Abstract
Background
Pregnancy has been associated with a decreased risk of HIV disease progression in the highly active antiretroviral therapy (HAART) era. The effect of timing of HAART initiation relative to pregnancy on maternal virologic, immunologic and clinical outcomes has not been assessed.
Methods
We conducted a retrospective cohort study from 1997–2005 among 112 pregnant HIV-infected women who started HAART before (N = 12), during (N = 70) or after pregnancy (N = 30).
Results
Women initiating HAART before pregnancy had lower CD4+ nadir and higher baseline HIV-1 RNA. Women initiating HAART after pregnancy were more likely to receive triple-nucleoside reverse transcriptase inhibitors. Multivariable analyses adjusted for baseline CD4+ lymphocytes, baseline HIV-1 RNA, age, race, CD4+ lymphocyte count nadir, history of ADE, prior use of non-HAART ART, type of HAART regimen, prior pregnancies, and date of HAART start. In these models, women initiating HAART during pregnancy had better 6-month HIV-1 RNA and CD4+ changes than those initiating HAART after pregnancy (−0.35 vs. 0.10 log10 copies/mL, P = 0.03 and 183.8 vs. −70.8 cells/mm3, P = 0.03, respectively) but similar to those initiating HAART before pregnancy (−0.32 log10 copies/mL, P = 0.96 and 155.8 cells/mm3, P = 0.81, respectively). There were 3 (25%) AIDS-defining events or deaths in women initiating HAART before pregnancy, 3 (4%) in those initiating HAART during pregnancy, and 5 (17%) in those initiating after pregnancy (P = 0.01). There were no statistical differences in rates of HIV disease progression between groups.
Conclusions
HAART initiation during pregnancy was associated with better immunologic and virologic responses than initiation after pregnancy.
Introduction
Women comprise an increasing proportion of HIV-infected persons, and most of these women are of child-bearing age [1]. Although mother-to-child transmission of HIV has been reduced to <2% of HIV-infected pregnancies due to universal prenatal HIV counseling and testing, antiretroviral therapy, scheduled Cesarean delivery, and avoidance of breastfeeding [2]–[4], challenges remain in improving primary prevention and antiretroviral treatment of HIV-infected women. Moreover, the effects of these interventions on maternal HIV disease progression have not been fully assessed.
There are conflicting data in the literature on the effect of pregnancy on HIV disease progression and survival among HIV-infected women. Studies conducted early in the HIV epidemic reported a possible association between pregnancy and accelerated HIV disease progression [5]–[7], particularly in developing countries [8], [9]. However, studies conducted in the United States and Europe did not find a detrimental effect of pregnancy [10]–[14]. These studies were conducted prior to the era of highly active antiretroviral therapy (HAART) and had significant methodological differences that made it difficult to asses the true effect of pregnancy on HIV disease progression [15].
A study conducted by Tai et al of women from our HIV clinic receiving care in the HAART era, found that pregnancy was associated with a lower risk of HIV disease progression [16]. Although the pregnant women were younger and healthier at HAART initiation than the women who did not become pregnant, pregnant women had lower rates of AIDS-defining illnesses and deaths after controlling for age, baseline CD4+ lymphocytes and HIV-1RNA, and durable virologic suppression. The findings persisted after including a propensity score for pregnancy, and in an analysis that matched pregnant and nonpregnant women according to date of cohort entry, baseline CD4+ lymphocyte count, receipt of HAART, and age at study entry. These results suggested a possible beneficial interaction between pregnancy and HAART in HIV-infected women.
However, to our knowledge, no study has examined the response to HAART among HIV-infected women according to timing of HAART initiation in relation to their pregnancy. We hypothesized that women starting HAART during pregnancy would have improved virologic, immunologic, and clinical responses. If proven clinically significant, improved maternal HIV outcomes would indicate the need to conduct similar studies in resource-limited settings. We therefore conducted a retrospective cohort study to evaluate changes in HIV-1 RNA and CD4+ lymphocytes after starting HAART before, during, or after pregnancy, and subsequent HIV disease progression while in care.
Materials and Methods
Study cohort
The study cohort was defined as HIV-1-infected women with at least one pregnancy while receiving care (>1 visit) between 1 January 1997 and 31 December 2005 at the Comprehensive Care Center (CCC) in Nashville, Tennessee. Only women who started their first HAART regimen and had HIV-1 RNA and CD4+ lymphocyte measurements <180 days prior to HAART initiation were included in this study. This cohort was drawn from part of a previously described cohort [16].
Clinical data were entered into an electronic medical record by medical providers at the time of the patient encounter, by automated data upload (e.g., laboratory results), or by clinic personnel (e.g., deaths). Laboratory and antiretroviral therapy data (including regimen and start and stop dates) were validated by systematic chart review. The informed consent process was waived in accordance with the Code of Federal Regulations (CFR) 45 CFR 46.116 (d). The study and the waiver of consent were approved by the Vanderbilt Institutional Review Board.
Definition of study exposure
Study patients were classified according to timing of first HAART initiation. Women were classified as initiating HAART before, during, or after first pregnancy while in care if they started first HAART ≥30 days before estimated date of conception (DOC), <30 days before DOC and ≤30 days after pregnancy event (defined as delivery, miscarriage, or pregnancy termination), or >30 days after pregnancy event, respectively. DOC was determined by last menstrual period and/or fetal ultrasound. The 30-day window before date of conception was used due to uncertainty of the estimated date of conception as determined by last menstrual period [17]–[20]. The 30-day window after date of pregnancy event was used because of continued changes in immunologic parameters seen after pregnancy event that might influence immunologic and virologic responses to HAART [21], [22]. Women who started HAART during pregnancy were presumed to start HAART for prevention of mother-to-child HIV transmission (PMTCT) and not for maternal health if they had no previous opportunistic infections, had nadir CD4+ lymphocyte count >350 cells/mm3, and peak HIV RNA level any time prior to HAART initiation while in care <100,000 copies/mL [2].
