Abstract
The aim of this study was to compare patterns of cerebral atrophy on MRI, and neurochemistry on magnetic resonance spectroscopy (MRS), in subjects with posterior cortical atrophy (PCA) and typical Alzheimer's disease (AD). Voxel-based morphometry was used to assess grey matter atrophy in 38 subjects with PCA, 38 subjects with typical AD, and 38 controls. Clinical data was assessed in all PCA subjects. Single-voxel 1H MRS located in the posterior cingulate was analyzed in a subset of subjects with PCA, typical AD, and control subjects. PCA showed a pattern of atrophy affecting occipital, parietal and posterior temporal lobes, compared to controls. The pattern was bilateral, but more severe on the right. Subjects with PCA showed greater atrophy in the right visual association cortex than subjects with typical AD, whereas those with AD showed greater atrophy in the left hippocampus than those with PCA. 1H MRS suggested loss of neuronal integrity and glial activation in subjects with PCA and AD. The differing patterns of atrophy on MRI suggest that PCA should be considered a distinct entity from typical AD.
Keywords: Posterior cortical atrophy, Alzheimer's disease, voxel-based morphometry, magnetic resonance imaging, magnetic resonance spectroscopy
1. Introduction
Posterior cortical atrophy (PCA) is a rare, slowly progressive, dementia characterized by the development of early higher order visuospatial and visual perceptual deficits, often accompanied, or followed by features of Balint's syndrome, Gerstmann syndrome, and transcortical sensory aphasia[4, 17, 54]. These early clinical features differ from those of typical Alzheimer's disease (AD) in which memory impairment is an early feature, although as both diseases progress there is considerable overlap. In addition, the most frequent pathological findings of PCA are neurofibrillary tangles (NFT) and neuritic plaques characteristic of typical AD [48], although distributed differently, leading some authors to suggest that PCA is simply an atypical presentation of AD [19]. These differences and similarities beg the question of whether PCA is a distinct entity from typical AD.
Visual assessments of MRI have been reported in cases of PCA and have typically shown a pattern of bilateral posterior cerebral atrophy, predominantly affecting the occipital, parietal, and the temporal lobes[1, 4, 21, 24, 32, 39, 41, 43]. However, a number of recent automated image analysis techniques have been applied to study patterns of cerebral atrophy on MRI. One such technique that has been used extensively in various neurodegenerative disorders [9, 45], is voxel-based morphometry (VBM). This technique uses unbiased statistics to assess patterns of cerebral atrophy throughout the whole brain in groups of subjects. A number of VBM studies have demonstrated atrophy of the hippocampus and medial temporal lobe regions, the posterior cingulate gyrus, precuneus, temporoparietal neocortex and prefrontal cortex in typical AD[3, 10, 30, 44, 52].
Proton MR spectroscopy (1H MRS) is a technique that provides information regarding the neurochemistry of the brain, and has been useful in differentiating the common degenerative dementias[14, 29]. The neuronal integrity marker N-acetyl aspartate (NAA) level is decreased, and the glial activity marker myo-inositol (mI) level is elevated in brain regions affected by the neurodegenerative pathologies in AD and frontotemporal lobar degeneration [14, 28].
Neither VBM nor 1H MRS has been applied to study the characteristics of PCA, or to compare features of PCA and typical AD. Therefore, the primary aim of this study was to assess the patterns of cerebral atrophy and 1H MRS findings in a large group of PCA patients, and then to compare this to the patterns identified in patients with typical AD and control subjects. Based on the pattern of pathological involvement, we hypothesize that the patterns of atrophy on MRI would differ between patients with PCA and AD, yet the pattern of single voxel 1H MRS acquired from the posterior cingulate would be similar.
2. Methods
2.1 Subjects
The Mayo Clinic medical records database was used to identify all cases with a clinical diagnosis of PCA and at least one volumetric MRI that had been evaluated between January 1st 1995 and December 31st 2005. Seventy cases were identified with a clinical diagnosis of PCA, of which 47 cases had at least one volumetric MRI.
The medical records of each of these 47 cases were reviewed by one behavioral neurologist (KAJ) to ensure that they fulfilled a set of rigorous clinical criteria. In order to avoid any circularity all cases were defined solely using clinical criteria, without reference to MRI, 1H MRS or PET/SPECT results. Inclusion criteria were as follows: 1) insidious onset of symptoms; 2) a chief complaint of at least two prominent symptoms referable to the occipital, parietal or posterior temporal lobes that has been liked to PCA; 3) no primary ocular disease accounting for the compliant after ophthalmologic evaluation by an ophthalmologist, 4) an evaluation by at least one behavioral neurologist and 5) progression of symptoms. The list of prominent symptoms linked to PCA include visuospatial deficit, visual perceptual deficit, visual agnosia, environmental disorientation, prosopagnosia, body schema distortion, or any feature suggestive of the Balint's or Gerstmann syndromes[4, 39, 48]. Patients were excluded if there was early loss of insight, or prominent cognitive impairment affecting language fluency, behavior, or episodic memory that preceded the onset of visual impairment, or exceeded the severity of the visual complaint at onset. A total of six patients were excluded. Three of these presented with significant episodic memory loss preceding the onset of visual complaints, one with the onset of symptoms characterized by an expressive or non-fluent aphasia, and two with the onset of progressive gait abnormalities years prior to the onset of visual symptoms.
In addition, all MRI scans were reviewed. Scans were rejected for poor quality (such as motion artifact) or the presence of other pathologies (such as stroke, tumor or other structural lesion) that may influence either the structural analysis or clinical presentation. Three cases were rejected due to the presence of a hemispheric infarct in the occipital lobe that could have contributed to the presenting syndrome.
A total of 38 cases of PCA were identified that fulfilled clinical criteria and had usable MRI. Detailed clinical features were extracted retrospectively from the medical records by a behavioral neurologist (KAJ) blinded to imaging data. Information was extracted from yearly clinical evaluations up to the one corresponding to the time of the MRI utilized in this study. While this study was retrospective in design this technique is standard for the collection of data in uncommon neurodegenerative diseases such as PCA. The aim of the study was not to collect data on the prevalence of clinical features in PCA but to perform a detailed anatomical assessment of the patterns of atrophy on MRI and assess correlations with clinical features. Detailed clinical features on nine of these cases were previously reported[48]. Only one case in this series had come to autopsy and was found to have NFT and neuritic plaques in a distribution typical of PCA[34, 48]. A subset of 19 PCA cases also had a single voxel 1H MRS study acquired from the posterior cingulate gyrus. All MRS studies were performed on the same date as the MRI.
