Abstract
Bacterial vaginosis (BV) is one of the most prevalent vaginal disorders in adult women and is associated with adverse pregnancy outcomes such as pre-term birth. Genetic factors, particularly in genes involved in inflammation and infection, are associated with this condition. Additionally, environmental risk factors including stress and smoking are associated with BV. The purpose of this study was to identify genetic variants in stress-related genes such as corticotropin-releasing hormone (CRH), receptor 1, receptor 2 and binding protein (CRH-BP) that associate with BV. Also gene–environment effects with smoking are determined. BV was quantified using the Nugent score in 82 white and 65 black women in the first trimester of pregnancy. Associations between Nugent score, genotype and smoking were analyzed using Kruskal–Wallis and Wilcoxon rank sum non-parametric tests. In white women, non-smokers with the CT genotype at CRH-BP + 17487 have lower Nugent scores (median: 0, range: 0–0) than non-smokers with the TT genotype (median: 2, range: 0–8) (P = 0.002); whereas smokers with the CT genotype have higher Nugent scores (median: 6, range: 0–10) than smokers with the TT genotype (median: 1, range: 0–10) (P = 0.021). In black women, the AG genotype at CRH + 3362 or CRH − 1667 is associated with lower Nugent scores (median for both: 3, range: 0–10) compared with the homozygous genotypes (median for each homozygous genotype: 8, range: 0–10). Also, in black women, models remain significant after adjusting for smoking (P = 0.04 for both). These data indicate that susceptibility to BV is affected by patterns of genetic variation in stress-related genes and smoking plays an important role.
Keywords: bacterial vaginosis, corticotropin-releasing hormone, smoking, genetic association
Introduction
Bacterial vaginosis (BV) is one of the most common types of lower genital tract disorders in adult women (Hay et al., 1994; Hillier et al., 1995; Llahi-Camp et al., 1996; Culhane et al., 2001). BV is defined by a decrease in lactobacilli and an overgrowth of anaerobic bacteria such as Gardnerella vaginalis, Mobiluncus and Prevotella species in the vaginal flora (Koumans and Kendrick, 2001). Approximately 10% of women with BV experience adverse pregnancy outcomes such as spontaneous pre-term delivery, premature rupture of membranes and amniotic fluid infection (Silver et al., 1989; Martius and Eschenbach, 1990; Kurki et al., 1992).
Women of low socioeconomic status who are young and unmarried are more likely to develop BV during pregnancy (Meis et al., 1995; Goldenberg et al., 1996). BV is also twice as common in black women as in white women (Ness et al., 2003). This disparity remains after controlling for known risk factors such as income, education, history of sexually transmitted diseases and feminine hygiene practices (Ness et al., 2003). One possible explanation for this disparity is that black women are exposed to significantly more physiological and psychological stressors than white women and this high level of chronic stress leads to an increased risk for BV (Culhane et al., 2001, 2002, 2006; Nansel et al., 2006). One study found that, after adjusting for maternal age, race, education, parity, douching, number of sexual partners, sexual practices and use of illicit drugs, women with high psychological stress were 2.2 times more likely to develop BV (Culhane et al., 2001).
Corticotropin-releasing hormone (CRH) is an important factor in the response to stress and in the regulation of inflammation and immune responses. During pregnancy it is primarily produced in the decidua, fetal membranes and placenta (Petraglia et al., 1987; Sasaki et al., 1987). CRH plays a critical role in regulating the interaction between stress hormones and inflammatory mediators on the hypothalamic–pituitary–adrenal (HPA) axis (Baerwald et al., 1999). CRH operates by activating two G-protein-coupled receptors: corticotropin-releasing hormone receptor 1 (CRH-R1) and corticotropin-releasing hormone receptor 2 (CRH-R2) (Challis et al., 2000). During pregnancy, CRH-R1 is primarily expressed in the myometrium and fetal membranes, whereas CRH-R2 is mainly expressed in the fetal membranes (Grammatopoulos et al., 1995; Karteris et al., 1998). CRH and CRH-R1 act together to increase the levels of cAMP and promote uterine relaxation (Grammatopoulos and Hillhouse, 1999). Corticotropin-releasing hormone binding protein (CRH-BP) is primarily produced by the liver and placenta during pregnancy and inhibits the ability of CRH to stimulate prostaglandin production (Potter et al., 1991; Challis et al., 1995, 2000).