HAART was defined as regimens of ≥7 days duration that contained two nucleoside reverse-transcriptase inhibitors (NRTI) plus a protease inhibitor (PI), a non-nucleoside reverse-transcriptase inhibitor (NNRTI), or a third NRTI; one NRTI, one PI, plus one NNRTI; 2 PIs plus one NNRTI or one NRTI; or any regimen containing enfuvirtide. Non-HAART antiretroviral therapy (ART) included mono- or dual-NRTI therapy.
Definition of study outcomes
Outcomes of interest were rates of HIV-1 RNA and CD4+ lymphocyte change during the first 180 days of HAART initiation and HIV disease progression, which was defined as time to AIDS-defining event (ADE) or death at any time after HAART initiation. ADEs were based on the 1993 US Centers for Disease Control and Prevention classification criteria [23], excluding CD4+ lymphocytes <200 cells/mm3.
Study variables
Maternal characteristics such as age, race, baseline CD4+ lymphocyte count and percentage, CD4+ lymphocyte count nadir (the lowest CD4+ lymphocyte count in care prior to pregnancy event), baseline HIV-1 RNA, date of HAART start, hepatitis C virus (HCV) serological status, hepatitis B virus (HBV) infection, history of injection drug use (IDU) as an HIV acquisition risk factor, history of ADE, prior use of non-HAART ART, date of HAART start, and date of conception, and data on prior (i.e., prior to the study period) and subsequent (i.e., after the first pregnancy while in care during the study period) pregnancies were assessed.
Study period
Baseline (time 0) was defined as the date of initiation of first HAART. For the analysis of HIV-1 RNA and CD4+ lymphocyte responses to first HAART, the study period ended when one of the following conditions were met: 1) 180 days after first HAART start (to measure early changes in HIV-1 RNA and CD4+ lymphocyte responses); 2) change in pregnancy status; 3) death; 4) 31 December 2005; 5) discontinuation of first HAART regimen; 6) last clinic encounter date if before 31 December 2005. Change in pregnancy status was defined as 30 days before DOC for women initiating HAART before pregnancy and 30 days after pregnancy event for women initiating HAART during pregnancy.
For the time to ADE or death analysis the study period ended with the first ADE or death, last clinic encounter, or 31 December 2005. All events were reviewed and confirmed by study investigators (PFR, GB, SPR).
Statistical analyses
STATA SE (version 9.2; Stata Corporation) was used for all analyses. Continuous variables were compared with the Kruskal-Wallis test. Categorical variables were compared with the Fisher's exact test.
HIV-1 RNA and CD4+ lymphocyte analyses
Linear mixed effects models [24] were used to calculate the rates of HIV-1 RNA and CD4+ lymphocyte change according to timing of first HAART initiation (before, during, or after pregnancy). We fit models which adjusted for baseline CD4+ lymphocytes, baseline HIV-1 RNA, age, race, CD4+ lymphocyte count nadir, history of ADE, prior use of non-HAART ART, type of HAART regimen, prior pregnancies, and date of HAART start. These variables were chosen for inclusion in the analyses because of their clinical significance. From these models, we derived the rates of HIV-1 RNA and CD4+ lymphocyte change from interaction terms between time and HAART initiation category (before, during, or after pregnancy). We fit models that did not assume a linear relationship between CD4+ lymphocytes, HIV-1 RNA and time using restricted cubic splines. There was insufficient evidence to suggest that these nonlinear models improved the fit of the data (P>0.05 for all tests), so we report only the results of the linear models.
We also performed secondary analyses of HIV-1 RNA and CD4+ lymphocyte change including variables for HCV sero-status and history of IDU on the subset of individuals for whom this information was available. We examined the sensitivity of our analyses by assuming that persons with missing HCV serological status data and IDU data all had or did not have these conditions. Due to the concern that differences in HIV-1 RNA detection limits (400 and 50 copies/mL) during the study period could explain the differences in HIV-1 RNA change between the groups, we also performed sensitivity analyses assuming that every HIV-1 RNA value of <400 copies/mL was equal to 399 copies/mL instead of actual number of virions for HIV-1 RNA level between 50 and 399. Additional analyses of HIV-1 RNA and CD4+ lymphocyte changes repeated the above analyses excluding women on triple-NRTI HAART regimens, since such regimens are now not recommended despite their prior widespread use [25]–[27]. Additionally, we repeated the above analyses after grouping subjects based on DOC and date of pregnancy event without the 30-day window periods.
HIV disease progression analyses
Cox proportional hazards models compared rates of HIV disease progression (ADE/death) according to timing of HAART initiation, both unadjusted and adjusted for the propensity of belonging to a particular HAART initiation category (before, during, or after pregnancy). Propensity scores [28] for starting first HAART before or after pregnancy versus during pregnancy were derived from separate logistic regression models including age, race, baseline CD4+ lymphocyte count and HIV-1 RNA level, CD4+ lymphocyte count nadir, use of non-HAART ART, type of HAART regimen, prior pregnancies, and date of conception. We were unable to use history of IDU and history of ADE because these variables perfectly predicted HAART initiation groups.
We performed secondary analyses comparing rates of HIV disease progression using HCV sero-status as an additional covariate on the subset of individuals who had this information available, and sensitivity analyses assuming that persons with missing HCV sero-status data all had or did not have HCV infection. We also repeated analyses excluding women on triple-NRTI HAART regimens. Additionally, we repeated the above analyses by grouping subjects based on DOC and date of pregnancy event without the 30-day window periods.