Each of the patients with PCA was age and gender matched to a patient with typical AD who fulfilled the NINCDS-ADRDA criteria for probable AD, and a healthy control[38]. The date/year that the scans were performed were also matched in an attempt to control for any temporal fluctuations associated with different scanner platform versions. All the AD and healthy control subjects were initially recruited into the Mayo Clinic Alzheimer's Disease Research Center (ADRC), or the Alzheimer's Disease Patient Registry (ADPR), and were identified from the ADRC/ADPR database. Twenty-one patients with typical AD, and 22 healthy controls, had an 1H MRS study. In all cases the MRS study was performed on the same date as the MRI.
2.2 Volumetric MRI
All subjects had a T1-weighted volumetric MRI scan acquired at 1.5T (TE = minimum full 5ms, TR = 23ms, 22×16.5cm FOV, 25° flip angle, 124 contiguous 1.6mm thick coronal slices). An optimized method of VBM was applied[2, 47], implemented using SPM2 (http://www.fil.ion.ucl.ac.uk/spm). In order to reduce any potential normalization bias across the disease groups' customized templates and prior probability maps were created from all subjects in the study. To create the customized template and priors all images were registered to the MNI template using a 12 degrees of freedom (dof) affine transformation and segmented into grey matter (GM), white matter (WM) and CSF using MNI priors. GM images were normalized to the MNI GM prior using a nonlinear discrete cosine transformation (DCT). The normalization parameters were applied to the original whole head and the images were segmented using the MNI priors. Average images were created of whole head, GM, WM and CSF, and smoothed using 8mm full-width at half-maximum (FWHM) smoothing kernel. All images were then registered to the customized whole brain template using a 12dof affine transformation and segmented using the customized priors. The GM images were normalized to the custom GM prior using a nonlinear DCT. The normalization parameters were then applied to the original whole head and the images were segmented once again using the customized priors. All images were modulated and smoothed with an 8mm FWHM smoothing kernel.
Two-sided t-tests were used to analyze the smoothed modulated images from the PCA group compared to controls, and from the AD group compared to controls. In addition, two direct comparisons were performed between the PCA and AD groups, firstly to identify regions that showed greater loss in PCA than AD, and second to identify regions that showed greater loss in AD than PCA. Grey matter differences were assessed at an uncorrected statistical threshold (p<0.001), and after correction for multiple comparisons over the whole brain volume (p<0.05).
2.3 Single Voxel 1H MRS
1H MRS studies were performed with the LX system automated single voxel 1H MRS package: PROBE /SV (General Electric Medical Systems, Milwaukee, WI). An eight cm3 midline parietal (2×2×2 cm) voxel was placed on a mid-sagittal T1-weighted image including right and left posterior cingulate gyri and inferior precunei [29]. Point resolved spectroscopy (PRESS) pulse sequence with TR = 2000 ms, TE=30 ms, 2048 data points and 128 excitations were used for the examinations. The prescan algorithm of PROBE automatically adjusted the transmitter and receiver gains and center frequency. The local magnetic field homogeneity was optimized with the three-plane auto-shim procedure, and the flip angle of the third water suppression pulse was adjusted for chemical-shift-water suppression (CHESS) prior to PRESS acquisition. Metabolite intensity ratios were automatically calculated at the end of each PROBE /SV acquisition. 1H MRS variables analyzed for this study were NAA /Creatine (Cr), Choline (Cho) /Cr, and mI /Cr ratios.
2.4 Statistics
To confirm the effectiveness of our age matching, we compared average age across the three groups using ANOVA. Differences in years of education among the three groups were tested with a nonparametric 3-group Kruskal-Wallis test due to skewness, while differences among the groups in handedness were tested using Fisher's exact test. We compared the AD and PCA patients on disease duration, the short test of mental status (STMS), mini mental status exam (MMSE), and clinical dementia rating (CDR) sum of boxes using Wilcoxon rank-sum tests because of skewness in these measures. Only cognitive scores within 120 days of the volumetric MRI were considered. Because the control group was by definition cognitively normal, we excluded controls from tests of differences in cognitive scores. ANOVA and post hoc two-sided two-sample t-tests were used to compare metabolite ratios across the three groups. All tests were two-sided and we used p<0.05 to indicate significance.
3. Results
3.1 Clinical
The demographics of all subjects are shown in Table 1. In each group of 38 subjects, 22 were female and the majority of subjects were right-handed. The AD and PCA groups only differed in terms of their STMS performance (Wilcoxon rank-sum; p=0.02).
Table 1.
Subject demographics
| Controls | AD | PCA | P values | |
|---|---|---|---|---|
| n | 38 | 38 | 38 | |
| Female/Male (% female) | 22:16 (57.9) | 22:16 (57.9) | 22:16 (57.9) | n/a |
| Age, mean (SD) years | 65.9 (7.0) | 65.3 (6.9) | 64.3 (7.2) | 0.61* |
| Right: left handed (% right handed) | 37:1 (97.4) | 29:3 (90.6) | 31:1 (96.9) | 0.52† |
| Education, median (range) years | 14.0 (8.0, 20.0) | 12.5 (12.0, 20.0) | 14.0 (8.0, 18.0) | 0.17§ |
| Disease duration, median (range) years | - | 4.7 (0.8, 11.0) | 4.0 (1.0, 9.0) | 0.24‡ |
| STMS, median (IQR) | 36.0 (36.0, 37.0) | 22.0 (16.2, 25.0) | 26.0 (19.5, 28.8) | 0.021‡ |
| MMSE, median (IQR) | 29.0 (28.0, 30.0) | 17.0 (13.0, 24.0) | 18.5 (14.8, 21.0) | 0.71‡ |
| CDR sum of boxes, median (IQR) | 0.0 (0.0, 0.0) | 5.0 (3.0, 10.0) | 5.5 (3.6, 7.8) | 0.91‡ |
3-group ANOVA test
Fisher's exact test
Wilcoxon rank-sum test comparing AD and PCA groups
3-group Kruskal-Wallis test
STMS = short test of mental status (available in N=19 C, N=32 AD, N=38 PCA); MMSE = Mini-mental status examination (N=38 C, N=36 AD, N=16 PCA); CDR = clinical dementia rating (N=38 C, N=37 AD, N=18 PCA); SD = standard deviation; IQR = interquartile range; n/a = not applicable since the gender ratios are the same across all three groups.
