Abstract
Background
Digital pulse amplitude augmentation in response to hyperemia is a novel measure of peripheral vasodilator function that partially depends on endothelium-derived nitric oxide. Baseline digital pulse amplitude reflects local peripheral arterial tone. The relation of digital pulse amplitude and digital hyperemic response to cardiovascular risk factors in the community is unknown.
Methods and Results
Using a fingertip peripheral arterial tonometry (PAT) device, we measured digital pulse amplitude in Framingham Third Generation Cohort participants (n=1957, mean age 40±9 years, 49% women) at baseline and in 30 second intervals for 4-minutes during reactive hyperemia induced by 5-minute forearm cuff occlusion. To evaluate the vascular response in relation to baseline, adjusting for systemic effects and skewed data, we expressed the hyperemic response (termed PAT ratio) as the natural logarithm of the post-deflation to baseline pulse amplitude ratio in the hyperemic finger divided by the same ratio in the contralateral finger that served as control. The relation of PAT ratio to cardiovascular risk factors was strongest in the 90-120 second post-deflation interval (overall model R2=0.159). In stepwise multivariable linear regression models, male sex, body mass index, total/HDL cholesterol, diabetes, smoking and lipid-lowering treatment were inversely related to PAT ratio; whereas increasing age was positively related to PAT ratio (all P<0.01).
Conclusions
Reactive hyperemia produced a time-dependent increase in fingertip pulse amplitude. Digital vasodilator function is related to multiple traditional and metabolic cardiovascular risk factors. Our findings support further investigations to define the clinical utility and predictive value of digital pulse amplitude.
Keywords: vascular, epidemiology, risk factors, cohort study
Endothelial dysfunction is a key component of atherogenesis and contributes to the development of clinical cardiovascular disease.1 In the presence of vascular risk factors, endothelial cells undergo phenotypic changes resulting in decreased nitric oxide bioactivity, thereby promoting vasoconstriction, inflammation and thrombosis.2 In human studies, risk factors for vascular disease have been associated with impaired vasomotor function and individuals with abnormal vasodilator function have increased cardiovascular event rates.3
Measurement of peripheral vasodilator response using a fingertip pulse amplitude tonometry (PAT) device is emerging as a useful method for assessing vascular function.4,5 In response to hyperemic flow, digital pulse amplitude increases, and this response has been shown to depend, in part, on nitric oxide synthesis.6 Augmentation of pulse amplitude in the finger with hyperemia is a complex response to ischemia and reflects both changes in digital flow and digital microvessel dilation. In prior clinical studies, impairment of pulse amplitude hyperemic response was associated with the presence of coronary artery endothelial dysfunction.4,7
Previous studies investigating the relations of digital pulse amplitude to clinical risk factors have been limited to small, selected samples. We sought to evaluate the correlates of digital pulse amplitude responses as a measure of peripheral vascular function in the large, community-based Framingham Heart Study.
Methods
Participants
The design for the Third Generation Cohort has been described elsewhere.8 There were 4,095 participants in the first examination cycle (2002-2005).8 The acquisition of PAT data began part way through the first examination; therefore, 2,217 participants were eligible for participation. Of those eligible for participation, we excluded 260 subjects due to: Raynaud's disease (n=2), finger probe unavailable (n=7), patient refusal (n=5), missing data file (n=8), sonographer error (n=1), anatomic issue (mastectomy, arm or hand abnormality, n=13), technically inadequate study (n=207), and missing covariate data (n=17). Technically inadequate studies included those with: inadequate flow occlusion (n=73), poor PAT signal quality (n=64), incomplete PAT data acquisition (n=64), computer error (n=6). Participants who had available PAT data were more likely to be male, smokers, on hypertensive medications, on lipid-lowering medications and had higher total cholesterol/HDL ratio (see Supplementary Table 1).
All participants underwent routine medical history, physical examination, and laboratory assessment of risk factors and C-reactive protein (CRP). Current cigarette smoking (within the year prior to examination) was determined by self-report. Waist circumference was measured at the umbilicus level. The Boston University Medical Center Institutional Review Board approved the study and all participants provided written informed consent.