Results
Patient characteristics
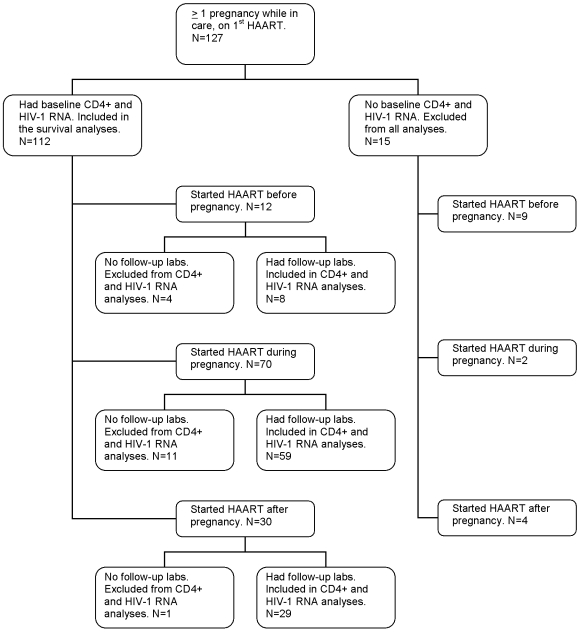
Of the 127 women who were pregnant and initiated their first HAART while followed at the Comprehensive Care Center during the study period, 112 had baseline HIV-1 RNA and CD4+ lymphocytes and were included in the HIV disease progression analysis: 12 women started HAART before pregnancy, 70 women started HAART during pregnancy, and 30 women started HAART after pregnancy. Of the 112, 96 additionally had follow-up labs available during the first 180 days after HAART start and were included in the analysis of HIV-1 RNA and CD4+ lymphocytes following HAART initiation (Figure 1). Fifteen women who did not meet inclusion criteria were similar to the study subjects according to age, HAART duration, and proportion with ADE or death. The 16 (of 112) women without follow-up labs were similar to the study subjects according to age, baseline CD4+ lymphocytes, baseline HIV-1 RNA, HAART duration, and proportion with ADE or death while in care.
Figure 1. Flow chart for patient selection.
HAART: Highly active antiretroviral therapy.
Table 1 shows demographic and clinical characteristics of the 112 patients. The groups were similar according to age and race. Women starting HAART before pregnancy had lower CD4+ lymphocyte count nadir, higher baseline HIV-1 RNA, higher proportion with a history of ADE, started their first HAART at an earlier date, were more likely to receive PI-based HAART, and had a longer study period for ADE and death analyses, than women who started HAART during or after pregnancy. Of those with available data, rates of HCV infection and history of IDU were similar across groups. A higher proportion of women who started HAART after pregnancy received HAART regimens other than PI- or NNRTI-based HAART (all were triple-NRTI-based HAART regimens). They also were more likely to have received non-HAART ART prior to HAART and conceived at an earlier date compared to the other two groups. There were no cases of HBV infection.
Table 1. Demographic and clinical characteristics of the study population (N = 112).
| Characteristic | Started HAART before pregnancy N = 12 | Started HAART during pregnancy* N = 70 | Started HAART after pregnancy N = 30 | P a |
| Age at first HAART start, median (IQR), years | 26.5 (23.5–35) | 25.5 (22–29) | 26 (23–29.5) | 0.42 |
| Black race, no. (%) | 5 (41.7) | 36 (51.4) | 12 (40.0) | 0.56 |
| Baseline CD4+ lymphocyte count, median (IQR), cells/mm3 | 284 (50–472) | 407 (306–494) | 430 (144–515) | 0.27 |
| Baseline CD4+ lymphocyte percentage, median (IQR), percent | 23 (10–34.5) | 27 (21–33) | 26 (14–34) | 0.50 |
| CD4+ lymphocyte count nadir, median (IQR), cells/mm3 | 220 (37–414) | 381 (272–465) | 284 (130–416) | 0.009 |
| Baseline HIV-1 RNA level, median (IQR), log10 copies/mL | 4.97 (4.20–5.17) | 3.89 (3.37–4.49) | 3.70 (2.97–4.58) | 0.006 |
| Prior ADE, no. (%) | 3 (25.0) | 1 (1.4) | 1 (3.3) | 0.01 |
| HCV, no. (%) | 2 (16.7) | 4 (6.0) | 5 (16.7) | 0.14 |
| Missing, no. (%) | 0 | 3 (4.3) | 0 | |
| IDU, no. (%) | 1 (10.0) | 3 (5.6) | 5 (16.7) | 0.24 |
| Missing, no. (%) | 2 (16.7) | 16 (22.9) | 0 | |
| HAART start date, median (IQR), month/year | 07/1999 (08/1998–04/2000) | 12/2001 (06/2000–01/2004) | 04/2001 (08/1999–02/2003) | 0.001 |
| First HAART regimen | 0.007 | |||
| PI-based, no. (%) | 7 (58.3) | 23 (32.9) | 7 (23.3) | |
| NNRTI-based, no. (%) | 3 (25.0) | 31 (44.3) | 6 (20.0) | |
| Other, no. (%) | 2 (16.7) | 16 (22.9) | 17 (56.6) | |
| First HAART duration, median (IQR), months | 24.1 (1.8–40.3) | 6.7 (4.1–32.7) | 11.2 (5.5–26.9) | 0.76 |
| Prior non-HAART ART use, no. (%) | 4 (33.3) | 20 (28.6) | 26 (86.7) | <0.001 |
| Used prior to pregnancy, no. (%) | 4 (33.3) | 20 (28.6) | 10 (33.3) | |
| Used during and/or after pregnancy, no. (%) | – | – | 16 (53.3) | |
| Date of conception, median (IQR), month/year | 05/2001 (07/1999–12/2002) | 07/2001 (01/2000–08/2003) | 08/1998 (10/1997–09/1999) | <0.001 |
| Pregnancy duration, median (IQR), weeks | 37.5 (24–38) | 38 (37–39) | 38 (36–39) | 0.05 |
| Number of pregnancies prior to the study period, median (IQR) | 1 (0–2.5) | 1 (1–2) | 2 (1–3) | 0.26 |
| Number of subsequent pregnancies during study period, median (IQR) | 0 (0–0.5) | 0 | 0 (0–1) | 0.13 |
| Number of provider visits during study period/month, median (IQR) | 0.50 (0.37–0.54) | 0.58 (0.35–0.95) | 0.39 (0.34–0.62) | 0.08 |
| Study period duration for ADE and death analyses, median (IQR), months | 77.5 (60.1–83.1) | 39.8 (17.8–57.4) | 42.1 (15.0–67.0) | 0.001 |
Note: HAART: highly active antiretroviral therapy. IQR: interquartile range. CD4+ lymphocyte count nadir: the lowest CD4+ lymphocyte count while in care. ADE: AIDS-defining event. IDU: history of injection drug use as a risk factor for HIV infection acquisition. HCV: hepatitis C virologic status prior to first HAART initiation. NA: not available. ART: antiretroviral therapy. PI: protease inhibitor. NNRTI: non-nucleoside reverse transcriptase inhibitor.