The clinical features of the patients with PCA are shown in Table 2. The most frequent sign or symptom documented in the PCA cohort was visuospatial deficits. Episodic memory loss occurred in 17 cases (45%), however in over 70% of these the onset was not early. Furthermore, in no case was the memory loss a prominent feature. In addition to these common signs and symptoms reported in PCA, other less commonly reported signs were also present including cerebellar signs, parkinsonism, myoclonic jerks, rapid eye movement (REM) sleep behavior disorder, and visual hallucinations, however none of these features occurred early in the disease course (Table 2). The cerebellar signs included terminal tremor on finger-nose-finger testing (N=3), and head titubation in one patient.
Table 2.
Frequency of reported signs and symptoms in PCA up to time of scan
| Clinical features | Total (N =38) |
|---|---|
| Visuospatial deficits* | 31 (82%) |
| Features of the Gerstmann syndrome | 25 (66%) |
| Anomia | 23 (61%) |
| Visual agnosia | 22 (58%) |
| Ideomotor apraxia | 22 (58%) |
| Environmental disorientation | 19 (50%) |
| Episodic memory loss | 17 (45%) |
| Dressing apraxia | 13 (34%) |
| Pure alexia | 10 (26%) |
| Prosopagnosia | 9 (24%) |
| Parkinsonism | 9 (24%) |
| Body schema distortion of spatial location of the body | 8 (21%) |
| Hemianopia | 7 (18%) |
| Visual hallucinations | 7 (18%) |
| Achromatopsia | 5 (13%) |
| Cortical sensory loss | 5 (13%) |
| Cerebellar signs | 4 (11%) |
| Myoclonic jerks | 4 (11%) |
| REM sleep behavior disorder | 4 (11%) |
Visuospatial deficits, encompasses features of the Balint syndrome and visual disorientation
3.2 Volumetric MRI
3.2.1 PCA versus controls
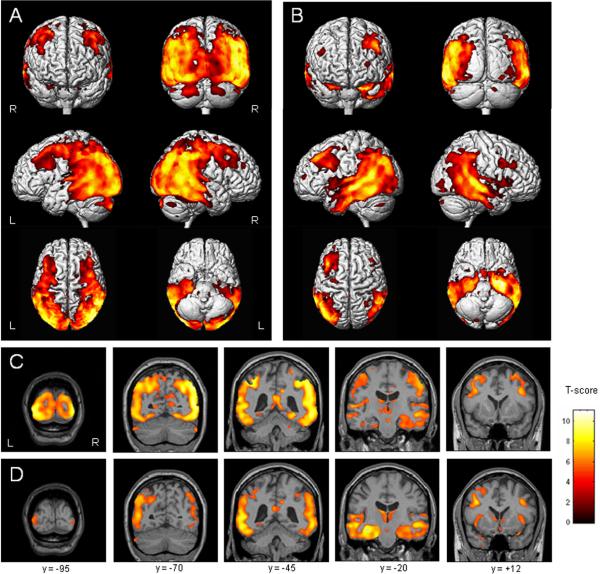
The PCA group showed a widespread pattern of bilateral grey matter loss predominantly affecting the posterior aspects of the brain, compared to controls (corrected, p<0.05, Table 3). The loss extended from the primary visual cortex and the visual association cortices (Brodmann areas (BA) 17, 18, 19) forward through the parietal lobes, involving the posterior cingulate and retrosplenial gyri, and into posterior temporal regions (corrected, p<0.05) (Figure 1A and C). The loss also extended superiorly and anteriorly across the primary sensory (BA 1–3) and motor cortices (BA 4). These regions of loss were bilateral but had a right-sided predominance. The atrophy of the temporal lobes was predominantly posterior, with relative sparing of the temporal poles, and again, with a right-sided predominance. On the right the inferior, middle and superior temporal gyri and hippocampus were involved. The left temporal lobe had a noticeable sparing of the hippocampus, and inferior and middle temporal gyri. Grey matter loss was also identified in the bilateral superior insula, thalamus, globus pallidus, putamen, and posterior middle and inferior frontal gyri, albeit less severe than the posterior atrophy (Figure 1A and C). There was a notable sparing of anterior prefrontal regions. A small region of grey matter loss was also identified in the posterior cerebellum. No regions showed greater loss in the control subjects than the PCA subjects (uncorrected, p<0.001).
Table 3.
Location of the voxel showing the most significant grey matter loss on VBM in each of the group comparisons
| Comparison | Region | MNI coordinates | Z score | Cluster size | P value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| PCA versus C | Right lateral temporoparietal | 49 | −61 | 42 | >8 | 389623 | <0.000 |
| AD versus C | Left medial temporal | −28 | −10 | −18 | >8 | 231019 | <0.000 |
| PCA versus AD | Right lateral occipital/temporal | 39 | −85 | −6 | 5.22 | 1041 | <0.000 |
| AD versus PCA | Left medial temporal | −26 | −9 | −20 | 5.47 | 2397 | <0.000 |
Voxel coordinates are in millimeters after transformation into standard Montreal Neurologic Institute (MNI) stereotactic space. Results have been corrected for multiple comparisons (p<0.05). PCA = Posterior Cortical Atrophy; AD = Alzheimer's disease; C = control; PCA versus AD = voxel showing greater atrophy in the PCA group than the AD group; AD versus PCA = voxel showing greater atrophy in the AD group than the PCA group.
Figure 1.
