manuscript submitted). Thus, our data could be explained by a model in which naïve alloreactive CD4 cells are required to upregulate PD-L1 in the presence of anti-CD154 on these APC populations that then interact with alloreactive PD-1-positive CD8 cells, rendering them tolerant. Pick-up and presentation of donor-derived MHC class I molecules to directly alloreactive CD8 cells by recipient B cells (and DCs) could be invoked to explain a role for these cell types in tolerizing directly alloreactive CD8 cells. Donor alloantigen pick-up by recipient dendritic cells has been described as an alternative pathway of alloantigen presentation to directly alloreactive recipient T cells (42-44). Interestingly, bone marrow-resident CD11c+ DCs promote the presence of recirculating mature B cells in bone marrow niches (45), an interaction that may have immunological consequences in the context of bone marrow transplantation.
Our previous studies showed that CD8 tolerance measured in vitro at 2 weeks in CML assays correlated with the achievement of initial and durable mixed allogeneic chimerism in mice receiving allogeneic BMT with 3 Gy TBI and anti-CD154 mAb (10). We have now shown that this tolerance is evident as early as 4 days post-BMT and cannot be overcome by the addition of exogenous IL-2. However, when CD4 cells are depleted from recipients, tolerance can be broken by the addition of IL-2. Thus, in these animals, induction of initial mixed chimerism reflects successful tolerization of pre-existing peripheral CD8 T cells, which is followed later by central deletional tolerance of T cells that develop de novo following the transplant (5). The CTL results presented here confirm in vitro the requirement for CD4 T cells to fully regulate donor-reactive CD8 cells early post-transplant, consistent with our previous in vivo results (8). Consistently, donor-reactive CD8 cells are not deleted by Day 4 and have an activated phenotype, with upregulation of CD69, CD25 and CD44 (Haspot, Fehr et al, manuscript submitted). Donor-reactive CD8 cells are fully deleted by Day 10 post-BMT, when CD4 cells are no longer required for their tolerance (8). It is of interest that the CTL “anergy” on Day 4 was not overcome by the addition of exogenous IL-2 unless CD4 cells were depleted from the recipient, in which case anti-donor responses were revealed by IL-2. CD4 cells in this system may promote the induction in CD8 cells of a previously-reported form of anergy that cannot be overcome by IL-2 (46). In combination, our data suggest that donor-reactive CD8 cells rapidly undergo anergy followed by death within 2 weeks of BMT in this model. It is possible that the death phase occurred in vitro in CD8 cells from animals in which CD4 cells were not depleted, thus explaining the failure to reverse unresponsiveness by the addition of exogenous IL-2.
Taken together, our studies show that: (i) host MHC class II recognition by CD4 T cells is critical for peripheral CD8 tolerance; (ii) CD8 T cell tolerance requires recipient B cells and DCs; and (iii) interactions with recipient class II MHC by recipient CD4 T cells are required to tolerize directly alloreactive CD8 T cells, suggesting cross-talk between host MHC restricted CD4 cells and donor MHC-restricted CD8 cells. These results provide new mechanistic insights into allograft tolerance. We believe that an in-depth understanding of the cellular interactions involved in tolerance induction will be essential to the development of reliable protocols for clinical tolerance.
Acknowledgements
We thank Prof. Rolf Zinkernagel, Dr. Thomas Wekerle and Ms. Anne-Laurence Blanc for critical review of this manuscript, Mr. Orlando Moreno for technical assistance, and Ms. Kelly Walsh for expert assistance with the manuscript.
Nonstandard abbreviations
- BM(T)
bone marrow (transplantation)
- CML
cell-mediated lympholysis
- DT(R)
diphtheria toxin (receptor)
- HSC
hematopoietic stem cell
- TBI
total body irradiation
- Treg
T regulatory cell
Footnotes
Supported by NIH grants R01 HL49915 and P01 HL18646. TF was supported by the Swiss Foundation for Medical and Biological Grants/Novartis Switzerland and the Walter and Gertrud Siegenthaler Foundation (University of Zurich). FH was supported by the Fondation pour la Recherche Medicale, France and the American Society of Transplantation/American Society of Blood and Marrow Transplantation.