The reference group.
Continuous data were compared by Kruskal-Wallis test. Categorical data were compared by 2-sided Fisher's exact test.
All women who initiated HAART before pregnancy presented to the Comprehensive Care Center prior to the date of conception, compared to 33% of women who initiated HAART during or after pregnancy (P<0.001). Thirty (43%) women who initiated HAART during pregnancy started HAART for PMTCT. Of the 30 women, 19 (63%) stopped HAART within 90 days after pregnancy event. Median (IQR) time between HAART initiation and date of conception among 12 women who initiated before pregnancy was 526 (364–1199) days. Median (IQR) time between date of HAART initiation and pregnancy event among 70 women who initiated HAART during pregnancy was 137 (109–169) days. Among 30 women who initiated HAART after pregnancy, median (IQR) time between a pregnancy event and HAART initiation was 682 (364–1563) days. Finally, there were no cases of mother-to-child transmission of HIV-1.
Among the 96 women included in the longitudinal analysis of HIV-1 RNA and CD4+ lymphocytes following HAART start, the study period (median (IQR)) was shorter for those initiating HAART during pregnancy (135 (88–164) days) compared to women initiating HAART before (180 (129–180) days) and after pregnancy (180 (167–180) days) (P<0.001). The most common reason for ending the study period was reaching 180 days of follow-up for women who started HAART before and after pregnancy and end of pregnancy for those who started HAART during pregnancy.
Fifty-nine women initiating HAART during pregnancy had more provider visits per month during the study period for HIV-1 RNA and CD4+ lymphocyte analysis (median 1.76 visits) compared to 8 women initiating HAART before (median 0.58) and 29 women initiating HAART after pregnancy (median 0.65) (P<0.001). The median (range) number of HIV-1 RNA measurements was 3 (2–4), 4 (2–9), and 3 (1–6) for those initiating HAART before, during, or after pregnancy, respectively (P<0.001). The median (range) number of CD4+ lymphocyte measurements was 3 (2–3), 4 (2–4), and 2 (2–2) for the three groups (P<0.001).
HIV-1 RNA following first HAART start
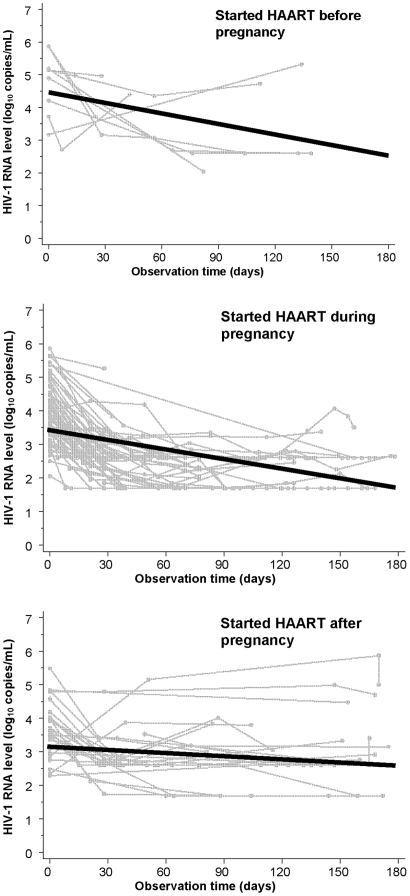
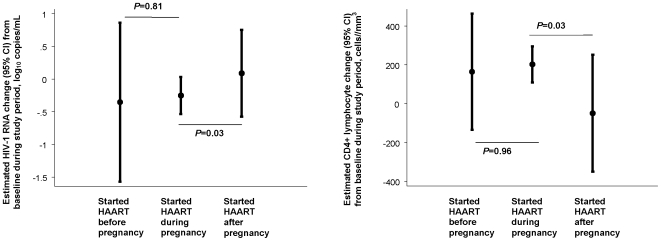
Figure 2 shows the unadjusted rate of HIV-1 RNA decline during the study period for all women in each of the three groups. After adjusting for baseline CD4+ lymphocytes, baseline HIV-1 RNA, age, race, CD4+ lymphocyte count nadir, history of ADE, prior use of non-HAART ART, type of HAART regimen, prior pregnancies, and date of HAART start, estimated HIV-1 RNA decline (95% confidence interval (CI)) over the 6 months after HAART initiation for those initiating during pregnancy was similar to that of women initiating prior to pregnancy (P = 0.96) but greater than that of women initiating after pregnancy (P = 0.03): −0.32 log10 copies/mL (95% CI−1.45, 0.81), −0.35 log10 copies/mL (95% CI −0.57, −0.13), and 0.10 log10 copies/mL (95% CI −0.46, 0.66) for women initiating HAART before, during, or after pregnancy, respectively (Figure 3 and Table 2).
Figure 2. Unadjusted HIV-1 RNA change.
Unadjusted HIV-1 RNA following first HAART initiation for each woman (gray lines) and average decline (solid black line) by timing of HAART initiation.