Determination of Digital Pulse Amplitude
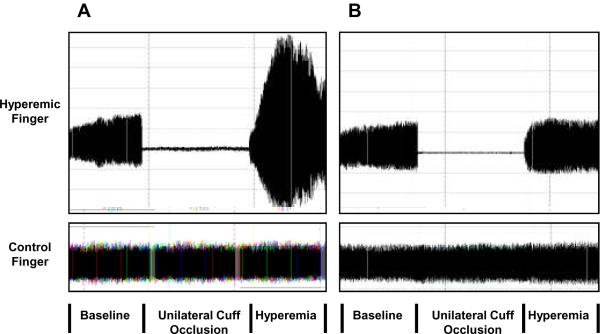
Digital pulse amplitude was measured in the fasting state using a PAT device placed on the tip of each index finger (Endo-PAT2000, Itamar-Medical, Caesarea, Israel). The PAT device is comprised of a pneumatic plethysmograph that applies uniform pressure to the surface of the distal finger allowing for measurement of pulse volume changes in the finger. Throughout the study, the inflation pressure of the digital device was electronically set to 10mmHg below diastolic blood pressure or 70mmHg (whichever was lower). Baseline pulse amplitude was measured from each fingertip for 2 minutes and 20 seconds. Arterial flow was interrupted for 5 minutes by cuff placed on one proximal forearm (Hokanson AG101) at whichever occlusion pressure would be higher: 200 mmHg or 60 mmHg plus systolic blood pressure. Pulse amplitude was recorded electronically in both fingers and analyzed by a computerized, automated algorithm (Itamar Medical, Caesarea, Israel) that provided the average pulse amplitude for each 30-second interval following forearm cuff deflation up to four minutes; see representative tracing in Figure 1.
Figure 1.
Panel A displays the pulse amplitude tracing in a participant with a PAT ratio in the highest tertile. Panel B displays the pulse amplitude tracing in a participant with a PAT ratio in the lowest tertile. As shown, in the arm undergoing hyperemia (upper tracing in A and B) baseline amplitude is recorded; subsequently during cuff inflation flow is occluded and rapidly rises after release during the hyperemic period in an individual with a high response (panel A), but in an individual with a low response (panel B). In the contralateral, control finger (lower tracing in A and B) flow continues throughout and there is minimal change in pulse amplitude.
Statistical Analysis
For each 30-second interval, pulse amplitude response to hyperemia was calculated from the hyperemic fingertip as the ratio of the post-deflation pulse amplitude to the baseline pulse amplitude (i.e. Xht/ Xh0: with h denoting hyperemic finger, t denoting time interval and 0 denoting baseline, X being the pulse amplitude). We divided this result by the corresponding ratio from the contralateral, control hand (i.e. Xct/ Xc0: with c denoting the control finger, t denoting time interval and 0 denoting baseline) to obtain the PAT ratio. In scatterplots, the relation between baseline and post-deflation variables was not linear and errors were not homoscedastic; therefore we used a natural logarithmic (ln) transformation of the PAT ratio, namely: PAT ratio = ln[ (Xht/ Xh0) / (Xct/ Xc0)].
We tabulated descriptive characteristics separately by sex. We examined age- and sex-adjusted linear regression models, to determine the correlates of 1) baseline pulse amplitude and 2) PAT ratio responses with the following clinical covariates: systolic and diastolic blood pressure, heart rate, body mass index, total/HDL cholesterol, triglycerides, glucose, diabetes, current smoking, hormone replacement therapy, hypertension treatment, lipid-lowering treatment, and prevalent cardiovascular disease. For each 30-second time interval following cuff release, we examined stepwise selection (with age and sex forced in) to create multivariable models, with the criterion P<0.05 for a variable to enter and stay in the model. Secondarily, we tested potential effect modification by age and sex for covariates retained in the stepwise models. All analyses were performed using SAS 8.1.9 Two-sided P<0.05 for primary analyses and P<0.01 for secondary analyses were considered statistically significant. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Participant Characteristics and PAT
The clinical characteristics of the 1957 study participants (mean age 40±9 years, 49% women) are shown in Table 1. Baseline pulse amplitude was higher in men than in women (Table 2). As shown in Table 2, baseline pulse amplitude was correlated with PAT ratio in both men and women.
Table 1.
Participant Characteristics
| Characteristic | Men (n=1003) | Women (n=954) |
|---|---|---|
| Age, years | 40±9 | 40±9 |
| Systolic blood pressure, mm Hg | 120±12 | 113±14 |
| Diastolic blood pressure, mm Hg | 78±9 | 73±9 |
| Heart rate, beats per minute | 61±9 | 63±9 |
| Body mass index, kg/m2 | 27.9±4.7 | 26.3±6.2 |
| Total cholesterol/HDL, ratio | 4.4±1.4 | 3.3±1.1 |
| Triglycerides, mg/dL | 136±102 | 99±71 |
| Fasting glucose, mg/dL | 99±18 | 92±18 |
| Diabetes, % | 4 | 2 |
| Smoking, % | 21 | 17 |
| Hypertension, % | 21 | 13 |
| Hormone replacement therapy, % | -- | 4 |
| Hypertension medication, % | 11 | 9 |
| Lipid-lowering medication, % | 12 | 5 |
| Prevalent cardiovascular disease, % | 2 | 1 |
Continuous variables, mean±SD
Table 2.