Patterns of grey matter atrophy in the PCA group (A and C) and AD group (B and D), compared to healthy controls (corrected for multiple comparisons, p<0.05). Results have been overlaid both on a 3D surface render (A and B) and on representative coronal MRI slices (C and D) from a healthy control. Warmer colors indicate statistically greater differences between groups.
3.2.2 AD versus controls
The pattern of grey matter loss in the AD group, compared to controls, was pronounced in the medial temporal lobes, and spread back into the parietal lobes (corrected, p<0.05, Table 3 and Figure 1B). The pattern was bilateral but, in contrast to the PCA group, showed a left-sided predominance. Grey matter loss within the temporal lobe was identified bilaterally in the hippocampus, amygdala, fusiform gyri, and middle and inferior temporal gyri, also with some involvement of the posterior superior temporal gyrus (Figure 1D). As in the PCA versus control comparison the temporal pole was relatively spared (Figure 1B). The posterior cingulate was involved bilaterally, although the loss did not spread into the retrosplenial gyrus (Figure 1D). Grey matter loss was also observed bilaterally in the insula, thalamus, basal forebrain and posterior middle frontal gyri (L>R). No regions showed greater loss in the control subjects than the AD subjects (uncorrected, p<0.001).
3.2.3 PCA versus AD
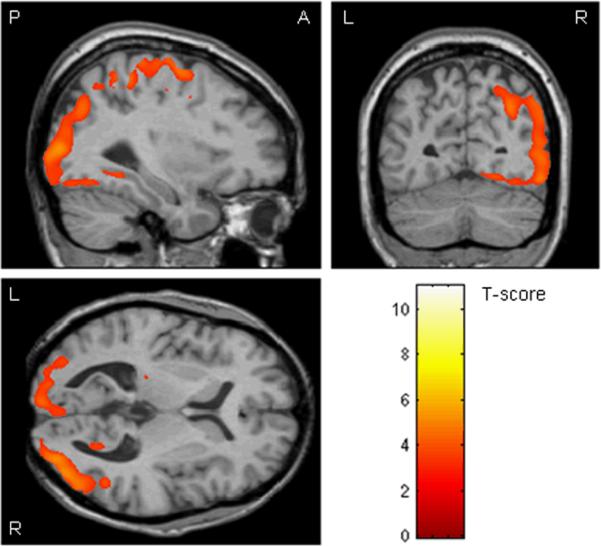
This comparison highlights regions that showed significantly greater atrophy in the PCA group than the AD group. These regions included the bilateral primary visual cortex (BA 17), visual association cortex (BA 18, 19), the bilateral primary sensory and motor cortex (BA 1–3, 4), and the right posterior parietal lobe (uncorrected, p<0.001) (Figure 2). The only region to survive correction for multiple comparisons was a small region in the right visual association cortex (BA 19) (corrected, p<0.05, see Table 3).
Figure 2.
Regions showing significantly greater grey matter atrophy in the PCA group than the AD group (uncorrected for multiple comparisons, p<0.001), overlaid on an MRI from a healthy control
3.2.4 AD versus PCA
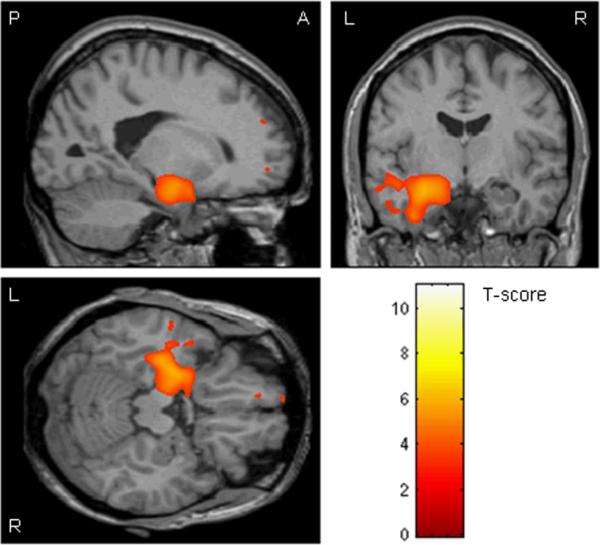
This comparison highlights regions that showed significantly greater atrophy in the AD group than the PCA group. These regions were found in the left medial (including hippocampus and amygdala), inferior and middle temporal lobe (uncorrected, p<0.001) (Figure 3). The only region to survive correction for multiple comparisons was in the left hippocampus (corrected, p<0.05, see Table 3)
Figure 3.
Regions showing significantly greater grey matter atrophy in the AD group than the PCA group (uncorrected for multiple comparisons, p<0.001), overlaid on an MRI from a healthy control
3.3 Single Voxel 1H MRS
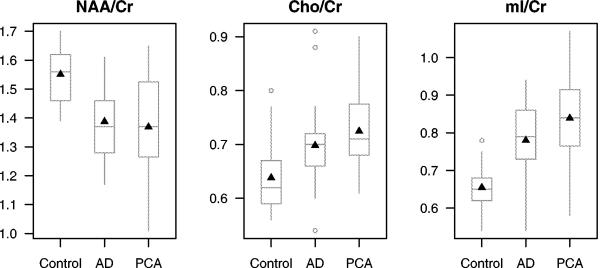
The 22 controls, 21 patients with AD and 19 patients with PCA who completed the 1H MRS studies did not differ in age, gender or education (Table 4). In addition, there was no difference in disease duration, or in any measure of clinical severity, between the PCA and AD patients. The 1H MRS metabolite ratios are shown in Table 4 and Figure 4. The neuronal integrity marker NAA /Cr ratio was lower in patients with AD and PCA compared to controls (t-tests p≤0.001). The membrane integrity marker Cho /Cr levels were elevated in patients with AD (t-tests; p=0.016) and PCA (t-tests; p<0.001) compared to controls. The glial marker mI /Cr levels were also elevated and in patients with AD and PCA (t-tests; p<0.001) compared to controls. Although there was a trend for higher mI /Cr ratio in PCA compared to AD (t-tests; p=0.066), there were no statistically significant 1H MRS metabolite differences between patients with AD and PCA in the posterior cingulate gyri and precunei (Table 4 and Figure 4).
Table 4.
