Abstract
Centrosome integrity is critically important for successful fertilization and embryo development. In humans, the sperm contributes the dominant centrosomal material containing centrioles and centrosomal components onto which oocyte centrosomal proteins assemble after sperm incorporation to form the sperm aster that is essential for uniting sperm and oocyte pronuclei. Increasingly, dysfunctional sperm centrosomes have been identified as a factor for sperm-derived infertility and heterologous Intracytoplasmic Sperm Injection (ICSI) has been used to assess centrosome and sperm aster formation and clearly established a relationship between infertility and sperm centrosomal dysfunction. ICSI has been used successfully to provide novel treatment to overcome male factor infertility and it may open up new possibilities to correct specific sperm-related centrosome dysfunctions at molecular levels. New data indicate that it is now possible to replace dysfunctional centrosomes with functional donor sperm centrosomes which may provide new treatment for couples in which infertility is a result of centrosome-related sperm dysfunctions.
Keywords: centrosomes, infertility, sperm aster, centrosome integrity
Introduction
The impact of centrosome integrity on successful fertilization has become a major point of concern in Assisted Reproductive Technology (ART), as the success of fertilization highly depends on centrosomal contributions by both sperm and oocyte. However, in most systems, the sperm centrosome contributes the dominant nucleating seed consisting of the proximal centriole surrounded by pericentriolar components onto which the oocyte's centrosomal material is assembled (reviewed in Manandhar et al., 2005; Sun and Schatten, 2006, 2007). The sperm centrosome is primarily responsible for nucleating and organizing the sperm aster, which pushes the sperm head toward the oocyte center and guides migration of the female pronucleus for union with the male pronucleus, completing the fertilization process.
The sperm aster is a highly dynamic structure that undergoes rapid shape changes facilitated by centrosome plasticity and structural flexibility, molecular reorganizations, microtubule polymerization and depolymerization, allowing sperm aster growth and modifications. These rapid dynamics allow growth of the sperm aster into the zygote aster that surrounds the fused or apposed pronuclei. Centrosomal defects will cause fertilization failure and developmental arrest at the pronuclear stage.
Centrosome structure and function during fertilization
Generally described as the cell's main microtubule organizing center (MTOC), the centrosome is a central station that plays a role in multiple important functions facilitated by its capability to directly and effectively communicate through the microtubule network. Translocation of signal transduction molecules, macromolecular complexes, enzyme-containing vesicles and cell organelles are among the numerous activities carried out by centrosome-organized microtubules aided by microtubule motor proteins such as kinesin and dynein, and by numerous other molecules that are important for cell cycle-specific functions. Rapid interactions and rapid responses to altered environments are facilitated by the centrosome's structural plasticity and its special nature as a subcellular non-membrane bound semi-conservative organelle. Approximately 1 µm in size, the centrosome is able to form and reshape whereas reorganizing its structure and centrosomal proteins as required for specific cellular functions.
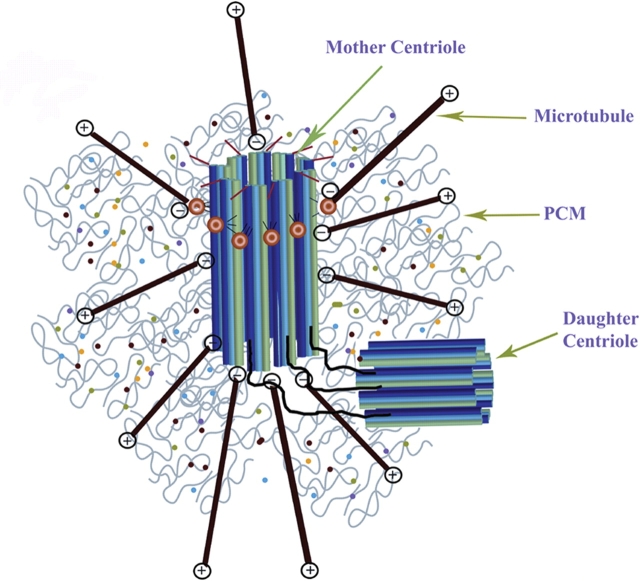
A typical centrosome is composed of a large number of centrosome proteins surrounding a pair of perpendicularly oriented cylindrical centrioles, therefore referred to as pericentriolar material (PCM) (Fig. 1). However, centrosomes without centrioles (acentriolar centrosomes) exist and are found for example in MII spindles of most mammalian species performing functions similar to centriole-containing centrosomes.
Figure 1.
Schematic diagram of a typical mammalian centrosome composed of two centrioles (mother and daughter centriole) surrounded by a meshwork of pericentriolar material (PCM).
Both centrioles are connected through interconnecting fibers. The mother centriole is distinguished from the daughter centriole by distal and subdistal appendages. The centrosomal material consists of a fibrous scaffolding lattice with a large amount of coiled-coil centrosome proteins and the centrosome's three-dimensional architecture is primarily maintained through specific protein–protein interactions. Microtubules are anchored with their minus ends to the centrosome core structure and microtubule growth is regulated by distal plus-end addition of tubulin subunits.
Unique to centrosomes compared with other cellular organelles are the lack of a membrane surrounding the organelle. The centrosomal material consists of a fibrous scaffolding lattice with a large amount of coiled-coil centrosome proteins and the centrosome's three-dimensional architecture is primarily maintained through specific protein–protein interactions. Microtubules are anchored with their minus ends to the centrosome core structure (Bornens, 2002) and microtubule growth is regulated by distal plus-end addition of tubulin subunits (McIntosh and Euteneuer, 1984). Microtubule numbers and lengths are regulated throughout the cell cycle to accommodate specific cellular requirements. Centrosome shape and microtubule organization are tightly correlated; highly compacted centrosomes are typically associated with focused microtubule asters although expanded centrosome structure results in the formation of various expanded microtubule organizations. These observations imply a close relationship and direct communication between microtubules and centrosomes that allow precise accommodations for cell-cycle-specific functions. A large amount of accessory proteins including centrosome-associated and microtubule-associated proteins play critical roles in rapid reorganizations that are especially apparent during sperm aster formation. Rapid reorganization is important for nuclear migrations in the fertilized oocyte to unite maternal and paternal genomes.