References
- 1.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr., Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. N.Engl.J.Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, Preffer F, Tolkoff-Rubin N, Dey BR, Saidman SL, Kraus A, Bonnefoix T, McAfee S, Power K, Kattleman K, Colvin RB, Sachs DH, Cosimi AB, Sykes M. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am.J.Transplant. 2006;6:2121–2133. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 3.Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl.Immunol. 2004;13:117–130. doi: 10.1016/j.trim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Sharabi Y, Sachs DH, Sykes M. In: Gergely J, Benczur M, Falus A, Fust G, Medgyesi G, Petranyi G, Rajnavolgyi E, editors. T cell subsets resisting induction of mixed chimerism across various histocompatibility barriers; Progress in Immunology VIII. Proceedings of the Eighth International Congress of Immunology, Budapest, 1992; Springer-Verlag, Heidelberg. 1992.pp. 801–805. [Google Scholar]
- 5.Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG, Zhao G, Sykes M. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp.Med. 1998;187:2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14:417–424. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- 7.Kurtz J, Shaffer J, Lie A, Anosova N, Benichou G, Sykes M. Mechanisms of early peripheral CD4 T-cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: evidence for anergy and deletion but not regulatory cells. Blood. 2004;103:4336–4343. doi: 10.1182/blood-2003-08-2642. [DOI] [PubMed] [Google Scholar]
- 8.Fehr T, Takeuchi Y, Kurtz J, Wekerle T, Sykes M. Early regulation of CD8 T cell alloreactivity by CD4(+)CD25(-)T cells in recipients of anti-CD154 antibody and allogeneic BMT is followed by rapid peripheral deletion of donor-reactive CD8(+) T cells, precluding a role for sustained regulation. Eur.J.Immunol. 2005;35:2679–2690. doi: 10.1002/eji.200526190. [DOI] [PubMed] [Google Scholar]
- 9.Kurtz J, Wekerle T, Sykes M. Tolerance in mixed chimerism - a role for regulatory cells? Trends Immunol. 2004;25:518–523. doi: 10.1016/j.it.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi Y, Ito H, Kurtz J, Wekerle T, Ho L, Sykes M. Earlier Low-Dose TBI or DST Overcomes CD8+ T-Cell-Mediated Alloresistance to Allogeneic Marrow in Recipients of Anti-CD40L. Am.J.Transplant. 2004;4:31–40. doi: 10.1046/j.1600-6135.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 11.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 12.Chang CH, Guerder S, Hong SC, Van Ewijk W, Flavell RA. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- 13.Perarnau B, Saron MF, San Martin BR, Bervas N, Ong H, Soloski MJ, Smith AG, Ure JM, Gairin JE, Lemonnier FA. Single H2Kb, H2Db and double H2KbDb knockout mice: peripheral CD8+ T cell repertoire and anti-lymphocytic choriomeningitis virus cytolytic responses. Eur.J.Immunol. 1999;29:1243–1252. doi: 10.1002/(SICI)1521-4141(199904)29:04<1243::AID-IMMU1243>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Jung S, Unutmaz D, Wong P, Sano G, De los SK, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould DS, Auchincloss H., Jr. Direct and indirect recognition: the role of MHC antigens in graft rejection. Immunol.Today. 1999;20:77–82. doi: 10.1016/s0167-5699(98)01394-2. [DOI] [PubMed] [Google Scholar]
- 16.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J.Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Sun YK, Xi YP, Maffei A, Reed E, Harris P, Suciu-Foca N. Contribution of direct and indirect recognition pathways to T cell alloreactivity. J.Exp.Med. 1993;177:1643–1650. doi: 10.1084/jem.177.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherwood RA, Brent L, Rayfield LS. Presentation of alloantigens by host cells. Eur.J.Immunol. 1986;16:569–574. doi: 10.1002/eji.1830160519. [DOI] [PubMed] [Google Scholar]
- 19.Popov IA, Fedoseyeva EV, Orr PL, Garovoy MR, Benichou G. Direct evidence for in vivo induction of CD8+ cytotoxic T cells directed to donor MHC class I peptides following mouse allotransplantation. Transplantation. 1995;60:1621–1624. [PubMed] [Google Scholar]
- 20.Kishimoto K, Yuan X, Auchincloss H, Jr., Sharpe AH, Mandelbrot DA, Sayegh MH. Mechanism of action of donor-specific transfusion in inducing tolerance: role of donor MHC molecules, donor co-stimulatory molecules, and indirect antigen presentation. J.Am.Soc.Nephrol. 2004;15:2423–2428. doi: 10.1097/01.ASN.0000137883.20961.2D. [DOI] [PubMed] [Google Scholar]
- 21.Yamada A, Chandraker A, Laufer TM, Gerth AJ, Sayegh MH, Auchincloss H., Jr. Recipient MHC class II expression is required to achieve long-term survival of murine cardiac allografts after costimulatory blockade. J.Immunol. 2001;167:5522–5526. doi: 10.4049/jimmunol.167.10.5522. [DOI] [PubMed] [Google Scholar]
- 22.Rulifson IC, Szot GL, Palmer E, Bluestone JA. Inability to induce tolerance through direct antigen presentation. Am.J.Transplant. 2002;2:510–519. doi: 10.1034/j.1600-6143.2002.20604.x. [DOI] [PubMed] [Google Scholar]
- 23.Richards DM, Zhang N, Dalheimer SL, Mueller DL. Allopeptide-specific CD4(+) T cells facilitate the differentiation of directly alloreactive graft-infiltrating CD8(+) T Cells. Am.J.Transplant. 2007;7:2269–2278. doi: 10.1111/j.1600-6143.2007.01934.x. [DOI] [PubMed] [Google Scholar]