Figure 3. Estimated rate of HIV-1 RNA and CD4+ lymphocyte change.
The estimated rate of HIV-1 RNA decline and CD4+ lymphocyte increase (small circles) and 95% confidence interval (vertical bars) by pregnancy group over the 6 months following HAART initiation, adjusted for baseline CD4+ lymphocyte count and HIV-1 RNA, age, race, CD4+ lymphocyte count nadir, prior ADE, prior use of non-HAART ART, HAART type, prior pregnancies, and date of HAART start. Horizontal lines represent p-values in a pair-wise comparison (women who started HAART during pregnancy as a reference). Left panel: The estimated rate of HIV-1 RNA decline: −0.32 log10 copies/mL (95% CI −1.45, 0.81) in women who started HAART before pregnancy, −0.35 log10 copies/mL (95% CI −0.57, −0.13) in women who started HAART during pregnancy, and 0.10 log10 copies/mL (95% CI −0.46, 0.66) in women who started HAART after pregnancy. Right panel: The estimated rate of CD4+ lymphocyte increase: estimates were 155.8 cells/mm3 (95% CI −107.6, 419.2) in women who started HAART before pregnancy, 183.8 cells/mm3 (95% CI 110.8, 256.9) in women who started HAART during pregnancy, and −70.8 cells/mm3 (95% CI −326.8, 185.3) in women who started HAART after pregnancy.
Table 2. Multivariable linear mixed effects models: independent predictors of HIV-1 RNA levels (log10 copies/mL) and CD4+ lymphocyte counts (cells/mm3) during 6 months following first HAART initiation* &.
| Independent Variables | HIV-1 RNA level predictors, Effect (95% CI) | P | CD4+ lymphocyte count predictors, Effect (95% CI) | P |
| Change per month, women who started HAART during pregnancy | −0.06 (−0.09, 0.02) | 0.002 | 30.6 (18.6, 42.9) | <0.001 |
| Interaction term, women who started HAART before pregnancy** | 0.03 (−1.03, 1.09) | 0.96 | −28.1 (−258.5, 202.5) | 0.81 |
| Interaction term, women who started HAART after pregnancy** | 0.45 (0.5, 0.85) | 0.03 | −254.5 (−477.0, −32.2) | 0.03 |
| Baseline CD4+ lymphocyte count, per 100 cells/mm3 increase | 0.01 (−0.12, 0.14) | 0.86 | 50.6 (27.0, 74.2) | <0.001 |
| CD4+ lymphocyte count nadir, per 100 cell/mm3 increase | −0.10 (−0.24, 0.05) | 0.25 | 72.5 (46.1, 99.0) | <0.001 |
| Baseline HIV-1 RNA level, per log10 copies/mL increase | 0.24 (0.06, 0.41) | 0.01 | −0.36 (−31.8, 31.08) | 0.98 |
| Age at first HAART start, per year | −0.02 (−0.04, 0.01) | 0.25 | 3.8 (−1.1, 8.8) | 0.13 |
| Black race | 0.18 (−0.09, 0.44) | 0.19 | 27.9 (−19.7, 75.5) | 0.25 |
| Prior ADE (yes/no) | 0.58 (−0.27, 1.43) | 0.18 | −80.9 (−238.7, 76.9) | 0.32 |
| Prior non-HAART ART use (yes/no) | 0.27 (−0.07, 0.60) | 0.16 | 40.8 (−20.0, 101.5) | 0.19 |
| HAART type | 0.01 (−0.16, 0.18) | 0.87 | −5.4 (−36.9, 26.1) | 0.74 |
| Prior pregnancies (yes/no) | −0.27 (−0.59, 0.05) | 0.10 | −46.1 (−104.6, 12.3) | 0.12 |
| Date of HAART initiation, per year | −0.0004 (−0.0006, −0.0002) | <0.001 | −0.004 (−0.04, 0.03) | 0.83 |
Note: 95% CI: 95% confidence interval. HAART: highly active antiretroviral therapy. CD4+ lymphocyte count nadir: the lowest CD4+ lymphocyte count while in care. ADE: AIDS-defining event. Non-HAART ART: non-HAART antiretroviral therapy.
Mixed effect model adjusted for baseline CD4+ lymphocyte count and HIV-1 RNA level, age, race, CD4+ lymphocyte count nadir, prior ADE, prior use of non-HAART ART, HAART type, prior pregnancies, and date of HAART initiation.
The reference group was women who started HAART during pregnancy.
Interaction terms are equal to the difference in slopes of HIV-1 RNA and CD4+ lymphocyte changes between women who started HAART during pregnancy and those who started HAART before or after pregnancy.
In secondary analyses that included persons in whom HCV serological status and history of IDU data were available, the difference in the estimated HIV-1 RNA decline between those initiating HAART during and after pregnancy was no longer statistically significant: −0.25 log10 copies/mL (95% CI −0.53, 0.03) for during and 0.09 log10 copies/mL (95% CI −0.57, 0.75) for after (P = 0.14). In all other secondary analyses described in the Methods, the estimated HIV-1 RNA decline was greater for those initiating HAART during pregnancy than after (P≤0.03 for all analyses, data not shown).