In most mammalian reproductive systems (except for rodents), the sperm contributes the centriole to the fertilized oocyte that duplicates during the pronuclear stage and separates after syngamy to serve as mitotic centers during first and all subsequent cell divisions (Figs 2 and 3). Therefore, the sperm centrosome plays essential and enormous roles in fertilization and impacts all stages of development. Studies by Palermo et al. (1997) assessed the role of physically separated sperm segments (head only, head and tail separated or isolated tail) and showed that physical disruption of spermatozoa compromises centrosome functions in the zygote. Based on a study of 10 patients with severe alterations in the head-tail junction and/or acephalic sperm, Chemes et al. (1999) revealed abnormal behavior of the spermatid centriole and tail anlage and showed that abnormal head-middle piece connections as result of abnormal neck development during spermiogenesis negatively impacts fertilization by Intracytoplasmic Sperm Injection (ICSI). These studies pointed to abnormal functions of centrioles that may have genetic origins. However, we will discuss later that other criteria such as environmental factors may not be excluded, as centrosomes are highly susceptible to toxic components which may affect sperm centrosomes and impair fertilization. Subsequent studies by Rawe et al. (2002) focused on pathologies of the sperm centriole as factors responsible for defective sperm aster formation, syngamy and cleavage. Other studies by Colombero et al. (1996) employed immunofluorescent staining of sperm centrosomes and revealed signals at the junction between the head and midpiece. Subsequent labeling of physically dissected spermatozoa head and tail pieces showed an approximately 50% labeling in isolated heads and 50% in isolated tails which opens the possibility to utilize isolated tails containing a proximal centriole as donor centrosome for patients with sperm-related centrosomal dysfunction. Previous studies have clearly shown that the formation of a sperm aster is possible when an isolated sperm tail (containing the proximal centriole) is injected into an oocyte (Van Blerkom and Davis, 1995).
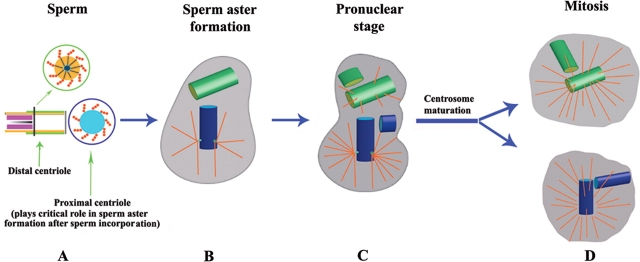
Figure 2.
Schematic diagram of centriole–centrosome organization and duplication from sperm incorporation through mitosis of the first cell cycle.
In most mammalian reproductive systems (except for rodents), the sperm contributes the centrioles to the fertilized oocyte that duplicate during the pronuclear stage and separate after syngamy to serve as mitotic centers during first and all subsequent cell divisions. Although only the proximal centriole displays microtubule organizing capabilities to form the sperm aster, the distal centriole may duplicate as explained in more detail in the text. (A) Prior to fertilization, spermatozoa display two distinct centriolar structures with the proximal centriole located within the connecting piece next to the basal plate of the sperm head. This centriole displays a pin-wheel structure of nine triplet microtubules surrounded by pericentriolar components. The degenerated distal centriole is organized perpendicular to the proximal centriole and aligned with the axoneme or sperm tail. (B) Shortly after sperm incorporation into the oocyte, a sperm aster is formed from the proximal centriole that allows pronuclear apposition. The sperm aster has several functions aside from guiding the two pronuclei into close apposition, as it also serves as railroad system for signal transduction molecules and for accumulating centrosomal components from the oocyte to the sperm's centriole–centrosome complex. (C) After pronuclear apposition, the sperm centrioles duplicate during the pronuclear stage (in subsequent cell cycles during the G1/S phases); mother and daughter centrioles form procentrioles, fibrous material that is associated with the proximal region and grows into daughter centrioles (in subsequent cell cycles during the S and G2 phases), resulting in two pairs of centrioles that indicate duplication of centrosomal material. This pattern of centriole duplication is termed semiconservative duplication, as each daughter cell retains one of the mother's centrioles although a new daughter centriole is formed. Centriole and centrosome cycles are tightly coupled and centrosome duplication occurs at the time centrioles duplicate. However, the composition of centrosomal material is complex and precisely regulated with other cell cycle events. (D) The duplicated centrioles separate and migrate around the zygote nucleus to form the opposite poles of the first mitotic spindle. Similar to somatic cells, major restructuring of the centrosome and microtubule nucleation takes place at the transition from G2/M to form the division-competent centers of the mitotic poles in a process termed centrosome maturation. Cell cycle specific centrosomal proteins become accumulated around the mitotic centriole–centrosome complex with the highest accumulation of γ-tubulin in mitosis to form microtubule-rich mitotic spindles.
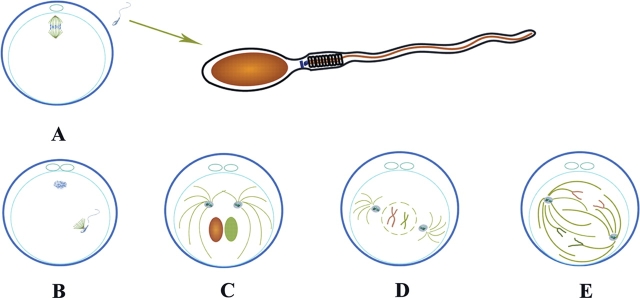
Figure 3.
Schematic diagram of the centriole–centrosome complex cycle within the first cell cycle.
(A) Sperm before fertilization contains a proximal and distal centriole. The meiotic spindle in the MII stage oocyte contains acentriolar centrosomes. (A) Extrusion of the second polar body; (B) Sperm aster formation from the sperm's proximal centriole–centrosome complex; (C) After pronuclear apposition, replication of the centriole at pronuclear stage; (D) After syngamy, the duplicated centriole–centrosome complex migrates around the zygote nucleus and relocates to opposite poles to form the centers of the mitotic spindle poles. (E) Mitosis of the first cell cycle.