- 24.Grossman Z, Paul WE. Adaptive cellular interactions in the immune system: the tunable activation threshold and the significance of subthreshold responses. Proc.Natl.Acad.Sci.U.S.A. 1992;89:10365–10369. doi: 10.1073/pnas.89.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhandoola A, Tai X, Eckhaus M, Auchincloss H, Mason K, Rubin SA, Carbone KM, Grossman Z, Rosenberg AS, Singer A. Peripheral expression of self-MHC-II influences the reactivity and self-tolerance of mature CD4(+) T cells: evidence from a lymphopenic T cell model. Immunity. 2002;17:425–436. doi: 10.1016/s1074-7613(02)00417-x. [DOI] [PubMed] [Google Scholar]
- 26.Dubois B, Vanbervliet B, Fayette J, Massacrier C, van Kooten C, Briere F, Banchereau J, Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J.Exp.Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang NN, Han SB, Hwang IY, Kehrl JH. B cells productively engage soluble antigen-pulsed dendritic cells: visualization of live-cell dynamics of B cell-dendritic cell interactions. J.Immunol. 2005;175:7125–7134. doi: 10.4049/jimmunol.175.11.7125. [DOI] [PubMed] [Google Scholar]
- 28.Diaz-de-Durana Y, Mantchev GT, Bram RJ, Franco A. TACI-BLyS signaling via B-cell-dendritic cell cooperation is required for naive CD8+ T-cell priming in vivo. Blood. 2006;107:594–601. doi: 10.1182/blood-2004-12-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, Adorini L. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J.Immunol. 2007;178:145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 30.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but Enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J.Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 31.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu.Rev.Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 32.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 34.Bennett SR, Carbone FR, Toy T, Miller JF, Heath WR. B cells directly tolerize CD8(+) T cells. J.Exp.Med. 1998;188:1977–1983. doi: 10.1084/jem.188.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niimi M, Roelen DL, Wong W, Hara M, Morris PJ, Wood KJ. Resting B cells as tolerogens in vivo but only for minor histocompatibility antigens: evidence for activation of resting B cells in vivo. Transplantation. 1997;64:1330–1335. doi: 10.1097/00007890-199711150-00016. [DOI] [PubMed] [Google Scholar]
- 36.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J.Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 37.Rowe V, Banovic T, MacDonald KP, Kuns R, Don AL, Morris ES, Burman AC, Bofinger HM, Clouston AD, Hill GR. Host B cells produce IL-10 following TBI and attenuate acute GVHD after allogeneic bone marrow transplantation. Blood. 2006;108:2485–2492. doi: 10.1182/blood-2006-04-016063. [DOI] [PubMed] [Google Scholar]
- 38.Deng S, Moore DJ, Huang X, Lian MM, Mohiuddin M, Velededeoglu E, Lee MK, Sonawane S, Kim J, Wang J, Chen H, Corfe SA, Paige C, Shlomchik M, Caton A, Markmann JF. Cutting edge: transplant tolerance induced by anti-CD45RB requires B lymphocytes. J.Immunol. 2007;178:6028–6032. doi: 10.4049/jimmunol.178.10.6028. [DOI] [PubMed] [Google Scholar]
- 39.Deng S, Moore DJ, Huang X, Mohiuddin M, Lee MK, Velidedeoglu E, Lian MM, Chiaccio M, Sonawane S, Orlin A, Wang J, Chen H, Caton A, Zhong R, Markmann JF. Antibody-induced transplantation tolerance that is dependent on thymus-derived regulatory T cells. J.Immunol. 2006;176:2799–2807. doi: 10.4049/jimmunol.176.5.2799. [DOI] [PubMed] [Google Scholar]
- 40.Mizoguchi A, Bhan AK. A case for regulatory B cells. J.Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 41.Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B cells activated by lipopolysaccharide, but not by anti-Ig and antiCD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J.Immunol. 2003;170:5897–5911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- 42.Smyth LA, Herrera OB, Golshayan D, Lombardi G, Lechler RI. A novel pathway of antigen presentation by dendritic and endothelial cells: Implications for allorecognition and infectious diseases. Transplantation. 2006;82:S15–S18. doi: 10.1097/01.tp.0000231347.06149.ca. [DOI] [PubMed] [Google Scholar]
- 43.Herrera OB, Golshayan D, Tibbott R, Salcido OF, James MJ, MarelliBerg FM, Lechler RI. A novel pathway of alloantigen presentation by dendritic cells. J.Immunol. 2004;173:4828–4837. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 44.Dolan BP, Gibbs KD, Jr., Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J.Immunol. 2006;177:6018–6024. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- 45.Sapoznikov A, Pewzner-Jung Y, Kalchenko V, Krauthgamer R, Shachar I, Jung S. Perivascular clusters of dendritic cells provide critical survival signals to B cells in bone marrow niches. Nat.Immunol. 2008;9:388–395. doi: 10.1038/ni1571. [DOI] [PubMed] [Google Scholar]
- 46.Wells AD, Walsh MC, Bluestone JA, Turka LA. Signaling through CD28 and CTLA-4 controls two distinct forms of T cell anergy. J.Clin.Invest. 2001;108:895–903. doi: 10.1172/JCI13220. [DOI] [PMC free article] [PubMed] [Google Scholar]