Subject demographics and 1H MRS metabolite ratios in patients who underwent single voxel 1H MRS studies
| Control | AD | PCA | P values | |
|---|---|---|---|---|
| n | 22 | 21 | 19 | |
| Female/Male (% female) | 13/9 (59.1) | 12/9 (57.1) | 11/8 (57.9) | 0.99** |
| Age, mean (SD) years | 65.3 (6.8) | 67.0 (6.6) | 63.6 (7.6) | 0.33* |
| Right: left handed (% right handed) | 21:1 (95.5) | 16:3 (84.2) | 16:2 (88.9) | 0.49† |
| Education, median (min., max.) | 14.5 (10.0, 20.0) | 14.0 (12.0, 20.0) | 14.0 (8.0, 17.0) | 0.95§ |
| Disease duration, median (min., max), years | - | 5.3 (0.9, 10.5) | 5.0 (1.0, 9.0) | 0.70‡ |
| STMS, median (IQR) | 37.0 (36.0, 38.0) | 21.5 (14.0, 24.8) | 19.0 (17.5, 25.0) | 0.96‡ |
| MMSE, median (IQR) | 29.0 (28.0, 30.0) | 17.0 (13.8, 22.8) | 17.0 (14.0, 21.0) | 0.82‡ |
| CDR sum of boxes, median (IQR) | 0.0 (0.0, 0.0) | 6.8 (3.8, 10.2) | 5.0 (3.8, 8.5) | 0.78‡ |
| NAA/Cr, mean (SD) | 1.55 (0.10) | 1.39 (0.13) | 1.37 (0.19) | <0.001* |
| Cho/Cr, mean (SD) | 0.64 (0.07) | 0.70 (0.09) | 0.72 (0.08) | 0.003* |
| MI/Cr, mean (SD) | 0.65 (0.06) | 0.78 (0.12) | 0.84 (0.12) | <0.001* |
ANOVA
Chi-squared test
Fisher's exact test
Wilcoxon rank-sum test between AD and PCA groups
Kruskal-Wallis
STMS = short test of mental status (available in N=9 C, N=18 AD, N=15 PCA); MMSE = Mini-mental status examination (N=22 C, N=20 AD, N=13 PCA); CDR = clinical dementia rating (N=22 C, N=20 AD, N=15 PCA); SD = standard deviation; IQR = interquartile range
Figure 4.
Boxplots of the distribution of 1H MRS metabolite ratios from a single voxel in the posterior cingulate and precunei by group. The boxes show the median and inter-quartile range with the means indicated by a triangle. The whiskers extend to the furthest data points that are not outliers (shown as separate points).
NAA= N-acetyl aspartate; Cr = creatine; Cho = choline; mI = myo-inositol
4. Discussion
This study investigated the patterns of atrophy on MRI, and 1H MRS neurochemistry, in a group of PCA subjects, and compared the findings to typical AD and healthy controls.
The clinical features of these 38 PCA patients were typical of other described cases and case series of PCA[4, 19, 39, 43, 48]. In all our patients the clinical features were characterized by predominant visuospatial, visuoperceptual deficits, and visual agnosia, as well as problems with reading and writing and environmental disorientation. We also found additional signs and symptoms that may extend the spectrum of features of PCA. These included hallucinations, parkinsonism, REM sleep behavior disorder and myoclonic jerks, however these features are not novel and have been reported in a previous clinical series on PCA[48]. It is not unexpected to find these features in PCA since PCA is a syndrome that may result from multiple different pathologies[43].
The pattern of grey matter loss on MRI in typical AD highlighted the medial temporal lobes, while loss in the PCA group emphasized the posterior hemispheres, including the primary visual cortex, the visual association cortex and parietal lobes, with a notable sparing of the anterior temporal and prefrontal cortex. These patterns were identified on visual assessment of the VBM results and confirmed by the specific locations of maximal grey matter loss shown in Table 3. These findings are not surprising given the clinical features of PCA which occur as a result of occipital, parietal and posterior temporal lobe dysfunction[46]. Similarly, the finding of medial temporal lobe atrophy in typical AD is in keeping with the classic and dominant feature of typical AD, episodic memory loss, which occurs as a result of hippocampal obliteration. The PCA group showed a predominantly right-sided pattern of loss, compared to a more left-sided pattern observed in the typical AD group. This finding is in agreement with most previous case reports which have demonstrated right hemisphere predominance in cases of PCA [21, 32, 41, 46, 53].
Both primary and secondary visual cortices were heavily involved in the PCA patients and showed significantly more atrophy in PCA than typical AD. Similar patterns have been reported using FDG-PET and SPECT techniques, showing greater hypometabolism in the visual cortex in PCA patients, compared to typical AD[5, 41]. In addition, pathological studies have revealed a high concentration of NFT in the primary visual cortex and visual association regions in PCA[48, 54]. In fact, NFT density in the visual association cortex has been shown to be greater in PCA than in typical AD[24]. These findings correlate with the visuospatial and visual perceptual deficits encountered in our patients with PCA, and the fact that patients with PCA typically perform worse on tests of visuospatial function than typical AD subjects[43].
The occipital and parietal lobes play an important role in visual perception and in the interpretation of sensory information in the brain. Damage in these regions may have contributed to a number of the problems observed in our cases, such as visual agnosia, anomia, and apraxia[20]. In particular, damage to the parietal lobes can cause Gerstmann's and Balint's syndrome, both of which were commonly found in our PCA patients. While the parietal lobe damage was bilateral in our PCA subjects; the right parietal lobe seemed more affected than the left. Damage to the right parietal lobe can cause deficits such as dressing apraxia, and body schema distortion, features which were common in our patients. While parietal lobe atrophy was also present in the patients with typical AD, it was much less severe than that observed in the PCA patients, and in contrast it seemed to affect the left parietal lobe greater than the right. Features such as agraphia, acalculia and apraxia typically result from left parietal lobe damage, and are relatively common findings in patients with typical AD[12, 39, 51].