To understand centrosome-related fertilization failures it is important to understand the centriole–centrosome complex itself, its regulation and functions during fertilization and cell division (Fig. 2). Prior to fertilization, human spermatozoa display two distinct centriolar structures (Sathananthan et al., 1991) with the proximal centriole located within the connecting piece next to the basal plate of the sperm head. This centriole displays a pin-wheel structure of nine triplet microtubules surrounded by pericentriolar components. The distal centriole is organized perpendicular to the proximal centriole and aligned with the axoneme or sperm tail (Holstein and Roosen-Runge, 1981; Sathananthan et al., 1991, 1996). Although the distal centriole progressively degenerates during spermiogenesis at the epididymal maturation stage in eutherian mammalian spermatozoa, the degeneration is not complete (Fawcett, 1965; Fawcett and Ito, 1965; Fawcett and Phillips, 1969; Holstein and Roosen-Runge, 1981; Manandhar et al., 2000) and the distal centriole is present as degenerated remnant in mature sperm. Manandhar et al. (2000) estimated using immunogold electron microscopy that about 50% of the microtubules of the distal centriole are lost. Shortly after sperm incorporation into the oocyte, a sperm aster is formed from the proximal centriole that allows pronuclear apposition. The proximal centriole maintains close connection to the decondensing sperm nucleus that gradually develops into a male pronucleus. There has been confusion regarding the number of centrioles contributed by sperm and regarding centriole functions shortly after fertilization. As has been reviewed by Manandhar et al. (2005), it is widely accepted that during animal fertilization the male gamete contributes two centrioles that organize a functional zygotic centrosome after recruiting centrosomal proteins from the oocyte's cytoplasm. As shown in Fig. 2, only the proximal centriole is able to organize microtubules whereas the distal degenerated (tail-associated) centriole is not. After pronuclear apposition, the sperm centrioles duplicate and the duplicated centrioles separate and migrate around the zygote nucleus to form the opposite poles of the first mitotic spindle (Sathananthan et al., 1991, 1996; reviewed in Manandhar et al., 2005) (Figs 2 and 3). The duplicated centriole–centrosome complex consists of a pair of centrioles surrounded by duplicated centrosome material. One complexity that has not yet been resolved in detail is asymmetric centriole–centrosome replication during fertilization as reported for various species including humans (Sathananthan et al., 1996; Crozet et al., 2000). As reviewed by Manandhar et al. (2005), this phenomenon relates to different replication of the proximal and distal centriole. As has been reported for sea urchin fertilization, the proximal centriole replicates normally, although the distal centriole also assembles a new centriole but without resurrecting microtubular triplets (Paweletz et al., 1987). This mode of replication may explain why different centriole numbers can be found at opposite poles of mitotic spindles during first division. Crozet et al. (2000) reported in sheep zygotes one centriole in one mitotic pole although two were found at the opposite mitotic pole and varying numbers of centrioles have been reported for humans by Sathananthan et al. (1996).
A typical centriolar duplex consists of two centrioles that are associated with each other in orthogonal orientation representing mother centriole and daughter centriole that are distinguished from each other by flap-like appendages at the distal end of the mother centriole and cone-shaped and striated appendages at the outer wall (Fig. 1). The daughter centriole displays ‘cartwheel’ organization in the proximal lumen (reviewed in Manandhar et al., 2005). Microtubule nucleation originates from the fibrous material around the mother centriole (Figs 2 and 3). During the pronuclear cell cycle stage (in subsequent cell cycles during the G1/S phases), mother and daughter centrioles form procentrioles, fibrous material that is associated with the proximal region and grows into daughter centrioles (in subsequent cell cycles during the S and G2 phases), resulting in two pairs of centrioles that indicate duplication of centrosomal material. This pattern of centriole duplication is termed semiconservative duplication, as each daughter cell retains one of the mother's centrioles although a new daughter centriole is formed (Kochanski and Borisy, 1990). Similar to somatic cells, major restructuring of the centrosome and microtubule nucleation takes place at the transition from G2/M to form the division-competent centers of the mitotic poles (Figs 2 and 3).
Sperm aster formation includes precise nucleation of microtubules. As discussed above, the sperm contains centrosomal proteins that are retained after centrosome reduction during gametogenesis. Centriole and centrosome cycles are tightly coupled and centrosome duplication occurs at the time centrioles duplicate. However, the composition of centrosomal material is complex and precisely regulated with other cell cycle events. Of the numerous centrosome and centrosome-associated proteins, four centrosome proteins [γ-tubulin, pericentrin, centrin, nuclear mitotic apparatus (NuMA)] will be discussed here that play critical roles in sperm aster and spindle formation and functions although it is clear that phosphoproteins (Palermo et al., 1997) and a variety of other centrosome-regulating components most certainly play a role in this dynamic event based on our knowledge of aster formation in somatic cells that are discussed in other reviews (Schatten, 2008; Schatten and Sun, 2009). We know from somatic cells that regulatory proteins including proteins or enzymes (kinases, phosphatases and others) associate with centrosomes and utilize centrosomes as platform for signaling but they are not centrosome or centrosome-associated proteins. In somatic cells, as many as 500 different proteins may be associated with the centrosome as determined by mass-spectrometry-based proteomic analysis (Andersen et al. 2003) and it is clear that not all of these proteins are centrosome proteins but some may be co-isolated because of their association with centrosomes as molecules that use the centrosome as docking station. However, as many as 100 different types of centrosome proteins may be involved in the dynamically changing centrosome composition during aster formation in the fertilized oocyte.
The γ-tubulin ring complexes, composed of γ-tubulin and accessory proteins, are centrosome proteins that are permanently associated with the centrosome core structure and nucleate microtubules throughout the cell cycle. The unfertilized oocyte contains γ-tubulin that is recruited to the sperm centrosome to nucleate increasing numbers of microtubules as the sperm aster grows into the zygote aster formed by the zygotic centrosome. Accurate recruitment of γ-tubulin is important, as over-recruitment will result in nucleation of too many microtubules although recruitment of insufficient amounts of γ-tubulin will result in sparse aster formation that both may result in aster formation abnormalities and decreased developmental potential as well as cell cycle abnormalities. Sperm aster formation and size has been analyzed during bovine fertilization and correlated to in vitro embryonic development to the blastocyst stage which showed a bull-dependent variation in the degree of sperm-derived centrosome and aster organization that affects male fertility and early development (Navara et al., 1996; Rawe et al., 2002). For more detailed analysis on centrosome components that play a role in the aster size variations, detailed molecular analysis will be necessary but may not be possible with our currently available techniques. More detailed knowledge would allow the selection of individual sperm for optimal ICSI procedures.