CD4+ lymphocyte count following first HAART start
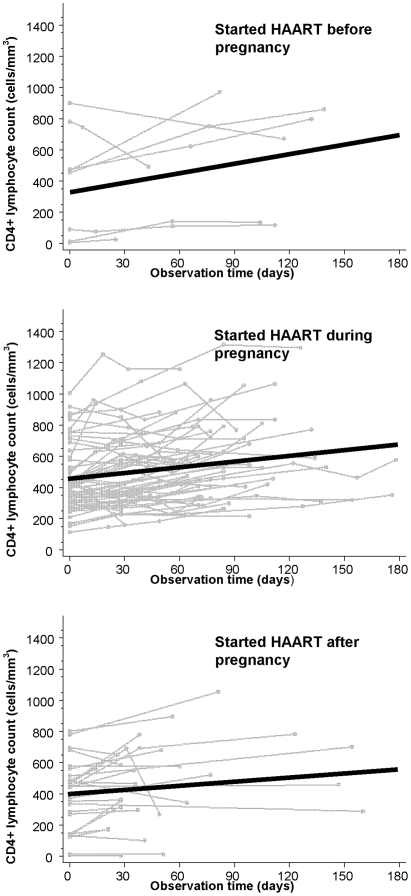
Figure 4 shows the unadjusted rate of CD4+ lymphocyte increase during the study period for all women in each of the three groups. After adjusting for baseline CD4+ lymphocytes, baseline HIV-1 RNA, age, race, CD4+ lymphocyte count nadir, history of ADE, prior use of non-HAART ART, type of HAART regimen, prior pregnancies, and date of HAART start, the estimated rate of CD4+ lymphocyte increase over the 6 months after HAART initiation for women initiating HAART during pregnancy was similar to that of women initiating before pregnancy (P = 0.81) and higher than that women initiating after pregnancy (P = 0.03): 155.8 cells/mm3 (95% CI −107.6, 419.2), 183.8 cells/mm3 (95% CI 110.8, 256.9), and −70.8 cells/mm3 (95% CI −326.8, 185.3) for those initiating HAART before, during, or after pregnancy (Figure 3 and Table 2).
Figure 4. Unadjusted CD4+ lymphocyte count change.
Unadjusted CD4+ lymphocyte count following first HAART initiation for each woman (gray lines) and average increase (solid black line) by timing of HAART initiation.
In all secondary analyses (described in Methods), the estimated CD4+ lymphocyte rise was greater for those initiating HAART during pregnancy than those initiating after pregnancy (P≤0.04 for all analyses, data not shown).
HIV disease progression
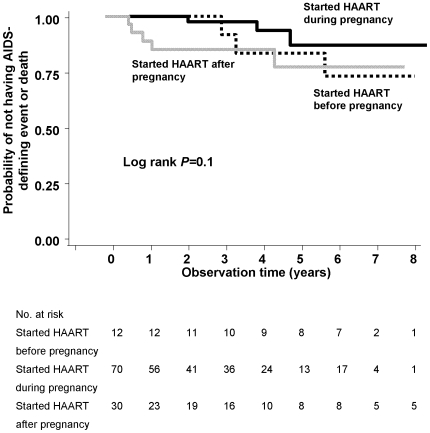
A lower proportion of women progressed to ADE or death among women starting first HAART during pregnancy compared to those starting HAART before or after pregnancy: 3 (25%), 3 (4%), and 5 (17%) for women who started HAART before, during, and after pregnancy, respectively (P = 0.01). When women were grouped based on DOC and date of pregnancy event without the 30-day window, the proportion of ADE/deaths did not change between the groups (P = 0.04). In Kaplan-Meier analysis using the original definition of the groups, the differences in HIV disease progression were not statistically significant (log-rank test, P = 0.10) (Figure 5). In multivariable Cox proportional hazards models that included propensity scores for initiating HAART before or after pregnancy (as described in the Methods), the hazard of ADE or death did not statistically differ between the groups: compared to those initiating HAART during pregnancy, the hazard ratio (HR) was 1.56 (95% CI 0.17, 14.79; P = 0.70) for initiating after pregnancy and 0.14 (95% CI 0.01, 3.09; P = 0.21) for initiating prior to pregnancy.
Figure 5. Kaplan-Meier survival curve of progression to new AIDS-defining event or death by timing of HAART initiation.
The numbers of women at risk each year are also given.
Results were similar in secondary analyses that included HCV sero-status in the propensity scores, analyses that excluded women starting triple-NRTI-based regimens, and analyses which grouped subjects based on DOC and date of pregnancy event without the 30-day window (data not shown).
Discussion
In this study, we compared 6-month changes in HIV-1 RNA and CD4+ lymphocyte and long-term rates of ADE/death in women starting first HAART before, during, or after pregnancy. We found that HIV-1 RNA decline and CD4+ lymphocyte increase were more rapid over the first 6 months among women initiating HAART during pregnancy than after pregnancy. However, there was insufficient evidence to conclude that the rate of disease progression differed according to the timing of HAART initiation relative to pregnancy. In a recent meta-analysis [29], a 0.3 log10 increase in HIV-1 RNA was associated with a 25% increase in progression to ADE. Therefore, the difference in virologic response that we noted could potentially have clinical significance. A study with a larger sample size and longer follow-up is needed to assess for significant difference in clinical outcomes.