Pulse Amplitude Measures
| Baseline Pulse Amplitude | PAT ratio | Correlation Baseline Pulse Amplitude and PAT ratio | |
|---|---|---|---|
| Mean±SD | Mean±SD | r | |
| Men | 6.08±0.72 | 0.58±0.34 | -0.67* |
| Women | 5.14±0.76 | 0.81±0.36 | -0.54* |
p<0.0001;
PAT measures are natural logarithm transformed
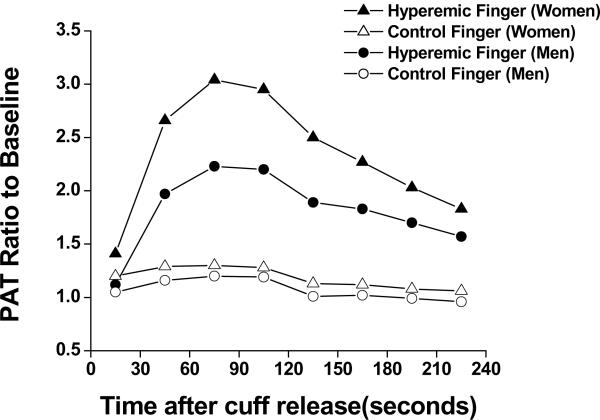
As shown in Figure 2, following forearm cuff deflation, the ratio of the pulse amplitude to baseline rose rapidly in the hyperemic fingertip with maximal response occurring in the 60-90 second post-deflation interval. Following forearm cuff deflation in the contralateral arm, in the control fingertip there was a minimal and non-sustained increase in pulse amplitude.
Figure 2.
Pulse amplitude response shown for the hyperemic finger and contralateral finger in women and men. Men had lower responses throughout in both fingers. Values are means. The minimum and maximum standard errors were 0.01 to 0.04.
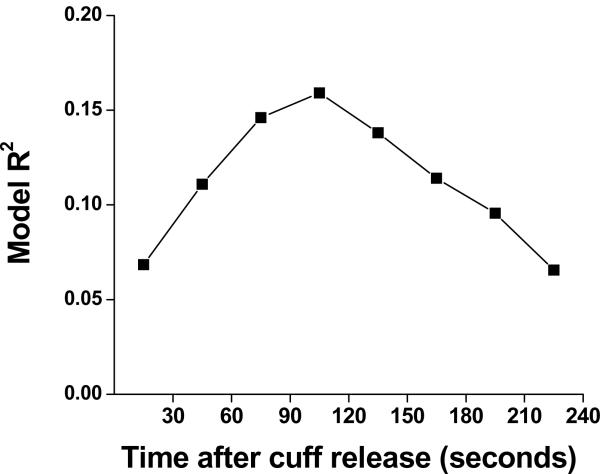
To determine the relation between the hyperemic response over time following cuff deflation and clinical cardiovascular risk factors, we performed stepwise regression models for the PAT ratio for each 30 second interval with age and sex forced in, selecting from systolic blood pressure, diastolic blood pressure, heart rate, body mass index, total/HDL cholesterol, triglycerides, glucose, diabetes, current smoking, hormone replacement therapy, hypertension treatment, lipid-lowering treatment, and prevalent cardiovascular disease. As shown in Figure 3, the overall model R2 (a representation of the proportion of variability in PAT ratio at each time interval that was explained by the regression on the model covariates) was maximized in the 90-120 second post-deflation interval. Previous investigators have used the ratio of pulse amplitude for 60 seconds beginning 1-minute following cuff release (an average of the 60-90 and 90-120 second intervals) to the baseline pulse amplitude divided by the corresponding ratio in the control finger.4,7 The overall model R2 for the mean PAT ratio 60-120 seconds was 0.152, which appeared lower than the model R2 for the PAT for the 90-120 second time interval. Therefore, we selected the 90-120 second time interval PAT ratio for subsequent evaluation of the relations between specific risk factors and digital pulse amplitude hyperemic response.
Figure 3.
Multivariable relation between cardiovascular risk factors including age, sex, systolic blood pressure, diastolic blood pressure, heart rate, body mass index, total/HDL cholesterol, triglycerides, glucose, diabetes, current smoking, hormone replacement therapy, hypertension treatment, lipid-lowering treatment, and prevalent cardiovascular disease, and the digital hyperemic response (PAT ratio) in the 30 second time intervals after cuff occlusion. As displayed, the strongest relation occurs in the 90-120 second post-deflation interval.