The centrosome protein pericentrin plays a role in centrosome and spindle organization (Doxsey et al., 1994; Dictenberg et al., 1998; Young et al., 2000) along with several other proteins. It forms a complex with γ-tubulin and depends on dynein for assembly onto centrosomes (Young et al., 2000). Mutation of the pericentrin gene results in loss of recruitment of structural and signaling proteins that are important for centrosome regulation (Griffith et al., 2008).
Centrins, members of a highly conserved subgroup of the EF-hand superfamily of Ca2+-binding proteins, are associated with centrioles (Bornens, 2002; Jurczyk et al., 2004). As shown by Manandhar et al. (2006), boar ejaculated spermatozoa contain centrin that is lost in zygotes; other species may retain centrin (reviewed in Manandhar et al., 2005). Studies in somatic cells have shown that centrin is an intrinsic component of centrosomes with an essential role in centrosome duplication (Salisbury, 1995; Levy et al., 1996; Lutz et al., 2001; reviewed in Salisbury et al., 2002; Manandhar et al., 2005).
A close relationship exists between centrosome and nuclear proteins which has clearly been shown for the NuMA protein (reviewed in Sun and Schatten, 2006). NuMA is localized in the decondensing sperm nucleus and in the oocyte nucleus and becomes a centrosomal protein during mitosis where it is crucial for tethering mitotic microtubules into the mitotic apparatus. NuMA is not localized to sperm centrosomes but it is prominent in the MII spindle of unfertilized oocytes and it is clearly localized to mitotic spindles after fertilization. In subsequent cell cycles, NuMA is not associated with the interphase centrosome but it strongly associates with mitotic centrosomes throughout development.
Taken together, during fertilization, the sperm centrosome serves as nucleating center onto which oocyte centrosomal material is precisely assembled to accurately form the sperm aster that develops into the zygote aster for pronuclear apposition. The centrosome further serves as unique signaling platform to recruit and distribute regulatory components and enzymes for cell cycle specific and adaptive regulations. Nuclear and centrosomal functions are tightly coupled and it is clear that accurate centrosomal functions are most important to ensure development of a fertilized oocyte into an entire well-functioning organism.
Centrosome reduction during gametogenesis
To understand centrosome-related fertilization failures it is important to understand centrosome reduction during gametogenesis which has been reviewed in detail by Manandhar et al. (2005) and Sun and Schatten (2006). A precise program of centrosome reduction has to take place during gametogenesis to ensure that a fully functional precisely composed centrosome is restored after fertilization. Excess or reduced centrosomal material will lead to fertilization failures or developmental abnormalities. The centrosome that is restored when sperm and oocyte centrosomal components unite at fertilization has to be able to form the sperm aster, duplicate during the pronuclear phase, mature by assembling cell cycle-specific centrosome proteins around the centrosome core, precisely separate during mitosis, and relocate to the divided daughter cells to ensure regulated cell divisions during all subsequent cell cycles. After completion of centrosome reduction during gametogenesis, spermatozoa are left with centrioles but have lost most of the PCM although the oocyte has lost centrioles but has retained a stockpile of centrosomal proteins that are dispersed throughout the ooplasm, as no centrioles are present within the oocyte to serve as focal center for centrosomal aggregation. Gamma-tubulin and centrin are present in soluble forms (Paoletti et al., 1996) and can be aggregated under activation conditions (Schatten et al., 1992).
During male meiotic divisions, haploid cells are formed, termed round spermatids. Round spermatids shed excess cytoplasm as residual bodies in a process termed spermiogenesis which is followed by spermiation during which fully formed spermatozoa are released into the seminiferous tubule that undergo maturation in the epididymis. The proximal centriole is retained although the distal centriole loses microtubule-nucleating functions in non-rodent mammalian sperm. Detailed analysis of centrosome reduction is provided in a review by Manandhar et al. (2005).
ICSI and assays for centrosome functions in ART
ICSI was first reported by Palermo et al. (1992) and has allowed a novel treatment overcoming male factor infertility. It has allowed sperm incorporation in cases in which in vitro fertilization (IVF) was difficult because of lack of sperm motility or other unknown factors. As many as 50% of IVF cycles are now employing ICSI; details regarding benefits and possible complexities associated with ICSI are reviewed by Hewitson (2004). In this section, we focus on the role and significance of centrosomes in ICSI and on possible therapies to restore defective centrosome functions. Various methods to determine sperm centrosome integrity, functioning and sperm aster formation have been designed and employed in recent years. Among them are studies that have evaluated the formation of the sperm aster within the fertilized oocyte to predict successful union of sperm and oocyte nuclei and positive developmental potential (Navara et al., 1996; Tachibana et al., 2009).
For human IVF, sperm centrosomal function and sperm aster formation is now being evaluated by insemination of rabbit or bovine oocytes which provides a more suitable and superior system compared with the mouse or hamster that had previously been used to assess sperm quality and the sperm's ability to form a functional sperm aster for apposition and/or union of male and female pronuclei. Several promising assays have been developed in recent years to assess sperm aster formation using heterologous ICSI systems (reviewed in Hayasaka et al., 2006; Terada, 2007) in which human sperm were microinjected into either rabbit (Terada et al., 2000, 2004) or bovine (Nakamura et al., 2001, 2002; Rawe et al., 2002; Yoshimoto-Kakoi et al., 2008) oocytes. Such assays clearly established a relationship between infertility and sperm centrosomal dysfunction (Terada et al., 2002). Recent studies by Ugajin et al. (2008) proposed selection of sperm with a morphologically normal looking midpiece containing the sperm's centrosome to improve the rate of fertilization when employing ICSI. Other experiments assessing centrosome functions in globozoospermia (characterized by sperm with round heads and lack of an acrosome and acrosomal enzymes and a disorganized midpiece; Dam et al., 2007) revealed low rates of sperm aster formation when heterologous ICSI with bovine oocytes was used (15.8%). Although oocyte activation with ethanol improved male pronuclear formation, the rate of sperm aster formation was only slightly improved (32.3%). In sperm with dysplasia of the fibrous sheath that are characterized by dysplastic development of the axonemal and periaxonemal cytoskeleton and midpiece disorganization, immunolabeling for centrin revealed negative results. Parthenogenesis of human oocytes followed by treatment with paclitaxel has recently been employed by Terada et al. (2009a) to induce MTOC and cytoplasmic aster formation. Previous studies on pig oocytes (Kim et al., 1996) and sea urchin oocytes (Schatten et al. 1992) had shown that MTOCs can be maternally induced and carry out centrosomal functions although they differed from sperm-induced MTOCs in that they were multiple aster formations that did not contain centrioles. Another study by Terada et al. (2009b) revealed that oocyte activation with calcium ionophore improved the rate of fertilization for couples with poor sperm centrosomal function. A case was reported in which successful pregnancy was achieved and a healthy baby was born when the oocyte was activated by exposure to ionomycin prior to ICSI although seven previous ICSI attempts of unactivated oocytes had failed.