Smoking may modify or confound the relation of stress and BV. Basal HPA neuroendocrine hormone concentrations and the physiological reaction to stress can be altered by continual exposure to nicotine which is a major component of cigarettes (Rohleder and Kirschbaum, 2006). Additionally, the response to psychological stress is diminished in smokers (Kirschbaum et al., 1993a, b).
Genetic associations with BV have been investigated mainly in genes related to immune response, specifically cytokine genes (Genc et al., 2004; Diaz-Cueto et al., 2005; Cauci et al., 2007). CRH and its related genes have not been examined for associations with BV even though stress is a strong independent risk factor for BV. Therefore, we assessed genetic variation in CRH, CRH-R1, CRH-R2 and CRH-BP for association with BV in white and black pregnant women. We also examined associations with BV for the interaction between genotype and smoking.
Materials and Methods
This is a cross-sectional study nested within an observational longitudinal cohort of women seeking prenatal care at the Magee-Women's Hospital prenatal clinic between April 2005 and June 2006. This study was approved by the Institutional Review Boards of the University of Pittsburgh and Vanderbilt University. Inclusion criteria for the cohort were singleton intrauterine gestation prior to 13 weeks. Median gestation at enrollment was 7 weeks (range 6–13 weeks). Exclusion criteria included vaginal bleeding, fetal anomalies, known thrombophilias, pre-gestational diabetes mellitus, chronic hypertension requiring medication, current or planned cervical cerclage, immune compromise (HIV positive, use of systemic steroids within 6 months and use of post-transplant immunosuppressive medication) and autoimmune disease (inflammatory bowel disease, systemic lupus erythematosus, rheumatoid arthritis and scleroderma). These exclusions were developed prior to study enrollment because they could confound the associations we proposed to examine. After provision of informed consent, all women provided demographic, medical, environmental exposures and clinical information through standardized, closed-question, interviews administered by research personnel. Women were instructed to abstain from vaginal intercourse for 48 h prior to the study pelvic exams in order to prevent vaginal pH and other measures from being compromised. Race was self-reported as white for women of Caucasian ancestry and black for women of African-American ancestry.
Women had cervical swabs obtained for Neiseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis and vaginal swabs were obtained for the diagnosis of BV. A diagnosis of BV was determined by vaginal pH ≥ 4.7 and a Nugent score of 7 through 10 from a Gram-stained vaginal smear of various types of bacteria (Nugent et al., 1991). Women were excluded if they were positive for N. gonorrhoeae, C. trachomatis or T. vaginalis (11.5%), had no gram stain score (0.5%), were not self-identified white or black (13.4%) or were under 18 (1.0%). A total of 82 white women and 65 black women were included in this study. The research microbiology laboratory used in this study is the laboratory of Dr Sharon Hillier. This laboratory has served as a reference laboratory for the identification of BV for many large US and international clinical trials. In this laboratory, Gram-stained vaginal smears have excellent test characteristics. In a blinded, quality control assessment, five trained microbiology technicians achieved a high degree of agreement (Cohen’s κ: 0.94) in the detection of BV.
For this analysis, we examined 25 single nucleotide polymorphisms (SNPs) in 4 genes: CRH (6 SNPs), CRH-R1 (2 SNPs), CRH-R2 (12 SNPs) and CRH-BP (5 SNPs). These samples and the SNPs were part of a larger study on genetic contributors to pre-term birth. TagSNPs were selected in whites and blacks separately and were chosen based on reported minor allele frequencies and relative distance to one another within each gene. Genotyping was performed using the Illumina platform (Illumina, San Diego, CA, USA).
Allelic and genotypic distributions were compared between ethnic groups using Fisher’s exact test. Hardy–Weinberg equilibrium (HWE) was calculated for each marker using Fisher’s exact test. The above were performed in Powermarker (Liu and Muse, 2005). Demographic characteristics were compared between ethnic groups with either a Wilcoxon rank sum test or Fisher’s exact test using Stata (Stata Version 9, Stata Corp., College Station, TX, USA). These results were not corrected for multiple testing.