Clinical correlates of Baseline Pulse Amplitude and Pulse Amplitude Hyperemic Response
In age- and sex-adjusted models, baseline pulse amplitude was directly related to most cardiovascular risk factors, and PAT ratio was inversely associated with most cardiovascular disease risk factors (Table 3). In stepwise multivariable regression models, the factors inversely associated with PAT ratio were male sex, body mass index, total/HDL cholesterol, diabetes, smoking and lipid-lowering treatment, whereas advancing age was associated with higher mean PAT ratio (Table 4). The overall model explained 15.9% of the variability in PAT ratio. The correlates of increasing baseline pulse amplitude were systolic blood pressure, body mass index, total/HDL cholesterol, smoking, and lipid-lowering treatment. Female sex and diastolic blood pressure were associated with lower baseline pulse amplitude.
Table 3.
Age- and Sex-Adjusted Models of Pulse Amplitude
| Characteristic | Mean Baseline | PAT Ratio* | ||
|---|---|---|---|---|
| β (SE) | P | β (SE) | P | |
| Age | 0.07 (0.02) | <0.001 | 0.01 (0.01) | 0.55 |
| Sex, female vs. male | -0.94 (0.03) | <0.0001 | 0.24 (0.02) | <0.0001 |
| Systolic blood pressure | 0.13 (0.02) | <0.0001 | -0.02 (0.01) | 0.03 |
| Diastolic blood pressure | 0.08 (0.02) | <0.0001 | -0.02 (0.01) | 0.06 |
| Heart rate | 0.05 (0.02) | 0.005 | -0.04 (0.01) | <0.0001 |
| Body mass index | 0.23 (0.02) | <0.0001 | -0.06 (0.01) | <0.0001 |
| Total/HDL cholesterol | 0.15 (0.02) | <0.0001 | -0.06 (0.01) | <0.0001 |
| Triglycerides | 0.13 (0.02) | <0.0001 | -0.05 (0.01) | <0.0001 |
| Fasting glucose | 0.11 (0.02) | <0.0001 | -0.04 (0.01) | <0.0001 |
| Diabetes | 0.52 (0.10) | <0.0001 | -0.24 (0.05) | <0.0001 |
| Smoking | 0.28 (0.04) | <0.0001 | -0.09 (0.02) | <0.0001 |
| Hypertension | 0.29 (0.05) | <0.0001 | -0.08 (0.02) | <0.001 |
| Hormone replacement therapy | 0.17 (0.12) | 0.15 | -0.07 (0.06) | 0.21 |
| Hypertension treatment | 0.29 (0.06) | <0.0001 | -0.11 (0.03) | <0.0001 |
| Lipid-lowering treatment | 0.30 (0.06) | <0.0001 | -0.14 (0.03) | <0.0001 |
| Prevalent cardiovascular disease | 0.22 (0.13) | 0.10 | -0.03 (0.06) | 0.62 |
The first two rows present models for age and sex separately, not adjusting for the other variable All continuous variables were standardized to mean 0, SD 1; all dichotomous variables were coded 1=presence and 0=absence of the factor.
Table 4.
Stepwise Models of Baseline and Hyperemic Digital Pulse Amplitude Tonometry
| Mean Baseline | PAT Ratio | |||||
|---|---|---|---|---|---|---|
| Characteristic | β (SE) | Partial R2 | P | β (SE) | Partial R2 | P |
| Age | 0.002 (0.017) | 0.000 | 0.89 | 0.03 (0.01) | 0.005 | 0.001 |
| Sex, female vs. male | -0.79 (0.03) | 0.161 | <0.0001 | 0.18 (0.02) | 0.049 | <0.0001 |
| Systolic blood pressure | 0.08 (0.02) | 0.004 | <0.001 | |||
| Diastolic blood pressure | -0.05 (0.02) | 0.002 | 0.03 | |||
| Body mass index | 0.19 (0.02) | 0.037 | <0.0001 | -0.04 (0.01) | 0.011 | <0.0001 |
| Total/HDL cholesterol | 0.07 (0.02) | 0.004 | <0.001 | -0.04 (0.01) | 0.009 | <0.0001 |
| Diabetes | -0.13 (0.05) | 0.004 | 0.004 | |||
| Smoking | 0.26 (0.04) | 0.013 | <0.0001 | -0.08 (0.02) | 0.007 | <0.0001 |
| Lipid-lowering treatment | 0.20 (0.06) | 0.004 | <0.001 | -0.10 (0.03) | 0.005 | 0.0008 |
| Model R2 | 0.387 | 0.159 | ||||
Age and sex were forced into all models. Variables allowed to enter into stepwise regression models were: systolic and diastolic blood pressure, heart rate, body mass index, total/HDL cholesterol, triglycerides, glucose, diabetes, current smoking, hormone replacement therapy, hypertension treatment, lipid-lowering treatment, and prevalent cardiovascular disease. All continuous variables were standardized to mean 0, SD 1; all dichotomous variables were coded 1=presence and 0=absence of the factor.