More recently, studies by Rawe et al. (2008) investigated the role of sperm proteasomes during sperm aster formation and revealed that centriolar pathology in patients was related to defective function of sperm proteasomes. Proteasomes were localized in the sperm acrosome and connecting-piece and in pronuclei of bovine and human zygotes. Defective human spermatozoa displayed decrease in proteasomal enzymatic activity. Pharmacological and immunological block of proteasomes in human or bovine spermatozoa and oocytes resulted in disrupted sperm aster formation and pronuclear development which was also shown in 28 discarded human post-ICSI fertilization failures. These findings suggest that this phenomenon may occur more frequently than has been recognized so far although precise numbers of incidences of centrosome dysfunctions are not available at the present time.
It has been proposed that sperm pathologies point to genetic and inherited defects (Baccetti et al., 1989; Chemes et al., 1999; Rawe et al., 2002; Porcu et al., 2003), particularly since in several cases sets of two brothers displayed the same centrosomal defects. Although genetic components most likely play a role in sperm pathologies and centrosome dysfunctions, pathologies of acquired origin also need to be considered. Centrosomes are extremely susceptible to environmental factors which may cause centrosomal defects as has clearly been shown for centrosomes in MII oocytes that are affected by toxic compounds such as bisphenol-A (a widely used environmental estrogen-like chemical), 2-methylestradiol or cocaine (reviewed in Schatten and Sun, 2009) that all destabilize centrosome structure. Although it is known that sperm count becomes reduced after exposure to toxic compounds, no studies so far have been performed to investigate the possible effects on the sperm's centriole–centrosome complex. Recent reviews highlight the impact of environmental factors on sperm DNA (Aitken, 2008) and the role of epigenetic factors and contribution of a functional centrosome for IVF have been addressed (Carrell, 2008). However, quantitative population studies are not yet available on the percentage of sperm centrosome pathologies that play a role in IVF. Individual study results are available and mainly relate to sperm aster formation that was ca. 19% lower in infertile males reported by Yoshimoto-Kakoi et al. (2008). Centriolar/centrosomal pathologies are emerging as major players in causes of infertility and further studies will be important to bring further insights in this area of human reproduction.
Because spermatozoa in mice, unlike in most other mammals, do not contribute centrosomes to zygotes, mouse ICSI only requires the sperm head, although ICSI in other species requires both the head and connected sperm centrioles. In rabbits, the importance of centrosomes was assessed in ICSI experiments in which the centrosome was removed from rabbit sperm by sonication and compared with fertilization with control sperm containing sperm centrosomes. As expected, the sperm head without centrosomes did not nucleate a sperm aster although organized microtubules were formed within the sperm-activated oocyte suggesting that the maternal centrosome was able to fulfill certain centrosomal functions leading to mitotic spindle formation. It should be emphasized that in rabbits, de novo formation of centrioles is possible and parthenogenesis is more readily induced than in other systems (Terada et al., 2000). Evidence that in rabbits a maternal MTOC can support spindle formation and subsequent development up to implantation has been shown by Ozil (1990) who induced oocyte activation by electrical stimulation and cytochalasin B to prevent second polar body extrusion to produce gynogenetic zygotes with two pronuclei. Szollosi and Ozil (1991) subsequently reported maternal origin for centrioles in parthenogenetically activated, diploidized rabbit embryos as a result of de novo formation of centrioles in this species. Human parthenogenetic embryos typically do not develop past the 8-cell stage (Winston et al., 1991) although mouse oocytes can develop further past implantation because of the maternal inheritance of centrosomes in mice lacking paternal centrosome contributions (Schatten et al., 1991). Birth of parthenogenetic mice that can develop to adulthood has been reported by Kono et al. (2004).
More recently, studies in the domestic cat analyzed the possible causes for lower developmental potential of testicular spermatozoa and examined centrosomal function and sperm aster formation after ICSI on first-cleavage timing, developmental rate and morula-blastocyst formation (Comizzoli et al. 2006). The results showed higher proportions of zygotes with short or absent sperm asters after ICSI with testicular spermatozoa compared with ejaculated spermatozoa that contained large sperm asters after ICSI. The poor pattern of aster formation from the testicular sperm centrosome was associated with delayed first cleavage, slower developmental rate, and reduced formation of morulae and blastocysts. Remarkably, improvement was reported when testicular sperm centrosome was replaced by a centrosome from an ejaculated spermatozoon which resulted in higher rates of embryo development comparable to data from ejaculated spermatozoa. These data indicate that sperm function therapy is possible by centrosome replacement from donor sperm which opens up immensely new possibilities for couples in which infertility is a result of centrosome-related sperm dysfunctions. However, genetic transmission of a sperm defect needs to be considered as is the case in Kartagener's syndrome and would require informed consent, as centrosome transplants may bypass a genetic defect for IVF but would inherit the defect. More research is clearly needed to assess defective centrosomes being the result of genetic transmission, developmental complexities during spermiogenesis, or environmental factors. Injection of round spermatids into oocytes (ROSI) works routinely in the mouse, but it is controversial in humans. Insufficient oocyte activation following ROSI and the functional immaturity of the centrosome could be responsible for human ROSI failure (Yanagimachi, 2005). ICSI procedures with round spermatids (ROSI) has not yet been explored in sufficient detail and it is possible that centrosome integrity may not always be maintained, explaining the failure to achieve viable offspring with ROSI (Sofikitis et al., 1994, 1995). The poor success rates can be explained by the use of cells from defective spermatogenesis. It is possible that the absence of a functional centrosome may be a factor in pregnancy failures after ROSI. Centrosome maturity is important for successful embryo development although more research is needed to determine the specific factors involved in the sperm's centrosome maturation. Based on our present knowledge, no difference in male centrosome conditions have been reported in fertilization by ICSI compared with normal fertilization. In both cases, the sperm provides the seed onto which oocyte centrosomal material aggregates. If the sperm centrosome is defective, in both cases fertilization is compromised. If the sperm displays centrosomal integrity, pronuclear apposition is ensured along with positive developmental potential. At present, there is no evidence that centrosome dysfunctions are increased after ICSI. Taken together, these results indicate that centrosomal functions significantly impact developmental potential.