We also demonstrated that the posterior hippocampus and posterior aspects of the temporal lobes, particularly on the right, are affected in patients with PCA. The right medial temporal lobe, particularly the hippocampus, plays a role in spatial navigation and topographical memory[8, 36, 37], and may therefore contribute to the feature of environmental disorientation which was observed in 50% of our PCA cohort. Similar clinical features have also been reported in previous studies[4]. Interestingly, the cingulate and retrosplenial cortex have also been suggested to play a role in spatial navigation[11, 23, 35] and these regions were found to be atrophied in our PCA cohort. It should be noted however that the PCA group did not have more atrophy in these regions on direct comparison than the typical AD group. This may reflect the fact that patients with typical AD also show deficits in spatial navigation and topographic memory[31, 42].
In addition, deficits in the recognition of familiar faces (prosopagnosia) are another feature typically reported in cases of PCA[40, 48] and has been linked to damage of the right temporal lobe[13, 15, 27]. Symptoms of prosopagnosia were present in 24% of our PCA cohort. We therefore suspect that damage to the right temporal lobe was contributing to a number of different clinical features in PCA.
In contrast to the right-sided temporal pattern in PCA the typical AD patients showed a more left-sided temporal predominance which has been reported before[3, 26]. In fact the typical AD subjects had significantly greater atrophy of the left medial temporal lobe (particularly the hippocampus) and inferior and middle temporal gyri than the PCA patients, reflecting the deficits in episodic memory that are found in patients with typical AD. This is perhaps unsurprising since patients were excluded from a diagnosis of PCA if they had significant and early episodic memory impairment, and AD patients perform worse on tests of episodic memory than PCA patients[39, 43].
The PCA patients also showed atrophy in a region spreading forward from the parietal lobes spanning the primary sensory and motor cortex. Conversely most studies have reported the absence of motor or primary sensory deficits in PCA[1, 4, 17, 21, 32]. However, cortical sensory loss and myoclonic jerks were present in a small proportion of our cases late in the disease course and has also been previously reported in PCA [25, 48]. The cases with cortical sensory loss had an average five classic features of PCA suggesting that these cases are not atypical. The posterior cerebellum was also found to be involved in PCA. This is difficult to explain, however it is possible that the loss may be secondary to degeneration of the afferent input from the motor cortex, since the motor cortex has extensive connections to the cerebellum. Cerebellar findings were also documented in a small number of our cases of PCA, and may correlate with this finding. Additional studies are needed to confirm this finding.
We also showed some minor frontal lobe involvement in both the PCA and typical AD groups. Frontal lobe atrophy is a common finding on MRI in typical AD[3, 18, 30], and case reports have similarly shown evidence that the frontal lobes can become affected over time in PCA, both clinically, with deficits in executive function and typical signs such as echopraxia and perseverations, and on FDG-PET and SPECT[21, 41, 46]. In fact the location of the frontal lobe atrophy in PCA coincides with regions of hypometabolism identified in the frontal eye fields in a recent FDG-PET study[41] where the authors hypothesized that damage to these regions may result from degeneration of the afferent input from the occipito-parietal cortices, and may contribute to ocular apraxia in PCA[41].
The single voxel 1H MRS acquisition in a subgroup of controls, patients with AD and PCA was from a midline parietal voxel, which included the right and left posterior cingulate gyri and precunei. We chose to study this voxel location because relatively high-quality, reproducible, short echo time 1H MR spectra can be obtained from this region [28], which is involved with the neurofibrillary pathology of AD[7]. We hypothesized that similar 1H MRS changes related to the degenerative pathology of PCA will be identified in the medial parietal region. We showed that the neuronal marker NAA /Cr levels were decreased, indicating loss of neuronal integrity and the glial marker mI /Cr levels were elevated, suggesting increased glial activity in both AD and PCA. The trend of higher mI /Cr levels in PCA compared to AD further suggests a more prominent glial activation in the medial parietal lobes of PCA than AD patients. Furthermore, elevation of the membrane marker Cho /Cr levels in both AD and PCA may indicate increased membrane turnover because of the degenerative process, cholinergic deficit, or both [29].
1H MRS metabolite ratios from the posterior cingulate gyri and precunei voxel did not differ significantly between the PCA and AD patients. This is in agreement with the VBM findings which showed posterior cingulate atrophy in both AD and PCA. The two techniques provide complimentary information on the degenerative pathology in the medial parietal lobes of AD and PCA patients demonstrating different aspects of the neurodegenerative pathology. Decreased NAA /Cr along with atrophy indicate neuronal loss or dysfunction, and elevated Cho /Cr and mI /Cr suggest increased glial activity and membrane turnover, which is common to both AD and PCA in the medial parietal lobes.
The strengths of this study include the fact that it used a large group of well characterized patients. The PCA subjects were originally evaluated by one, sometimes two, experienced behavioral neurologists and an ophthalmologist during the course of their illness. In addition, both VBM and 1H MRS techniques were applied to assess similarities and differences between PCA and AD. However, the study also had a few limitations that should be discussed. First, pathological confirmation was only available in one PCA subject. However, a number of studies have demonstrated that PCA can have multiple pathologies[43, 48–50]. Secondly, clinical data abstraction was retrospective, and the historical evaluations were not entirely standardized, although the neurological examinations were. It is also difficult to match the PCA and AD groups for disease severity as tests such as the MMSE[16] and Short test of mental status[33] are heavily weighted towards memory. The acquisition of MRI across a number of years may inevitably lead to slight fluctuations in scan quality over time. Although we attempted to correct for such fluctuations by matching scan date across groups this additional noise may well have lead to an underestimation of group differences in the VBM analysis. In addition, there are a number of limitations inherent to the techniques of normalization and segmentation within VBM, which can be a particular problem in the analysis of atrophic brains[6, 22]. However, these issues are not specific to this study and apply to all VBM studies on atrophic brains.
These results suggest that subjects with PCA have different patterns of cerebral loss on MRI than subjects with AD even though neurochemically they have a similar degenerative pattern in the medial parietal lobes. The PCA subjects showed greater loss to the primary visual cortex, visual association cortices and right parietal lobes, yet less severe loss of the left medial temporal lobe, than subjects with AD. In addition, while atrophy in typical AD seems to be left hemisphere predominant, atrophy in PCA favors the right hemisphere. These differences suggest that PCA should be considered as a separate entity from typical AD.