Secondary Analyses
Obesity and Baseline Pulse Amplitude and Pulse Amplitude Hyperemic Response
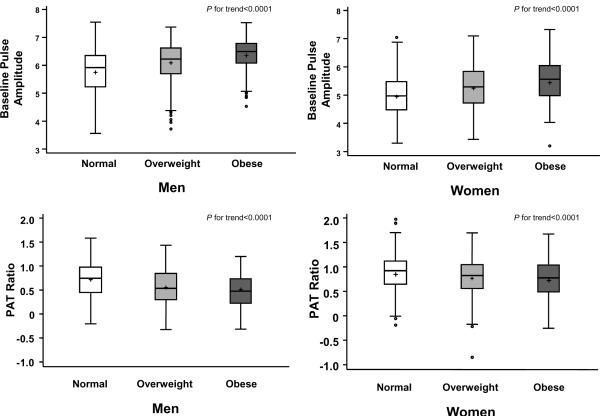
As displayed in Figure 4, there was a significant increase in mean age-adjusted PAT ratio with increasing weight category (trend test of normal, overweight, obese, P<0.0001) in both men and women.
Figure 4.
Boxplot of age-adjusted baseline and hyperemic pulse amplitude according to body mass index category and sex (Normal, <25 kg/m2; Overweight, 25 to <30 kg/m2; Obese ≥30 kg/m2). For men n=283, 471, 249 and for women n=501, 253, 200 for normal, overweight and obese, respectively, P for trend <0.0001.
To evaluate the role of abdominal obesity in the relation between obesity and digital vascular function, we assessed the relation of waist circumference to the PAT measures. In age- and sex adjusted analyses increasing waist circumference was related to higher baseline pulse amplitude (β=0.23, standard error 0.02, P<0.0001) and to lower PAT ratio (β=-0.07, standard error 0.01, P<0.0001). Waist circumference did not enter either the stepwise multivariable model relating cardiovascular risk factors to baseline pulse amplitude or to PAT ratio.
Inflammation and Baseline Pulse Amplitude and Pulse Amplitude Hyperemic Response
To explore the possibility that systemic inflammation may adversely affect digital vascular function, we examined the relation of CRP to PAT measures. Overall the mean CRP was 2.5±4.6mg/L. Due to a skewed distribution, CRP was log transformed for analysis. In age- and sex-adjusted analyses, there was a trend toward a direct relation between CRP and baseline pulse amplitude (β=0.02, standard error 0.01, P=0.09). In age- and sex-adjusted analyses, there was no significant relation between CRP and PAT ratio (β=-0.006, standard error 0.006, P=0.36). CRP did not enter either the stepwise multivariable model relating cardiovascular risk factors to baseline pulse amplitude or to PAT ratio.
Interactions
In multivariable models, for PAT ratio, we observed a sex-body mass index interaction (P<0.01); the body mass index estimated effect size was -0.07 for men and -0.03 for women, confirming that obesity-related decrease in PAT ratio was greater in men than women (Figure 4). In multivariable models, for baseline pulse amplitude, there were significant interactions of sex-body mass index and sex-total/HDL cholesterol ratio (both P<0.01); for body mass index the increase was greater in men than women (0.27 in men and 0.14 in women) whereas for total/HDL cholesterol ratio the increase was greater in women than in men (0.02 in men and 0.15 in women).
The pre-specified interaction tests for between age and the clinical covariates for baseline and PAT ratio were not significant, and there was not a significant relation between age and PAT ratio in sex-adjusted models. However, in the multivariable model we observed an unanticipated positive relation between age and PAT ratios. We examined the step by step details of the stepwise regression analysis to reveal which covariate(s) uncovered a positive age-PAT ratio relation. With age and sex forced in, the first selected covariate was body mass index, revealing a significant positive association between age and PAT ratio (p=0.036). Age was positively correlated with body mass index after adjusting for sex (partial r=0.176; p<0.0001) suggesting negative confounding between age and sex-adjusted PAT ratio.