The Kruskal–Wallis test was used to determine if median Nugent scores differ by genotype in whites and blacks, separately. Non-parametric tests were performed because the Nugent score is an ordinal variable ranging from 0 to 10 at intervals of 1. Additionally, transformation with the natural log does not normalize the distribution (Shapiro Wilk P-value < 1 × 10−5). Additive, dominant and recessive models were analyzed for all SNPs. Dominant and recessive models were defined by the minor allele. For example, if the minor allele (allele frequency < 50%) is T then the dominant model is defined as TT + CT compared with CC. The recessive model would then be CC + CT compared with TT. Analyses were not performed if there were less than five observations in any cell. The Kruskal–Wallis test was also used to determine if Nugent score differed by genotype and smoking status. Therefore, for additive models, there were six strata (smokers with three genotypic classes and non-smokers with three genotypic classes) and for dominant and recessive models there were four strata (smokers with two genotypic classes and non-smokers with two genotypic classes). Smokers were defined as women who reported any smoking within the 3 months prior to pregnancy. If the Kruskal–Wallis test was positive, Wilcoxon rank sum tests were performed to identify which groups determined the significance. To correct for multiple testing, false discovery rate (FDR) was used (Benjamini and Hochberg, 1995). A total of 186 Kruskal–Wallis and Wilcoxon rank sum tests were performed and an FDR of 0.2 was used to determine the threshold value. Due to the large number of tests performed, only results that are significant after correction for multiple testing will be discussed.
To further evaluate our results, a case–control analysis was performed. There were 22 white women and 34 black women with BV and 52 white women and 23 black women without BV. A total of 16 women (8 white and 8 black) were excluded because of an intermediate BV score (Nugent score of 4–6). Allelic and genotypic distributions were compared between cases and controls, and dominant and recessive models were also assessed. Fisher’s exact tests were performed in all instances. For the significant results (α< 0.05), logistic regression was performed with and without smoking as a covariate. Likelihood ratio tests were used to determine if smoking was an important factor in the model. Due to small sample sizes, interactions between smoking and genotype could not be tested with logistic regression. These tests were not corrected for multiple testing.
Pair-wise linkage disequilibrium using r2 was assessed using HaploView statistical software (Barrett et al., 2005). Haplotype blocks were defined by an algorithm developed by Gabriel et al. (2002). Dark gray shading indicates strong evidence of LD, light gray indicates uninformative LD structure and white indicates low confidence of LD. Haplotype analysis was done for each gene that demonstrated significance in the single locus analysis or the analysis adjusted for smoking. A sliding window was used to capture two, three or four locus effects. Haplotype frequencies were determined with HaploStats (Schaid et al., 2002). Score tests were used to test associations between haplotypes and BV score as an ordinal variable (Schaid et al., 2002). Haplotype effects were adjusted for smoking (Harrell, 2001).
Results
Demographic characteristics for the study population are presented in Supplementary Table S1. White women are more likely to smoke before or during pregnancy and less likely to be unmarried, been previously pregnant or have BV than black women. Significant differences in allelic and genotypic distributions and different patterns of linkage disequilibrium are observed between white and black women, with whites having more linkage disequilibrium as expected (Supplementary Table S2, Figures S1 and S2). All markers are in HWE with the exception of CRH-R2 + 12421 (P = 0.01) in whites and CRH-R2 + 8288 in blacks (P = 0.04).
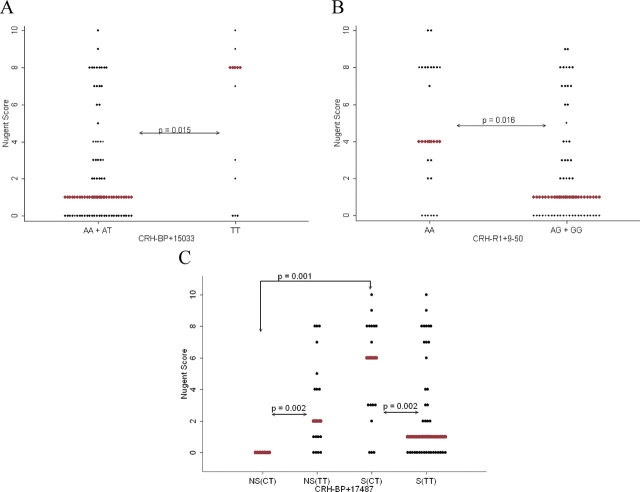
After correction for multiple testing, there are three SNPs (CRH-BP + 15033, CRH-R1 + 9-50 and CRH-R2 + 5253) in white women associated with Nugent score regardless of smoking; however, only two of these (CRH-BP + 15033 and CRH-R1 + 9-50) were significant in the case–control analysis (Table IA, Supplementary Tables S3 and S4). Individuals with the TT genotype (median Nugent score: 8, range: 0–10, number of individuals (n) = 13) at CRH-BP + 15033 have significantly higher Nugent scores (P = 0.02) and increased risk of BV (OR = 5.4, P = 0.009) compared with those with the AA or AT genotypes (median Nugent score: 1, range: 0–10, n = 69) (Table IA and Fig. 1A). Women with the AA genotype (median Nugent score: 4, range: 0–10, n = 23) at CRH-R1 + 9-50 also have significantly higher Nugent scores (P = 0.02) and increased risk for BV (OR = 4.0, P = 0.01) compared with those with the AG or GG genotypes (median Nugent score: 1, range: 0–9, n = 57) (Table IA and Fig. 1B). Smoking was not significantly associated with BV status in these models (CRH-BP + 15033: P = 0.34 and CRH-R1 + 9-50: P = 0.22). Additionally, the likelihood ratio tests indicate that these models are not affected by smoking status. However, under the dominant model associations with CRH-BP + 15033 are marginally affected by smoking (P = 0.03) (Table IIA).