Conclusions and future perspectives
The study of centrosomes in germ cells has opened exciting new possibilities to treat infertility related to centrosomal defects and an inability to form a sperm aster and mitotic apparatus for cell division to assure proper development. Studies in the cat have shown that it is possible to use donor sperm centrosomes to fulfill sperm functions that are impaired in couples with infertility problems. Although intracytoplasmic human sperm injection therapy with donor centrosomes is a complex process, reconstituting defective centrosomal material is a promising new avenue to pursue, and it may open new aspects for infertility treatments.
Funding
Parts of the authors' studies cited in this review were supported by the National Basic Research Program of China (2006CB944001, 2006CB504004) and NIH grants (R03-HD43829-02 and R03HD047309-02).
Acknowledgements
The authors gratefully acknowledge Dr Yi-Liang Miao for his excellent help with the schematic representations (Figs 1–3).
References
- Aitken RJ. Just how safe is assisted reproductive technology for treating male factor infertility? Expert Rev Obstet Gynecol. 2008;3:267–271. [Google Scholar]
- Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- Baccetti B, Burrini AG, Collodel G, Magnano AR, Piomboni P, Renieri T, Sensini C. Morphogenesis of the decapitated and decaudated sperm defect in two brothers. Gamete Res. 1989;23:181–188. doi: 10.1002/mrd.1120230205. [DOI] [PubMed] [Google Scholar]
- Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14:25–34. doi: 10.1016/s0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- Carrell DT. Paternal genetic and epigenetic influences on IVF outcome. Expert Rev Obstet Gynecol. 2008;3:359–367. [Google Scholar]
- Chemes HE, Puigdomenech ET, Carizza C, Brugo Olmedo S, Zanchetti F, Hermes A. Acephalic spermatozoa and abnormal development of the head-neck attachment. A human syndrome of genetic origin. Hum Reprod. 1999;14:1811–1818. doi: 10.1093/humrep/14.7.1811. [DOI] [PubMed] [Google Scholar]
- Colombero LT, Moomjy M, Rosenwaks Z, Palermo GD. Indirect evidence of centrosome and spindle development in embryos generated by sperm segments. Hum Reprod. 1996;11:21–22. [Google Scholar]
- Comizzoli P, Wildt DE, Pukazhenthi BS. Poor centrosomal function of cat testicular spermatozoa impairs embryo development in vitro after intracytoplasmic sperm injection. Biol Reprod. 2006;75:252–260. doi: 10.1095/biolreprod.106.051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozet N, Dahirel M, Chesne P. Centrosome inheritance in sheep zygotes; centrioles are contributed by the sperm. Microsc Res Tech. 2000;49:445–450. doi: 10.1002/(SICI)1097-0029(20000601)49:5<445::AID-JEMT6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Dam AHDM, Feenstra I, Westphal JR, Ramos L, van Golde RJT, Kremer JAM. Globozoospermia revisited. Hum Reprod Update. 2007;13:63–75. doi: 10.1093/humupd/dml047. [DOI] [PubMed] [Google Scholar]
- Dictenberg J, Zimmerman W, Sparks C, Young A, Vidair C, Zheng Y, Carrington W, Fay F, Doxsey SJ. Pericentrin and gamma tubulin form a protein complex and are organized into a novel lattice at the centrosome. J Cell Biol. 1998;141:163–174. doi: 10.1083/jcb.141.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey SJ, Stein P, Evans L, Calarco P, Kirschner M. Pericentrin, a highly conserved protein of centrosomes involved in microtubule organization. Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Fawcett DW. The anatomy of the mammalian spermatozoon with particular reference to the guinea pig. Z Zellforsch. 1965;67:279–296. doi: 10.1007/BF00339376. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Ito S. The fine structure of bat spermatozoa. Am J Anat. 1965;116:567–609. doi: 10.1002/aja.1001160306. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Phillips DM. The fine structure and development of the neck region of the mammalian spermatozoon. Anat Rec. 1969;165:153–184. doi: 10.1002/ar.1091650204. [DOI] [PubMed] [Google Scholar]
- Griffith E, Walker S, Martin C-A, Vagnarelli P, Stiff T, Vernay B, Al Sanna N, Saggar A, hamel B, Earnshaw WC, et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet. 2008;40:232–236. doi: 10.1038/ng.2007.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Terada Y, Morita J, Tachibana M, Shima-Morito Y, Kakoi-Yoshimoto T, Nakamura S, Murakami T, Yaegashi N, Okamura K. Post-ICSI cytoskeletal dynamics during fertilization. J Mamm Ova Res. 2006;23:21–26. [Google Scholar]
- Hewitson L. Primate models for assisted reproductive technologies. Reproduction. 2004;128:293–299. doi: 10.1530/rep.1.00242. [DOI] [PubMed] [Google Scholar]
- Holstein AF, Roosen-Runge EC. Atlas of Human Spermatogenesis. Berlin: Grosse Verlag; 1981. [Google Scholar]
- Kim NH, Moon SJ, Prather RS, Day BN. Cytoskeletal alteration in aged porcine oocytes and parthenogenesis. Mol Reprod Dev. 1996;43:513–518. doi: 10.1002/(SICI)1098-2795(199604)43:4<513::AID-MRD14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kochanski R, Borisy G. Mode of centriole duplication and distribution. J Cell Biol. 1990;110:1599–1605. doi: 10.1083/jcb.110.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T, Obata Y, Wu Q, Niwa K, Ono Y, Yamamoto Y, Park ES, Seo JS, Ogawa H. Birth of parthenogenetic mice that can develop to adulthood. Nature. 2004;428:860–864. doi: 10.1038/nature02402. [DOI] [PubMed] [Google Scholar]