5. Acknowledgment
This study was supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078, by grants P50 AG16574, U01 AG06786 and R01 AG11378 from the National Institute on Aging, Bethesda MD, NIRG-03-4842 from the Alzheimer's Association, and the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer s Disease Research Program of the Mayo Foundation, U.S.A.
Footnotes
Disclosures: The authors have reported no conflicts of interest
References
- [1].Aharon-Peretz J, Israel O, Goldsher D, Peretz A. Posterior cortical atrophy variants of Alzheimer's disease. Dement Geriatr Cogn Disord. 1999;10(6):483–7. doi: 10.1159/000017194. [DOI] [PubMed] [Google Scholar]
- [2].Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- [3].Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001;14(2):298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- [4].Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45(7):789–93. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- [5].Bokde AL, Pietrini P, Ibanez V, Furey ML, Alexander GE, Graff-Radford NR, Rapoport SI, Schapiro MB, Horwitz B. The effect of brain atrophy on cerebral hypometabolism in the visual variant of Alzheimer disease. Arch Neurol. 2001;58(3):480–6. doi: 10.1001/archneur.58.3.480. [DOI] [PubMed] [Google Scholar]
- [6].Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14(6):1454–62. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- [7].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- [8].Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–41. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- [9].Burton EJ, Karas G, Paling SM, Barber R, Williams ED, Ballard CG, McKeith IG, Scheltens P, Barkhof F, O'Brien JT. Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. Neuroimage. 2002;17(2):618–30. [PubMed] [Google Scholar]
- [10].Busatto GF, Garrido GE, Almeida OP, Castro CC, Camargo CH, Cid CG, Buchpiguel CA, Furuie S, Bottino CM. A voxel-based morphometry study of temporal lobe gray matter reductions in Alzheimer's disease. Neurobiol Aging. 2003;24(2):221–31. doi: 10.1016/s0197-4580(02)00084-2. [DOI] [PubMed] [Google Scholar]
- [11].Cammalleri R, Gangitano M, D'Amelio M, Raieli V, Raimondo D, Camarda R. Transient topographical amnesia and cingulate cortex damage: a case report. Neuropsychologia. 1996;34(4):321–6. doi: 10.1016/0028-3932(95)00108-5. [DOI] [PubMed] [Google Scholar]
- [12].Croisile B. Agraphia in Alzheimer's disease. Dement Geriatr Cogn Disord. 1999;10(3):226–30. doi: 10.1159/000017124. [DOI] [PubMed] [Google Scholar]
- [13].De Renzi E, Perani D, Carlesimo GA, Silveri MC, Fazio F. Prosopagnosia can be associated with damage confined to the right hemisphere--an MRI and PET study and a review of the literature. Neuropsychologia. 1994;32(8):893–902. doi: 10.1016/0028-3932(94)90041-8. [DOI] [PubMed] [Google Scholar]
- [14].Ernst T, Chang L, Melchor R, Mehringer CM. Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology. 1997;203(3):829–36. doi: 10.1148/radiology.203.3.9169712. [DOI] [PubMed] [Google Scholar]
- [15].Evans JJ, Heggs AJ, Antoun N, Hodges JR. Progressive prosopagnosia associated with selective right temporal lobe atrophy. A new syndrome? Brain. 1995;118(Pt 1):1–13. doi: 10.1093/brain/118.1.1. [DOI] [PubMed] [Google Scholar]
- [16].Folstein MF, Folstein SE, McHugh PR. “Mini-Mental state”. A practical method for grading the mental state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [17].Freedman L, Selchen DH, Black SE, Kaplan R, Garnett ES, Nahmias C. Posterior cortical dementia with alexia: neurobehavioural, MRI, and PET findings. J Neurol Neurosurg Psychiatry. 1991;54(5):443–8. doi: 10.1136/jnnp.54.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Frisoni GB, Testa C, Zorzan A, Sabattoli F, Beltramello A, Soininen H, Laakso MP. Detection of grey matter loss in mild Alzheimer's disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2002;73(6):657–64. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123(Pt 3):484–98. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- [20].Giannakopoulos P, Gold G, Duc M, Michel JP, Hof PR, Bouras C. Neuroanatomic correlates of visual agnosia in Alzheimer's disease: a clinicopathologic study. Neurology. 1999;52(1):71–7. doi: 10.1212/wnl.52.1.71. [DOI] [PubMed] [Google Scholar]
- [21].Goethals M, Santens P. Posterior cortical atrophy. Two case reports and a review of the literature. Clin Neurol Neurosurg. 2001;103(2):115–9. doi: 10.1016/s0303-8467(01)00114-7. [DOI] [PubMed] [Google Scholar]
- [22].Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, Crum WR, Rossor MN, Frackowiak RS. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17(1):29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- [23].Harker KT, Whishaw IQ. A reaffirmation of the retrosplenial contribution to rodent navigation: reviewing the influences of lesion, strain, and task. Neurosci Biobehav Rev. 2004;28(5):485–96. doi: 10.1016/j.neubiorev.2004.06.005. [DOI] [PubMed] [Google Scholar]
- [24].Hof PR, Bouras C, Constantinidis J, Morrison JH. Balint's syndrome in Alzheimer's disease: specific disruption of the occipito-parietal visual pathway. Brain Res. 1989;493(2):368–75. doi: 10.1016/0006-8993(89)91173-6. [DOI] [PubMed] [Google Scholar]
- [25].Hsu JL, Chen WH, Chiu HC. Cortical sensory loss in a patient with posterior cortical atrophy: a case report. Neurocase. 2004;10(1):48–51. doi: 10.1080/13554790490960486. [DOI] [PubMed] [Google Scholar]
- [26].Janke AL, de Zubicaray G, Rose SE, Griffin M, Chalk JB, Galloway GJ. 4D deformation modeling of cortical disease progression in Alzheimer's dementia. Magn Reson Med. 2001;46(4):661–6. doi: 10.1002/mrm.1243. [DOI] [PubMed] [Google Scholar]