Table I.
Nugent score and single locus case control results in (A) white women and (B) black women
| Gene | Location | Nugent P-value | Modela | Case–control OR (95% CI), P-value |
|
|---|---|---|---|---|---|
| Unadjusted for smoking | Adjusted for smoking | ||||
| (A) White women | |||||
| CRH-BP | 15033 | 0.02 | TT versus AA/AT | 5.4b (1.5–19.1), <0.01 | 5.0 (1.4–18.0), 0.01 |
| CRH-R1 | 9-50 | 0.02 | AA versus AG/GG | 4.0b (1.4–11.9), 0.01 | 3.9 (1.3–11.7), 0.02 |
| (B) Black women | |||||
| CRH | 3362 | 5 × 10−3 | AG versus AA | 0.2b (0.03–0.68), 0.01 | 0.2b (0.03–0.94), 0.04 |
| CRH | −1667 | 5 × 10−3 | CT versus CC | 0.2b (0.03–0.68), 0.01 | 0.2b (0.03–0.94), 0.04 |
aSecond genotype given indicates the referent group.
bIndicates that likelihood ratio test was significant.
Figure 1.
Genetic associations with Nugent score in white women for (A) CRH-BP + 15033, (B) CRH-R1 + 9-50 and (C) CRH-BP + 17487. Red diamonds indicate median.
Table II.
Nugent score comparisons between genotypes for non-smokers (NS) and smokers (S) and between smoking status for each genotype in white women (A) and black women (B)
| Gene | Location | Model (genotype 1 versus 2) | Genotype 1 versus 2 |
Non-smokers versus smokers |
||
|---|---|---|---|---|---|---|
| NS | S | Genotype 1 | Genotype 2 | |||
| (A) White women | ||||||
| CRH-BP | 15033 | AA versus AT/TT | 0.08 | 0.03a | 0.18 | 0.02a |
| CRH-BP | 17487 | CT versus TT | 2 × 10−3a | 0.02a | 1 × 10−3a | 0.42 |
| (B) Black women | ||||||
| CRH | 3362 | AA/AG versus GG | 0.02a | 0.93 | 3 × 10−3a | 0.91 |
| CRH | −1667 | CC/CT versus TT | 0.02a | 0.93 | 3 × 10−3a | 0.91 |
| CRH | −2468 | CC versus CT/TT | 0.04 | 0.54 | 0.15 | 9 × 10−3a |
| CRH-R2 | 8288 | AA/AG versus GG | 0.26 | 0.25 | 0.01a | 0.178 |
| CRH-R2 | 5253 | CC versus CT/TT | 0.41 | 0.17 | 8 × 10−3a | 0.36 |
| CRH-R2 | 4853 | AA/AG versus GG | 0.28 | 0.06 | 0.11 | 0.02a |
aIndicates result is significant after correction for multiple testing.
Non-smokers with the CT genotype (median Nugent score: 0, range: 0–0, n = 7) at CRH-BP + 17487 have significantly lower Nugent scores compared with those with the TT genotype (median Nugent score: 2, range: 0–8, n = 18) (P = 0.002) (Table IIA, Fig. 1C and Supplementary Table S5A). However, in smokers, the opposite is true; those with the CT genotype (median Nugent score: 6, range: 0–10, n = 17) have significantly higher Nugent scores than those with the TT genotype (median Nugent score: 1, range: 0–10, n = 40) (P = 0.02). Also, in women with the CT genotype, non-smokers have significantly lower Nugent scores compared with smokers (P = 0.001).
There are four haplotypes in CRH-BP associated with Nugent score (Table III, Supplementary Table S6A). The most significant of these is a two locus haplotype including CRH-BP + 2012 and CRH-BP + 15033 (P = 0.007). The A/T haplotype is significantly associated with Nugent score (P = 0.002). When smoking is added to the model, the only haplotype effect that is present is at +2012/+15033 (P = 0.01) (Table IV, Supplementary Table S7A). Again the A/T haplotype is significantly associated with Nugent score (P = 0.002).