The etiology of the improved virologic and immunologic responses associated with HAART initiation during pregnancy is likely multifactorial. The improved response could be due in part to the differences among women in the three patient groups. Women who started HAART after pregnancy were more likely to have been previously exposed to non-HAART ART, to have started triple-NRTI-based HAART regimens, and to have started HAART during an earlier HAART era. These factors are known to lead to poorer responses to HAART [25]–[27], [30]–[32]. Additionally, high proportion of women who started HAART during pregnancy for presumed PMTCT rather than their own health. However, the better virologic and immunologic responses in the pregnant group persisted after adjusting for these factors in multivariable models and after excluding persons on triple-NRTIs. Second, improved health-related behavior during pregnancy, and resumption of unhealthy behaviors postpartum [33], [34] could explain the better virologic and immunologic responses in this group. Pregnancy also has been independently associated with greater adherence to HAART [35]–[37]. Although we were unable to directly assess for adherence, we measured the intensity of clinical care via the number of provider visits per month. Women initiating HAART during pregnancy were seen more frequently during the study period. This in turn provided a better opportunity to provide adherence counseling, which is a part of routine care of pregnant women at the Comprehensive Care Center. Improved adherence in turn could lead to virologic suppression [38], [39], prevention of ADE and death [40], [41] and of mother-to-child transmission [42]. The above factors may explain the poorer virologic and immunologic responses to HAART in women starting HAART after pregnancy. Third, the enhanced virologic and immunologic response among women who started HAART while pregnant could be related to the elevated levels of progesterone and estrogen during pregnancy, which lead to an increase in CD4+ CD25+ regulatory T cells and tolerance to alloantigens such as fetal antigens [21], [43]. Increased levels of regulatory T cells might limit CD8+ lymphocyte effector function that in turn could limit HIV-1 infection-associated immune dysregulation and reduce immune cell exhaustion and programmed cell death [44], [45]. Thus, the effect of pregnancy on regulatory T cells could possibly lead to a better virologic response to HAART among women starting first HAART during pregnancy
Due to the observational nature of this study, we cannot make causal inferences between timing of HAART initiation and HIV-related outcomes. First, our study had low power to detect differences in clinical outcomes between pregnancy groups. The number of women who started HAART before pregnancy was particularly low. Larger studies with longer follow-up may show statistically significant differences in HIV disease progression. Second, women have different indications for HAART initiation during pregnancy [2] compared to the indications when women are not pregnant [46]. Although we adjusted for important baseline characteristics that are associated with immune recovery and virologic suppression (such as baseline CD4+ lymphocyte count and HIV-1 RNA, CD4+ lymphocyte count nadir [47], injection drug use [48], [49], and hepatitis C virus co-infection [50]) residual confounding by indication for HAART initiation might still remain. Third, it should be recognized that of women who started HAART prior to becoming pregnant only those who survived were able to get pregnant at a later date and, therefore, were included in our study. Fourth, we were unable to assess and control for HIV-1 resistance that could be associated with prior non-HAART ART exposure and may contribute to poorer responses to HAART [51]–[56].
With the above noted, this study found an improved virologic and immunologic response among women who started HAART during pregnancy compared to women who started HAART after pregnancy. Regardless of the underlying etiology, it is an important finding that women initiating HAART during pregnancy had excellent virologic and immunologic responses; this presumably benefits both the mother and the fetus. Larger studies are warranted to assess for possible differences in subsequent HIV disease progression, particularly in resource-limited settings, and to investigate the factors associated with the improved outcomes during pregnancy, such as adherence or underlying biologic factors.
Acknowledgments
We would like to thank providers and all patients at the Comprehensive Care Center whose participation made these analyses possible. In particular, we would like to thank Beverly Byram, NP. We also would like to thank Drs. Richard D'Aquila and Todd Hulgan for their review of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the National Institutes of Health: the Vanderbilt-Meharry Center for AIDS Research (P30 AI54999) - BES, SES, PFR, GB, TRS; K24 A1065298 - SES, TRS; the NIH National Research Service Award Institutional Research Training Grant (T32 AI07474-10) - VVM; Bristol-Myers Squibb Virology Fellows Research Training Grant - VVM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.United Nations Joint Programme on HIV/AIDS. 2007 AIDS epidemic update. 2008. Available at http://www.unaids.org/en/. Accessed 20 July, 2009.
- 2.Perinatal HIV Guidelines Working Group. Public Health Service Task Force Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. July 8, 2008. pp. 1–98. Available at http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf. Accessed 20 July, 2009.
- 3.Achievements in public health. Reduction in perinatal transmission of HIV infection–United States, 1985–2005. MMWR Morb Mortal Wkly Rep. 2006;55:592–597. [PubMed] [Google Scholar]
- 4.Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Scott GB, Fischl MA, Klimas N, Fletcher MA, Dickinson GM, et al. Mothers of infants with the acquired immunodeficiency syndrome. Evidence for both symptomatic and asymptomatic carriers. JAMA. 1985;253:363–366. [PubMed] [Google Scholar]
- 6.Minkoff H, Nanda D, Menez R, Fikrig S. Pregnancies resulting in infants with acquired immunodeficiency syndrome or AIDS-related complex. Obstet Gynecol. 1987;69:285–287. [PubMed] [Google Scholar]
- 7.Biggar RJ, Pahwa S, Minkoff H, Mendes H, Willoughby A, et al. Immunosuppression in pregnant women infected with human immunodeficiency virus. Am J Obstet Gynecol. 1989;161:1239–1244. doi: 10.1016/0002-9378(89)90674-1. [DOI] [PubMed] [Google Scholar]
- 8.Deschamps MM, Pape JW, Desvarieux M, Williams-Russo P, Madhavan S, et al. A prospective study of HIV-seropositive asymptomatic women of childbearing age in a developing country. J Acquir Immune Defic Syndr. 1993;6:446–451. [PubMed] [Google Scholar]