- Jurczyk A, Gromley A, Redick S, Augustin JS, Witman G, Pazour GJ, Peters DJM, Doxsey S. Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly. J Cell Biol. 2004;166:637–643. doi: 10.1083/jcb.200405023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy YY, Lai EY, Remillard SP, Heintzelman MB, Fulton C. Centrin is a conserved protein that forms diverse associations with centrioles and MTOCs in Naegleria and other organisms. Cell Motil Cytoskeleton. 1996;33:298–323. doi: 10.1002/(SICI)1097-0169(1996)33:4<298::AID-CM6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lutz W, Lingle WL, McCormick D, Greenwood TM, Salisbury JL. Phosphorylation of centrin during the cell cycle and its role in centriole separation preceding centrosome duplication. J Biol Chem. 2001;276:20774–20780. doi: 10.1074/jbc.M101324200. [DOI] [PubMed] [Google Scholar]
- Manandhar G, Simerly C, Schatten G. Highly degenerated distal centrioles in rhesus and human spermatozoa. Hum Reprod. 2000;15:256–263. doi: 10.1093/humrep/15.2.256. [DOI] [PubMed] [Google Scholar]
- Manandhar G, Schatten H, Sutovsky P. Centrosome reduction during gametogenesis and its significance. Biol Reprod. 2005;72:2–13. doi: 10.1095/biolreprod.104.031245. [DOI] [PubMed] [Google Scholar]
- Manandhar G, Feng D, Yi YJ, Lai L, Letko J, Laurincik J, Sutovsky M, Salisbury JL, Prather RS, Schatten H, et al. Centrosomal protein centrin is not detectable during early pre-implantation development but re-appears during late blastocyst stage in porcine embryos. Reproduction. 2006;132:423–434. doi: 10.1530/rep.1.00983. [DOI] [PubMed] [Google Scholar]
- McIntosh JR, Euteneuer U. Tubulin hooks as probes for microtubule polarity: an analysis of the method and an evaluation of data on microtubule polarity in the mitotic spindle. J Cell Biol. 1984;98:525–533. doi: 10.1083/jcb.98.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Terada Y, Horiuchi T, Emuta C, Murakami T, Yaegashi N, Okamura K. Human sperm aster formation and pronuclear decondensation in bovine eggs following intracytoplasmic sperm injection using a Piezo-driven pipette. Biol Reprod. 2001;65:1359–1363. doi: 10.1095/biolreprod65.5.1359. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Terada Y, Horiuchi T, Emuta C, Murakami T, Yaegashi N, Okamura K. Analysis of the human sperm centrosomal function and the oocyte activation ability in a case of globozoospermia, by ICSI into bovine oocytes. Hum Reprod. 2002;17:2930–2934. doi: 10.1093/humrep/17.11.2930. [DOI] [PubMed] [Google Scholar]
- Navara CS, First NL, Schatten G. Phenotypic variations among paternal centrosomes expressed within the zygote as disparate microtubule lengths and sperm aster organization: Correlations between centrosome activity and developmental success. Proc Natl Acad Sci USA. 1996;93:5384–5388. doi: 10.1073/pnas.93.11.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozil JP. The parthenogenetic development of rabbit oocytes after repetitive pulsatile electrical stimulation. Development. 1990;109:117–127. doi: 10.1242/dev.109.1.117. [DOI] [PubMed] [Google Scholar]
- Palermo G, Joris H, Devroey P, Van Steirteghem A. Pregnancies after intracytoplasmic sperm injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- Palermo GD, Colombero LT, Rosenwaks Z. The human sperm centrosome is responsible for normal syngamy and early embryonic development. Rev Reprod. 1997;2:19–27. doi: 10.1530/ror.0.0020019. [DOI] [PubMed] [Google Scholar]
- Paoletti A, Moudjou M, Paintrand M, Salisbury JL, Bornens M. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J Cell Sci. 1996;109:3089–3102. doi: 10.1242/jcs.109.13.3089. [DOI] [PubMed] [Google Scholar]
- Paweletz N, Mazia D, Finze EM. Fine structural studies of the bipolarization of the mitotic apparatus in the fertilized sea urchin egg, I. The structure and behavior of centrosomes before fusion of the pronuclei. Eur J Cell Biol. 1987;44:195–204. [PubMed] [Google Scholar]
- Porcu G, Mercier G, Boyer P, Achard V, Banet J, Vasserot M, Melone C, Saias-Magnan J, D’Ercole C, Chau C, et al. Pregnancies after ICSI using sperm with abnormal head-tail junction from two brothers: Case report. Hum Reprod. 2003;18:562–567. doi: 10.1093/humrep/deg121. [DOI] [PubMed] [Google Scholar]
- Rawe V, Terada Y, Nakamura S, Chillik CF, Brugo Olmedo S, Chemes HE. A pathology of the sperm centriole responsible for defective sperm aster formation, sygamy and cleavage. Hum Reprod. 2002;17:2344–2349. doi: 10.1093/humrep/17.9.2344. [DOI] [PubMed] [Google Scholar]
- Rawe VY, Díaz ES, Abdelmassih R, Wójcik C, Morales P, Sutovsky P, Chemes HE. The role of sperm proteasomes during sperm aster formation and early zygote development: implications for fertilization failure in humans. Human Reprod. 2008;23:573–580. doi: 10.1093/humrep/dem385. [DOI] [PubMed] [Google Scholar]
- Salisbury JL. Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol. 1995;7:39–45. doi: 10.1016/0955-0674(95)80043-3. [DOI] [PubMed] [Google Scholar]
- Salisbury JL, Suino KM, Busby R, Springett M. Centrin-2 is required for centriole duplication in mammalian cells. Curr Biol. 2002;12:1287–1292. doi: 10.1016/s0960-9822(02)01019-9. [DOI] [PubMed] [Google Scholar]
- Sathananthan AH, Osborne IKJ, Trounson A, Bongso SC, Ng A, Ratnam SS. Centrioles in the beginning of human development. Proc Natl Acad Sci USA. 1991;88:4806–4810. doi: 10.1073/pnas.88.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathananthan AH, Ratnam SS, Ng SC, Tarin JJ, Gianaroli L, Trounson A. The sperm centriole: its inheritance, replication and perpetuation in early human embryos. Hum Reprod. 1996;11:345–356. doi: 10.1093/humrep/11.2.345. [DOI] [PubMed] [Google Scholar]