Table III.
Frequencies and P-values for significant haplotypes in CRH-BP in whites
| Haplotype frequency | Permuted P-value | Global permuted P-value | |||
|---|---|---|---|---|---|
| 2012 | 15033 | 7 × 10−3 | |||
| A | A | 0.59 | 0.03 | ||
| G | T | 0.12 | 0.44 | ||
| A | T | 0.29 | 2 × 10−3 | ||
| 15033 | 16022 | 0.02 | |||
| A | A | 0.59 | 0.03 | ||
| T | G | 0.39 | 0.13 | ||
| 2012 | 15033 | 16022 | 0.01 | ||
| A | A | A | 0.59 | 0.03 | |
| G | T | G | 0.12 | 0.43 | |
| A | T | G | 0.27 | 0.02 | |
| 15033 | 16022 | 17487 | 0.05 | ||
| A | A | T | 0.59 | 0.04 | |
| T | G | C | 0.15 | 0.57 | |
| T | G | T | 0.24 | 0.17 |
Table IV.
Frequencies and P-values for significant haplotypes in CRH-BP in whites adjusted for smoking
| Haplotype frequency | Permuted P-value | Global permuted P-value | ||
|---|---|---|---|---|
| 2012 | 15033 | 0.01 | ||
| A | A | 0.59 | 0.05 | |
| G | T | 0.12 | 0.34 | |
| A | T | 0.29 | 2 × 10−3 |
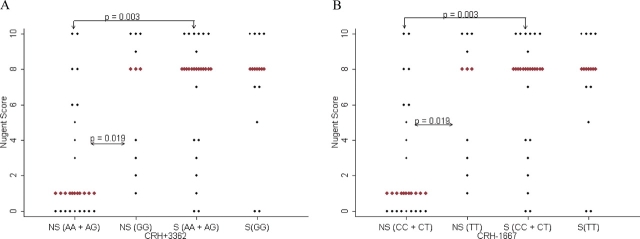
In black women, there are two SNPs in CRH (+3362 and −1667) associated with Nugent score and an increased risk of BV (Table IB, Supplementary Tables S3 and S4). Individuals with the AG genotype at CRH + 3362 or the CT genotype at CRH − 1667 (median Nugent score for either: 3, range: 0–10, n = 28) have significantly lower Nugent scores (P = 0.005) and lower risk of BV (OR = 0.2, P = 0.01) compared with the homozygous genotypes (median Nugent score for any of the homozygous genotypes: 8, range: 0–10, n = 15 for AA or CC and 21 for GG or TT) (Table IB). Smoking did not affect the significance of these results; however, smoking status was significantly associated with BV in these models (OR = 5.7, 95% CI = 1.6–20.4, P = 0.008) and the likelihood ratio test indicates that smoking is an important factor in these models. Non-smokers with the AA or AG genotype (median Nugent score: 1, range: 0–10, n = 21) at CRH + 3362 have significantly lower Nugent scores than those with the GG genotype (median Nugent score: 8, range: 0–10, n = 9) (P = 0.02) (Table IIB, Fig. 2A and Supplementary Table S5B). Additionally, non-smokers with the AA or AG genotype have significantly lower median Nugent scores compared with smokers with the same genotype (median Nugent score: 8, range: 0–10, n = 22) (P = 0.003). Similar results are observed at CRH − 1667 (P = 0.02 and P = 0.003) (Table IIB and Fig. 2B). Additionally, there are significant differences in Nugent score between smokers and non-smokers depending on the genotype at CRH − 2468, CRH-R2 + 8288, CRH-R2 + 5253 and CRH-R2 + 4853; this indicates a possible interactive effect between smoking and genotype (Table IIB). There are no haplotypes significantly associated with Nugent score adjusted or unadjusted for smoking (Supplementary Tables S6B and S7B).
Figure 2.
Genetic associations with Nugent score in black women for (A) CRH + 3362 and (B) CRH − 1667. Red diamonds indicate median.