Discussion
In our large community-based cohort, we evaluated the relations of baseline digital pulse amplitude and digital pulse amplitude hyperemic response, a non-invasive measure of peripheral vascular function, to clinical cardiovascular risk factors. We observed a time-dependent increase in fingertip pulse amplitude that peaked in the 60-90 second interval following induction of reactive hyperemia. In order to identify the most clinically relevant portion of the response, we related the pulse amplitude increase during each 30-second interval to a multivariable risk factor model and observed that the relation was maximized in the interval 90-120 seconds after cuff release. We observed that digital pulse amplitude hyperemic response was higher in women than in men and with advancing age, and was inversely related to multiple risk factors particularly diabetes, body mass index, higher cholesterol concentrations and smoking. Digital vasodilator function was lower with increasing weight category Baseline pulse amplitude was higher in men than in women and was also directly related to several cardiovascular risk factors.
Prior studies support the use of digital PAT as a measure of peripheral vasomotor function. Nitric oxide has been shown to be an important contributor to the augmentation in fingertip pulse amplitude following ischemia; administration of an endothelial nitric oxide synthase inhibitor blunted the PAT hyperemic response.6 Previous investigators also have demonstrated a correlation between the degree of hyperemic response in the fingertip and measures of endothelial vasomotor function. In patients referred for chest pain evaluation, PAT hyperemic response correlated with flow-mediated dilation in the conduit, brachial artery as well as with overall vascular risk factor burden.4 In addition, Bonetti et al. reported that reduced PAT hyperemic response predicted the presence of abnormal coronary artery endothelial function in 94 patients undergoing cardiac catheterization.7 However, in the Bonetti et al. study there were no correlations between individual cardiovascular risk factors and PAT hyperemic response in a multivariable model. An improvement in PAT hyperemic response has also been observed following treatments that are potentially beneficial for cardiovascular health.10,11
In the present study, we extended prior work by comprehensively examining the relations between specific cardiovascular risk factors and the time course of the PAT hyperemic response in a large, community-based cohort. Our observations indicate that the relation between cardiovascular risk factors and PAT hyperemic response was strongest in the 90-120 second interval after fingertip flow was restored. The logarithmic transformation of the PAT ratio and selection of the 90-120 second time interval increased the overall association with risk factors suggesting this may be the optimal method for assessing the PAT response to hyperemia. The selected time period includes the portion of hyperemic response that has previously been shown to depend, in part, on nitric oxide. Further studies are needed to evaluate the possibility that risk factors impair the PAT hyperemic response by decreasing endothelial nitric oxide bioavailability.6
As has been observed with other methods for assessing vasomotor function, we observed expected relations between hyperemic PAT response and a number of classical cardiovascular risk factors. For example, men had a lower PAT hyperemic response than women, which may be related to sex-specific determinants of endothelial function or alternatively to the presence of higher baseline pulse amplitude in men as compared to women. The unexpected inverse relation between lipid-lowering therapy and digital vasodilation is analogous to reports of flow-mediated dilation,12 and is likely attributable to indication bias in our observational, cross-sectional analysis. We speculate that individuals with more severe hyperlipidemia and higher risk factor burden were placed on drug treatment.
Our findings highlight metabolic risk factors including obesity, diabetes and total/HDL cholesterol as important correlates of digital vasodilator function. Previous studies have established a relation between metabolic risk factors and vascular dysfunction. Obese animals have an impaired hyperemic response attributable to both decreased microvessel distensibility and microvascular structural remodeling that occur in the setting of heightened oxidant stress and reduced endothelium-derived nitric oxide.13-15 In human participants, obesity and diabetes along with the associated dyslipidemia and insulin resistance have been linked with impaired vasodilator responses.16-19 The lower digital hyperemic response in obese participants is consistent with impaired microvessel flow reserve that may contribute to impaired blood flow supply in the setting of increased metabolic demands.19 Whereas we did not find a relation between abdominal obesity, reflected in waist circumference, and digital vascular function, it remains possible that a more detailed characterization of fat distribution would provide additional mechanistic understanding of the effect of obesity on microvessel function.
Several traditional risk factors were not related to the PAT hyperemic response. In contrast to studies using alternate methods to evaluate peripheral vasomotor function, there was no significant relation between hypertension and digital vasodilation.12 Systolic blood pressure was marginally associated with lower PAT hyperemic response in age- and sex-adjusted analysis but not in the multivariable analysis. It is possible that systemic blood pressure has limited effects on the distal microcirculation; however, this finding could also be explained by the low prevalence of hypertension in the relatively young Third Generation cohort or the use of antihypertensive agents. Blood pressure may also have a predominate influence on the baseline amplitude without additional modification of the hyperemic response when represented as a ratio of hyperemic to baseline amplitude.