- [27].Joubert S, Felician O, Barbeau E, Sontheimer A, Guedj E, Ceccaldi M, Poncet M. Progressive prosopagnosia: clinical and neuroimaging results. Neurology. 2004;63(10):1962–5. doi: 10.1212/01.wnl.0000144347.40132.6a. [DOI] [PubMed] [Google Scholar]
- [28].Kantarci K, Jack CR, Jr., Xu YC, Campeau NG, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: A 1H MRS study. Neurology. 2000;55(2):210–7. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kantarci K, Petersen RC, Boeve BF, Knopman DS, Tang-Wai DF, O'Brien PC, Weigand SD, Edland SD, Smith GE, Ivnik RJ, Ferman TJ, Tangalos EG, Jack CR., Jr. 1H MR spectroscopy in common dementias. Neurology. 2004;63(8):1393–8. doi: 10.1212/01.wnl.0000141849.21256.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Karas GB, Burton EJ, Rombouts SA, van Schijndel RA, O'Brien JT, Scheltens P, McKeith IG, Williams D, Ballard C, Barkhof F. A comprehensive study of gray matter loss in patients with Alzheimer's disease using optimized voxel-based morphometry. Neuroimage. 2003;18(4):895–907. doi: 10.1016/s1053-8119(03)00041-7. [DOI] [PubMed] [Google Scholar]
- [31].Kavcic V, Duffy CJ. Attentional dynamics and visual perception: mechanisms of spatial disorientation in Alzheimer's disease. Brain. 2003;126(Pt 5):1173–81. doi: 10.1093/brain/awg105. [DOI] [PubMed] [Google Scholar]
- [32].Kim E, Lee Y, Lee J, Han SH. A case with cholinesterase inhibitor responsive asymmetric posterior cortical atrophy. Clin Neurol Neurosurg. 2005;108(1):97–101. doi: 10.1016/j.clineuro.2004.11.022. [DOI] [PubMed] [Google Scholar]
- [33].Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc. 1987;62(4):281–8. doi: 10.1016/s0025-6196(12)61905-3. [DOI] [PubMed] [Google Scholar]
- [34].Levine DN, Lee JM, Fisher CM. The visual variant of Alzheimer's disease: a clinicopathologic case study. Neurology. 1993;43(2):305–13. doi: 10.1212/wnl.43.2.305. [DOI] [PubMed] [Google Scholar]
- [35].Maguire EA. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol. 2001;42(3):225–38. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- [36].Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280(5365):921–4. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- [37].Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97(8):4398–403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- [39].Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;14(1):33–40. doi: 10.1159/000058331. [DOI] [PubMed] [Google Scholar]
- [40].Mizuno M, Sartori G, Liccione D, Battelli L, Campo R. Progressive visual agnosia with posterior cortical atrophy. Clin Neurol Neurosurg. 1996;98(2):176–8. doi: 10.1016/0303-8467(95)00091-7. [DOI] [PubMed] [Google Scholar]
- [41].Nestor PJ, Caine D, Fryer TD, Clarke J, Hodges JR. The topography of metabolic deficits in posterior cortical atrophy (the visual variant of Alzheimer's disease) with FDG-PET. J Neurol Neurosurg Psychiatry. 2003;74(11):1521–9. doi: 10.1136/jnnp.74.11.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pai MC, Jacobs WJ. Topographical disorientation in community-residing patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2004;19(3):250–5. doi: 10.1002/gps.1081. [DOI] [PubMed] [Google Scholar]
- [43].Renner JA, Burns JM, Hou CE, McKeel DW, Jr., Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63(7):1175–80. doi: 10.1212/01.wnl.0000140290.80962.bf. [DOI] [PubMed] [Google Scholar]
- [44].Rombouts SA, Barkhof F, Witter MP, Scheltens P. Unbiased whole-brain analysis of gray matter loss in Alzheimer's disease. Neurosci Lett. 2000;285(3):231–3. doi: 10.1016/s0304-3940(00)01067-3. [DOI] [PubMed] [Google Scholar]
- [45].Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- [46].Schmidtke K, Hull M, Talazko J. Posterior cortical atrophy: variant of Alzheimer's disease? A case series with PET findings. J Neurol. 2005;252(1):27–35. doi: 10.1007/s00415-005-0594-5. [DOI] [PubMed] [Google Scholar]
- [47].Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR., Jr. Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26(2):600–8. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, Caselli RJ, Knopman DS, Petersen RC. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168–74. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- [49].Tang-Wai DF, Josephs KA, Boeve BF, Dickson DW, Parisi JE, Petersen RC. Pathologically confirmed corticobasal degeneration presenting with visuospatial dysfunction. Neurology. 2003;61(8):1134–5. doi: 10.1212/01.wnl.0000086814.35352.b3. [DOI] [PubMed] [Google Scholar]
- [50].Tang-Wai DF, Josephs KA, Boeve BF, Petersen RC, Parisi JE, Dickson DW. Coexistent Lewy body disease in a case of “visual variant of Alzheimer's disease”. J Neurol Neurosurg Psychiatry. 2003;74(3):389. doi: 10.1136/jnnp.74.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Teixeira F, Alonso E, Romero V, Ortiz A, Martinez C, Otero E. Clinicopathological correlation in dementias. J Psychiatry Neurosci. 1995;20(4):276–82. [PMC free article] [PubMed] [Google Scholar]
- [52].Testa C, Laakso MP, Sabattoli F, Rossi R, Beltramello A, Soininen H, Frisoni GB. A comparison between the accuracy of voxel-based morphometry and hippocampal volumetry in Alzheimer's disease. J Magn Reson Imaging. 2004;19(3):274–82. doi: 10.1002/jmri.20001. [DOI] [PubMed] [Google Scholar]
- [53].Wakai M, Honda H, Takahashi A, Kato T, Ito K, Hamanaka T. Unusual findings on PET study of a patient with posterior cortical atrophy. Acta Neurol Scand. 1994;89(6):458–61. doi: 10.1111/j.1600-0404.1994.tb02666.x. [DOI] [PubMed] [Google Scholar]
- [54].Zakzanis KK, Boulos MI. Posterior cortical atrophy. Neurologist. 2001;7(6):341–9. doi: 10.1097/00127893-200111000-00003. [DOI] [PubMed] [Google Scholar]