Discussion
In the present study, we explored the impact of genetic variation in CRH and related genes on Nugent score and determined if smoking affects these associations. In white women, there are two SNPs in CRH-BP associated with Nugent score. CRH-BP + 17487 is located in the 3’UTR; whereas CRH-BP + 15033 is located in an intron. There is significant linkage disequilibrium between these two SNPs, and the haplotype effect, including these two SNPs, is marginally significant, suggesting that these SNPs are not acting independently or are tagging another variant. It is important to note that CRH-BP is known to inhibit the effects of CRH on increased prostaglandin production. In a recent study of white mothers, CRH-BP + 15033 was found to associate with pre-term birth (allele P-value: 5.9 × 10−4) (Velez et al., 2008). The pre-term birth risk genotype was AT/TT with an OR of 2.0 (95% CI: 1.3–3.1, P < 0.01). In our study, the risk genotype for BV was TT with an odds ratio of 5.4 (95% CI: 1.5–19.1, P < 0.01). The fact that the TT genotype is associated with an increased risk of both disorders suggests a pleiotropic effect for this gene and may explain part of the correlation between BV and pre-term birth. Further studies on this gene will need to be performed to elucidate the biological mechanism relating this polymorphism to these reproductive disorders.
In black women, one 3′UTR SNP and two promoter SNPs in CRH are associated with Nugent score. There is significant linkage disequilibrium between these SNPs (Supplementary Figure S1B) but there are not any significant haplotype effects, indicating that the effects of these SNPs are independent or these SNPs interact to confer risk for BV in a way that is not being captured by the haplotype analysis. Additionally, CRH is an important factor in the regulation of inflammation and immune responses. Although BV is not characterized by the presence of inflammation; recent studies demonstrate that in white women cervical pro-inflammatory cytokines (chemokine (C-X-C motif) ligand 10 or IP10 and monocyte chemotactic protein (MCP1)) are lower in those with BV, but in black women cervical pro-inflammatory cytokines (Interleukin IL-1α) are higher in women with BV (Ryckman et al., 2008). It is possible that these SNPs regulate an increased inflammatory response in black women but not white women. However, further studies relating CRH genotypes to protein concentrations are needed.
Black women are at a much greater risk for developing BV than white women. One explanation for this disparity involves differences in the functioning of the HPA axis. Several studies have shown that, in pregnancy, CRH concentrations are lower in black women compared with white women (Holzman et al., 2001; Glynn et al., 2007). Studies exploring only promoter polymorphisms in the CRH gene found marked differences between white subjects from the UK and African subjects (Baerwald et al., 1999; Baerwald et al., 2000). Another study comparing whites and blacks found that SNPs in CRH were significantly different in frequency in these two populations (Shimmin et al., 2007). Of the 25 polymorphisms studied in CRH, CRH-R1, CRH-R2 and CRH-BP, 18 of these have significant (<0.05) allelic or genotypic differences between ethnic groups; 14 of which have a P-value <0.001 for either genotypic or allelic differences (Supplementary Table S2). In our study, there were more significant associations with Nugent score and higher allele frequencies of the associating allele in blacks compared with whites. This indicates that these SNPs may be involved in the racial disparity observed in the prevalence of BV.
For example, all of the polymorphisms in CRH have significantly different allelic and genotypic distributions between white and black women. In two of the four SNPs that associated with BV in whites, the protective allele is more common in whites than blacks (CRH-BP + 15033 and CRH-R1 + 50689); while allele frequencies for the other two (CRH + 16022 and CRH-R1 + 9-50) did not significantly differ between whites and blacks. Although not definitive, these results are consistent with the risk genotypes/alleles being more common in blacks than whites, supporting the argument for a genetic contribution to the disparity. Additionally, while not being directly tested, this may indicate that these SNPs could be contributing to the differences in CRH concentration observed between white and black women.
Our study is limited in that we do not connect measures of psychological or physiological stress to genotype. In order to assess this genotype in either BV or pre-term birth PTB, it will be necessary to also have data on CRH and CRH-BP proteins. These studies are planned as they will be important in elucidating the functional role of the genetic variation on physiology.
In this study, we demonstrated that variation in stress-related genes is associated with BV and that these variants and association patterns differ by race. This supports the idea that physiological stress may explain some of the racial disparity observed in BV in pregnant women. Polymorphisms in CRH-BP are associated with BV in white women and polymorphisms in CRH and CRH-R2 are associated with BV in black women. We have also demonstrated that smoking affects genetic susceptibility for BV.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Funding
Supported by NICHD 1 R01 HD41 663-01A1, 1 R01 HD052732-01 and MO1-RR000056.