As previous studies have suggested an association between inflammation and vascular dysfunction, we considered the possibility that systemic inflammation may be related to the PAT measures.20 However, we did not observe a relation between systemic inflammation assessed by CRP levels and digital vascular function. These findings suggest that inflammation may not play an important role in determining vasodilator function in the finger in a young to middle-aged cohort. However, it remains possible that local microvessel inflammation not reflected in a circulating marker may influence digital vascular function.
There was a minimal but paradoxically positive relation between advancing age and PAT hyperemic response. One possible explanation for the counterintuitive positive association with advancing age may be the relatively narrow age range of the participants in this study sample. Alternatively, there may be differential age-related changes in hyperemic response in the fingertip microvessels as compared to other vascular beds. The exploratory analyses suggest that the lack of association between age and PAT ratio in the sex-adjusted model may reflect negative confounding by body mass index and other covariates. The age and PAT ratio association will need to be examined in additional cohorts with a broader age distribution. The differences between our findings in the fingertip and previous findings in conduit vessels emphasize that arterial physiology and mechanisms of vasodilation may differ importantly according to vascular bed and to the stimulus for vasodilation, and may have implications for the clinical utility of the readily-accessible digital circulation.
Baseline pulse amplitude was directly related to multiple cardiovascular risk factors. In multivariable analyses, male sex and higher body mass index were strongly related to elevated pulse amplitude at baseline, whereas modest positive relations were observed with increasing systolic blood pressure, increasing total/HDL cholesterol ratio, and active smoking. Higher baseline pulse amplitude was observed in obese and overweight individuals compared to those of normal weight. Decreased digital microvessel tone, increased pulse pressure, increased blood flow, or altered microvascular structure may all contribute to higher resting pulse amplitude. Baseline pulse amplitude is highly dependent on digital blood flow and sympathetic tone as is evidenced by a marked reduction in digital pulse amplitude after the administration of phenylephrine, an alpha-adrenergic vasoconstrictor agent.6 Our findings are consistent with the observation that both obesity and smoking are associated with higher resting blood flow in the brachial artery.19 Similarly increased peripheral blood flow occurs with acute elevation in circulating free fatty acid levels after ingesting a fatty meal.21 The presence of increased basal blood flow is consistent with the presence of hyperperfusion that may contribute to microvascular hypertension and microvessel damage in obese individuals.19,22,23
Strengths and Limitations
The present study has several limitations. Due to the cross-sectional design, we cannot establish causal relations between risk factors and digital vascular function; however, our findings support the possibility of a link between certain risk factors and lower digital hyperemic response. We acknowledge that much of the variability in pulse amplitude remains unexplained by clinical factors. Because of the large sample size many of the cardiovascular risk factors were significantly associated with PAT measures but provided only minimal incremental contribution to the variability in pulse amplitude after accounting for age and sex. As our study sample was predominantly white individuals of European descent, our findings cannot be readily generalized to different ethnic or racial groups. As would be anticipated, we observed an inverse relation between baseline pulse amplitude and the PAT response to hyperemia. Expressing the hyperemic response as a ratio to the baseline pulse amplitude accounts, in part, for this association. Because of the community-based nature of our cohort, we were unable to administer nitroglycerin; therefore, we do not have a measure of endothelium-independent vasodilation. Thus, we acknowledge that we are unable to comment directly on the relative proportion of the PAT response to ischemia that is endothelium-dependent. Our study has several strengths that counterbalance these limitations including a large sample size and community-based design that reduced selection biases. The standardized evaluation of cardiovascular risk factors in a large number of individuals allowed for evaluation of multiple covariates and provided excellent power.
In conclusion, digital vascular dysfunction, as assessed by the pulse amplitude hyperemic response, occurs in association with multiple cardiovascular risk factors, in particular obesity and associated metabolic risk factors. The association between cardiovascular risk factors and digital arterial tonometry assessed by the PAT ratio suggests that PAT may be a useful measure of peripheral vascular function. The association with obesity and diabetes supports the possibility that metabolic risk factors particularly are reflected in microvessel responses. Future longitudinal studies are warranted to provide additional information about the clinical utility of measuring vasodilator function in the finger tip as well as the pathways connecting vascular dysfunction, metabolic risk and cardiovascular disease.