- Schatten H. The mammalian centrosome and its functional significance. Histochem Cell Biol. 2008;129:667–686. doi: 10.1007/s00418-008-0427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten H, Sun QY. The functional significance of centrosomes in mammalian meiosis, fertilization, development, nuclear transfer, and stem cell differentiation. Environ Mol Mutagen. 2009 doi: 10.1002/em.20493. Apr 28 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Schatten G, Simerly C, Schatten H. Maternal inheritance of centrosomes in mammals? Studies on parthenogenesis and polyspermy in mice. Proc Natl Acad Sci USA. 1991;88:6785–6789. doi: 10.1073/pnas.88.15.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten H, Walter M, Biessmann H, Schatten G. Activation of maternal centrosomes in unfertilized sea urchin eggs. Cell Motil Cytoskeleton. 1992;23:61–70. doi: 10.1002/cm.970230107. [DOI] [PubMed] [Google Scholar]
- Sofikitis N, Miyagawa I, Sharplip I, Hellstrom W, Mekras G, Mastelou E. Human pregnancies achieved by intra-ooplasmic injections of round spermatid (RS) nuclei isolated from testicular tissue of azoospermic men. J Urol. 1995;153(Suppl 1):258A. [Google Scholar]
- Sun QY, Schatten H. Multiple roles of NuMA in vertebrate cells: review of an intriguing multi–functional protein. Front Biosci. 2006;11:1137–1146. doi: 10.2741/1868. [DOI] [PubMed] [Google Scholar]
- Sun QY, Schatten H. Centrosome inheritance after fertilization and nuclear transfer in mammals. In: Sutovsky Peter., editor. Somatic Cell Nuclear Transfer. Vol. 591. Landes Bioscience, Adv Exp Med Biol; 2007. pp. 58–71. [DOI] [PubMed] [Google Scholar]
- Szollosi D, Ozil JP. De novo formation of centrioles in parthenogenetically activated, diploidized rabbit embryos. Biol Cell. 1991;72:61–66. doi: 10.1016/0248-4900(91)90079-3. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Terada Y, Ogonuki N, Ugajin T, Ogura A, Murakami T, Yaegashi N, Okamura K. Functional assessment of centrosomes of spermatozoa and spermatids microinjected into rabbit oocytes. Mol Reprod Dev. 2009;76:270–277. doi: 10.1002/mrd.20951. [DOI] [PubMed] [Google Scholar]
- Terada Y, Nakamura S, Simerly C, Hewitson L, Murakami T, Yaegashi N, Okamura K, Schatten G. Centrosomal function assessment in human sperm using heterologous ICSI with rabbit eggs; a new male factor infertility assay. Mol Reprod Dev. 2004;67:360–365. doi: 10.1002/mrd.20024. [DOI] [PubMed] [Google Scholar]
- Terada Y. Functional analyses of the sperm centrosome in human reproduction: implications for assisted reproductive technique. Soc Reprod Fertil Suppl. 2007;63:507–513. [PubMed] [Google Scholar]
- Terada Y, Simerly CR, Hewitson L, Schatten G. Sperm aster formation and pronuclear decondensation during rabbit fertilization and development of a functional assay for human sperm. Biol Reprod. 2000;62:557–563. doi: 10.1095/biolreprod62.3.557. [DOI] [PubMed] [Google Scholar]
- Terada Y, Nakamura S, Hewitson L, Simerly CR, Horiuchi T, Murakami T, Okamura K, Schatten G. Human sperm aster formation after intracytoplasmic sperm injection with rabbit and bovine eggs. Fertil Steril. 2002;77:1283–1284. doi: 10.1016/s0015-0282(02)03106-0. [DOI] [PubMed] [Google Scholar]
- Terada Y, Hasegawa H, Ugajin T, Murakami T, Yaegashi N, Okamura K. Microtubule organization during human parthenogenesis. Fertil Steril. 2009;a 90:1271–1272. doi: 10.1016/j.fertnstert.2008.05.051. [DOI] [PubMed] [Google Scholar]
- Terada Y, Hasegawa H, Takahashi A, Ugajin T, Yaegashi N, Okamura K. Successful pregnancy after oocyte activation by a calcium ionophore for a patient with recurrent intracytoplasmic sperm injection failure, with an assessment of oocyte activation and sperm centrosomal function using bovine eggs. Fertil Steril. 2009;b 91:935.e11–935.e14. doi: 10.1016/j.fertnstert.2008.09.043. [DOI] [PubMed] [Google Scholar]
- Ugajin T, Terada Y, Hasegawa H, Murakami T, Yaegashi N, Okamura K. Novel strategy of ICSI – selection of sperm with morphologically normal midpiece by using IMSI. Fertil Steril. 2008;90 abstract P-284. [Google Scholar]
- Van Blerkom J, Davis P. Evolution of the sperm aster microinjection of isolated human sperm centrosomes into meiotically mature human oocytes. Hum Reprod. 1995;10:2179–2182. doi: 10.1093/oxfordjournals.humrep.a136264. [DOI] [PubMed] [Google Scholar]
- Winston N, Johnson M, Pickering S, Braude P. Parthenogenetic activation and development of fresh and aged human oocytes. Fertil Steril. 1991;56:904–912. [PubMed] [Google Scholar]
- Yanagimachi R. Intracytoplasmic injection of spermatozoa and spermatogenic cells: Its biology and applications in humans and animals. Reproductive Biomedicine Online. 2005;10:247–288. doi: 10.1016/s1472-6483(10)60947-9. [DOI] [PubMed] [Google Scholar]
- Yoshimoto-Kakoi T, Terada Y, Tachibana M, Murakami T, Yaegashi N, Okamura K. Assessing centrosomal function of infertile males using heterologous ICSI. Syst Biol Reprod Med. 2008;54:135–142. doi: 10.1080/19396360802043091. [DOI] [PubMed] [Google Scholar]
- Young A, Dictenberg JB, Purohit A, Tuft R, Doxsey SJ. Cytoplasmic dynein-mediated assembly of pericentrin and γ-tubulin onto centrosomes. Mol Biol Cell. 2000;11:2047–2056. doi: 10.1091/mbc.11.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]