References
- Baerwald CG, Mok CC, Fife MS, Tikly M, Lau CS, Wordsworth BP, Ollier B, Panayi GS, Lanchbury JS. Distribution of corticotropin-releasing hormone promoter polymorphism in different ethnic groups: evidence for natural selection in human populations. Immunogenetics. 1999;49:894–899. doi: 10.1007/s002510050570. [DOI] [PubMed] [Google Scholar]
- Baerwald CG, Mok CC, Tickly M, Lau CS, Wordsworth BP, Ollier B, Panayi GS, Lanchbury JS. Corticotropin releasing hormone (CRH) promoter polymorphisms in various ethnic groups of patients with rheumatoid arthritis. Z Rheumatol. 2000;59:29–34. doi: 10.1007/s003930050002. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57:289–300. [Google Scholar]
- Cauci S, Di SM, Casabellata G, Ryckman K, Williams SM, Guaschino S. Association of interleukin-1β and interleukin-1 receptor antagonist polymorphisms with bacterial vaginosis in non-pregnant Italian women. Mol Hum Reprod. 2007;13:243–250. doi: 10.1093/molehr/gam002. [DOI] [PubMed] [Google Scholar]
- Challis JR, Matthews SG, Van MC, Ramirez MM. Current topic: the placental corticotrophin-releasing hormone-adrenocorticotrophin axis. Placenta. 1995;16:481–502. doi: 10.1016/s0143-4004(05)80001-3. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Culhane JF, Rauh V, McCollum KF, Hogan VK, Agnew K, Wadhwa PD. Maternal stress is associated with bacterial vaginosis in human pregnancy. Matern Child Health J. 2001;5:127–134. doi: 10.1023/a:1011305300690. [DOI] [PubMed] [Google Scholar]
- Culhane JF, Rauh V, McCollum KF, Elo IT, Hogan V. Exposure to chronic stress and ethnic differences in rates of bacterial vaginosis among pregnant women. Am J Obstet Gynecol. 2002;187:1272–1276. doi: 10.1067/mob.2002.127311. [DOI] [PubMed] [Google Scholar]
- Culhane JF, Rauh VA, Goldenberg RL. Stress, bacterial vaginosis, and the role of immune processes. Curr Infect Dis Rep. 2006;8:459–464. doi: 10.1007/s11908-006-0020-x. [DOI] [PubMed] [Google Scholar]
- Diaz-Cueto L, Cuica-Flores A, Ziga-Cordero F, rechavaleta-Velasco ME, rechavaleta-Velasco F. Genetic variation in the interleukin-8 gene promoter and vaginal concentrations of interleukin-8 are not associated with bacterial vaginosis during pregnancy. J Reprod Immunol. 2005;66:151–160. doi: 10.1016/j.jri.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Genc MR, Vardhana S, Delaney ML, Onderdonk A, Tuomala R, Norwitz E, Witkin SS. Relationship between a toll-like receptor-4 gene polymorphism, bacterial vaginosis-related flora and vaginal cytokine responses in pregnant women. Eur J Obstet Gynecol Reprod Biol. 2004;116:152–156. doi: 10.1016/j.ejogrb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Chicz-DeMet A, Hobel CJ, Sandman CA. Ethnic differences in adrenocorticotropic hormone, cortisol and corticotropin-releasing hormone during pregnancy. Peptides. 2007;28:1155–1161. doi: 10.1016/j.peptides.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Klebanoff MA, Nugent R, Krohn MA, Hillier S, Andrews WW. Bacterial colonization of the vagina during pregnancy in four ethnic groups. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1996;174:1618–1621. doi: 10.1016/s0002-9378(96)70617-8. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos D, Thompson S, Hillhouse EW. The human myometrium expresses multiple isoforms of the corticotropin-releasing hormone receptor. J Clin Endocrinol Metab. 1995;80:2388–2393. doi: 10.1210/jcem.80.8.7629235. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Hillhouse EW. Role of corticotropin-releasing hormone in onset of labour. Lancet. 1999;354:1546–1549. doi: 10.1016/S0140-6736(99)03418-2. [DOI] [PubMed] [Google Scholar]
- Harrell FE. Regression Modeling Strategies. NY: Springer-Verlag; 2001. [Google Scholar]
- Hay PE, Morgan DJ, Ison CA, Bhide SA, Romney M, McKenzie P, Pearson J, Lamont RF, Taylor-Robinson D. A longitudinal study of bacterial vaginosis during pregnancy. Br J Obstet Gynaecol. 1994;101:1048–1053. doi: 10.1111/j.1471-0528.1994.tb13580.x. [DOI] [PubMed] [Google Scholar]
- Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG, Rao AV, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol. 2001;97:657–663. doi: 10.1016/s0029-7844(00)01209-6. [DOI] [PubMed] [Google Scholar]