Supplementary Material
Acknowledgments
Sources of Funding The Framingham Heart Study is funded by NIH/NHLBI contract N01-HC25195. The project was supported NIH grants HL70100, HL076784, AG028321, RO1 HL 080124, 2K24 HL 4334 and the Donald W. Reynolds Foundation. Dr. Hamburg is supported by an American College of Cardiology Foundation/Merck Fellowship Award and NIH grant HL083781. The Peripheral Arterial Tonometry (PAT) device and measurements were provided by Itamar Medical Ltd. at no cost to the study.
Footnotes
Disclosures Dr. Sheffy is Senior Vice President for research and development and Chief Technical Officer at Itamar Medical. Dr Mitchell is owner of Cardiovascular Engineering, Inc., a company that designs and manufactures vascular stiffness measurement devices.
The other authors report no conflicts.
REFERENCES
- 1.Widlansky ME, Gokce N, Keaney JF, Jr., Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 2.Hamburg NM, Vita JA. Endothelial dysfunction in atherosclerosis: Mechanisms of impaired nitric oxide bioactivity. In: Loscalzo J, editor. Molecular mechanisms of atherosclerosis. Taylor & Francis; London: 2006. [Google Scholar]
- 3.Gokce N, Keaney JF, Jr., Menzoian JO, Watkins M, Hunter L, Duffy SJ, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 4.Kuvin JT, Patel AR, Sliney KA, Pandian GP, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 5.Kuvin JT, Mammen A, Mooney P, Alsheikh-Ali AA, Karas RH. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007;12:13–16. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- 6.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 7.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 8.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr., Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the NationalHeart Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 9.SAS Institute Inc. SAS/STAT User's Guide. Version 8 SAS Institute, Inc; Cary, N.C.: 1999. [Google Scholar]
- 10.Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, Schnall RP, Holmes DR, Higano ST, Lerman A. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 11.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr., Lehman B, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of endothelial function in the community: The Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 13.Frisbee JC. Vascular adrenergic tone and structural narrowing constrain reactive hyperemia in skeletal muscle of obese Zucker rats. Am J Physiol Heart Circ Physiol. 2006;290:H2066–H2074. doi: 10.1152/ajpheart.01251.2005. [DOI] [PubMed] [Google Scholar]
- 14.Frisbee JC. Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2005;289:R307–R316. doi: 10.1152/ajpregu.00114.2005. [DOI] [PubMed] [Google Scholar]
- 15.Phillips SA, Sylvester FA, Frisbee JC. Oxidant stress and constrictor reactivity impair cerebral artery dilation in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R522–R530. doi: 10.1152/ajpregu.00655.2004. [DOI] [PubMed] [Google Scholar]
- 16.de Jongh RT, Serne EH, Ijzerman RG, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–2535. doi: 10.1161/01.CIR.0000129772.26647.6F. [DOI] [PubMed] [Google Scholar]
- 17.Gokce N, Vita JA, Donnell M, Forse AR, Istfan N, Stoeckl M, Lipinska I, Keaney JF, Jr., Apovian CM. Weight reduction improves endothelial vasomotor function in overweight and obese patients. Am J Cardiol. 2005;95:266–268. doi: 10.1016/j.amjcard.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr., Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: The Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 20.Vita JA, Keaney JF, Larson MG, Keyes MJ, Massaro JM, Lipinska I, Fan S, Osypiuk E, Wilson PW, Vasan RS, Mitchell GF, Benjamin EJ. Brachial artery vasodilator function and systemic inflammation in the Framingham Heart Study. Circulation. 2004;110:3722–3728. doi: 10.1161/01.CIR.0000148821.97162.5E. [DOI] [PubMed] [Google Scholar]
- 21.Gokce N, Duffy SJ, Hunter LM, Keaney JF, Jr., Vita JA. Acute hypertriglyceridemia is associated with peripheral vasodilation and increased basal flow in healthy young adults. Am J Cardiol. 2001;88:153–159. doi: 10.1016/s0002-9149(01)01610-1. [DOI] [PubMed] [Google Scholar]
- 22.Serne EH, de Jongh RT, Eringa EC, Ijzerman RG, Stehouwer CD. Microvascular dysfunction: a potential pathophysiological role in the metabolic syndrome. Hypertension. 2007;50:204–211. doi: 10.1161/HYPERTENSIONAHA.107.089680. [DOI] [PubMed] [Google Scholar]
- 23.Sandeman DD, Shore AC, Tooke JE. Relation of skin capillary pressure in patients with insulin-dependent diabetes mellitus to complications and metabolic control. N Engl J Med. 1992;327:760–764. doi: 10.1056/NEJM199209103271103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.