- Karteris E, Grammatopoulos D, Dai Y, Olah KB, Ghobara TB, Easton A, Hillhouse EW. The human placenta and fetal membranes express the corticotropin-releasing hormone receptor 1α (CRH-1α) and the CRH-C variant receptor. J Clin Endocrinol Metab. 1998;83:1376–1379. doi: 10.1210/jcem.83.4.4705. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;a 28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrar J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacol Biochem Behav. 1993;b 44:527–531. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- Koumans EH, Kendrick JS. Preventing adverse sequelae of bacterial vaginosis: a public health program and research agenda. Sex Transm Dis. 2001;28:292–297. doi: 10.1097/00007435-200105000-00011. [DOI] [PubMed] [Google Scholar]
- Kurki T, Sivonen A, Renkonen OV, Savia E, Ylikorkala O. Bacterial vaginosis in early pregnancy and pregnancy outcome. Obstet Gynecol. 1992;80:173–177. [PubMed] [Google Scholar]
- Liu K, Muse S. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21:2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- Llahi-Camp JM, Rai R, Ison C, Regan L, Taylor-Robinson D. Association of bacterial vaginosis with a history of second trimester miscarriage. Hum Reprod. 1996;11:1575–1578. doi: 10.1093/oxfordjournals.humrep.a019440. [DOI] [PubMed] [Google Scholar]
- Martius J, Eschenbach DA. The role of bacterial vaginosis as a cause of amniotic fluid infection, chorioamnionitis and prematurity—a review. Arch Gynecol Obstet. 1990;247:1–13. doi: 10.1007/BF02390649. [DOI] [PubMed] [Google Scholar]
- Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, Johnson F, Iams JD, Thom E, Andrews WW. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1995;173:1231–1235. doi: 10.1016/0002-9378(95)91360-2. [DOI] [PubMed] [Google Scholar]
- Nansel TR, Riggs MA, Yu KF, Andrews WW, Schwebke JR, Klebanoff MA. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol. 2006;194:381–386. doi: 10.1016/j.ajog.2005.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness RB, Hillier S, Richter HE, Soper DE, Stamm C, Bass DC, Sweet RL, Rice P. Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc. 2003;95:201–212. [PMC free article] [PubMed] [Google Scholar]
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia F, Sawchenko PE, Rivier J, Vale W. Evidence for local stimulation of ACTH secretion by corticotropin-releasing factor in human placenta. Nature. 1987;328:717–719. doi: 10.1038/328717a0. [DOI] [PubMed] [Google Scholar]
- Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991;349:423–426. doi: 10.1038/349423a0. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Kirschbaum C. The hypothalamic–pituitary–adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. 2006;59:236–243. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Ryckman KK, Williams SM, Krohn MA, Simhan HN. Racial differences in cervical cytokine concentrations between pregnant women with and without bacterial vaginosis. J Reprod Immunol. 2008;78:166–171. doi: 10.1016/j.jri.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Shinkawa O, Margioris AN, Liotta AS, Sato S, Murakami O, Go M, Shimizu Y, Hanew K, Yoshinaga K. Immunoreactive corticotropin-releasing hormone in human plasma during pregnancy, labor, and delivery. J Clin Endocrinol Metab. 1987;64:224–229. doi: 10.1210/jcem-64-2-224. [DOI] [PubMed] [Google Scholar]
- Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002;70:425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmin LC, Natarajan S, Ibarguen H, Montasser M, Kim DK, Hanis CL, Boerwinkle E, Wadhwa PD, Hixson JE. Corticotropin releasing hormone (CRH) gene variation: comprehensive resequencing for variant and molecular haplotype discovery in monosomic hybrid cell lines. DNA Seq. 2007;18:432–442. doi: 10.1080/10425170701388719. [DOI] [PubMed] [Google Scholar]
- Silver HM, Sperling RS, St Clair PJ, Gibbs RS. Evidence relating bacterial vaginosis to intraamniotic infection. Am J Obstet Gynecol. 1989;161:808–812. doi: 10.1016/0002-9378(89)90406-7. [DOI] [PubMed] [Google Scholar]
- Velez DR, Fortunato SJ, Thorsen P, Lombardi SJ, Williams SM, Menon R. Preterm birth in Caucasians is associated with coagulation and inflammation pathway gene variants. PLoS ONE. 2008;3:e3283. doi: 10.1371/journal.pone.0003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.