- 9.Kumar RM, Uduman SA, Khurranna AK. Impact of maternal HIV-1 infection on perinatal outcome. Int J Gynaecol Obstet. 1995;49:137–143. doi: 10.1016/0020-7292(95)02356-h. [DOI] [PubMed] [Google Scholar]
- 10.Hocke C, Morlat P, Chene G, Dequae L, Dabis F. Prospective cohort study of the effect of pregnancy on the progression of human immunodeficiency virus infection. The Groupe d'Epidemiologie Clinique Du SIDA en Aquitaine. Obstet Gynecol. 1995;86:886–891. doi: 10.1016/0029-7844(95)00257-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alliegro MB, Dorrucci M, Phillips AN, Pezzotti P, Boros S, et al. Incidence and consequences of pregnancy in women with known duration of HIV infection. Italian Seroconversion Study Group. Arch Intern Med. 1997;157:2585–2590. [PubMed] [Google Scholar]
- 12.Bessinger R, Clark R, Kissinger P, Rice J, Coughlin S. Pregnancy is not associated with the progression of HIV disease in women attending an HIV outpatient program. Am J Epidemiol. 1998;147:434–440. doi: 10.1093/oxfordjournals.aje.a009468. [DOI] [PubMed] [Google Scholar]
- 13.Weisser M, Rudin C, Battegay M, Pfluger D, Kully C, et al. Does pregnancy influence the course of HIV infection? Evidence from two large Swiss cohort studies. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:404–410. doi: 10.1097/00042560-199804150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Saada M, Le Chenadec J, Berrebi A, Bongain A, Delfraissy JF, et al. Pregnancy and progression to AIDS: results of the French prospective cohorts. SEROGEST and SEROCO Study Groups. AIDS. 2000;14:2355–2360. doi: 10.1097/00002030-200010200-00017. [DOI] [PubMed] [Google Scholar]
- 15.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 1998;105:836–848. doi: 10.1111/j.1471-0528.1998.tb10227.x. [DOI] [PubMed] [Google Scholar]
- 16.Tai JH, Udoji MA, Barkanic G, Byrne DW, Rebeiro PF, et al. Pregnancy and HIV disease progression during the era of highly active antiretroviral therapy. J Infect Dis. 2007;196:1044–1052. doi: 10.1086/520814. [DOI] [PubMed] [Google Scholar]
- 17.Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:E35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 18.Dietz PM, England LJ, Callaghan WM, Pearl M, Wier ML, et al. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California livebirth and prenatal screening records. Paediatr Perinat Epidemiol. 2007;21(Suppl 2):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 19.Savitz DA, Terry JW, Jr, Dole N, Thorp JM, Jr, Siega-Riz AM, et al. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187:1660–1666. doi: 10.1067/mob.2002.127601. [DOI] [PubMed] [Google Scholar]
- 20.Waller DK, Spears WD, Gu Y, Cunningham GC. Assessing number-specific error in the recall of onset of last menstrual period. Paediatr Perinat Epidemiol. 2000;14:263–267. doi: 10.1046/j.1365-3016.2000.00275.x. [DOI] [PubMed] [Google Scholar]
- 21.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Ramon S, Navarro AJ, Aristimuno C, Rodriguez-Mahou M, Bellon JM, et al. Pregnancy-induced expansion of regulatory T-lymphocytes may mediate protection to multiple sclerosis activity. Immunol Lett. 2005;96:195–201. doi: 10.1016/j.imlet.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance care definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 24.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 25.Golub ET, Benning L, Sharma A, Gandhi M, Cohen MH, et al. Patterns, predictors, and consequences of initial regimen type among HIV-infected women receiving highly active antiretroviral therapy. Clin Infect Dis. 2008;46:305–312. doi: 10.1086/524752. [DOI] [PubMed] [Google Scholar]
- 26.Kuritzkes DR, Ribaudo HJ, Squires KE, Koletar SL, Santana J, et al. Plasma HIV-1 RNA dynamics in antiretroviral-naive subjects receiving either triple-nucleoside or efavirenz-containing regimens: ACTG A5166s. J Infect Dis. 2007;195:1169–1176. doi: 10.1086/512619. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Elias MJ, Moreno A, Moreno S, Lopez D, Antela A, et al. Higher virological effectiveness of NNRTI-based antiretroviral regimens containing nevirapine or efavirenz compared to a triple NRTI regimen as initial therapy in HIV-1-infected adults. HIV Clin Trials. 2005;6:312–319. doi: 10.1310/B3NK-V5XQ-6VX9-5DEK. [DOI] [PubMed] [Google Scholar]
- 28.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 29.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008;22:2179–2185. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arrive E, Newell ML, Ekouevi DK, Chaix ML, Thiebaut R, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 31.Chaix ML, Ekouevi DK, Rouet F, Tonwe-Gold B, Viho I, et al. Low risk of nevirapine resistance mutations in the prevention of mother-to-child transmission of HIV-1: Agence Nationale de Recherches sur le SIDA Ditrame Plus, Abidjan, Cote d'Ivoire. J Infect Dis. 2006;193:482–487. doi: 10.1086/499966. [DOI] [PubMed] [Google Scholar]
- 32.Giuliano M, Palmisano L, Galluzzo CM, Amici R, Germinario E, et al. Selection of resistance mutations in pregnant women receiving zidovudine and lamivudine to prevent HIV perinatal transmission. AIDS. 2003;17:1570–1572. doi: 10.1097/00002030-200307040-00022. [DOI] [PubMed] [Google Scholar]
- 33.Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. Am J Public Health. 1990;80:541–544. doi: 10.2105/ajph.80.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ebrahim SH, Floyd RL, Merritt RK, 2nd, Decoufle P, Holtzman D. Trends in pregnancy-related smoking rates in the United States, 1987–1996. JAMA. 2000;283:361–366. doi: 10.1001/jama.283.3.361. [DOI] [PubMed] [Google Scholar]
- 35.Vaz MJ, Barros SM, Palacios R, Senise JF, Lunardi L, et al. HIV-infected pregnant women have greater adherence with antiretroviral drugs than non-pregnant women. Int J STD AIDS. 2007;18:28–32. doi: 10.1258/095646207779949808. [DOI] [PubMed] [Google Scholar]
- 36.Bardeguez AD, Lindsey JC, Shannon M, Tuomala RE, Cohn SE, et al. Adherence to antiretrovirals among US women during and after pregnancy. J Acquir Immune Defic Syndr. 2008;48:408–417. doi: 10.1097/QAI.0b013e31817bbe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mellins CA, Chu C, Malee K, Allison S, Smith R, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care. 2008;20:958–968. doi: 10.1080/09540120701767208. [DOI] [PubMed] [Google Scholar]
- 38.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 39.Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 41.Hogg RS, Heath K, Bangsberg D, Yip B, Press N, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 42.Thea DM, Steketee RW, Pliner V, Bornschlegel K, Brown T, et al. The effect of maternal viral load on the risk of perinatal transmission of HIV-1. New York City Perinatal HIV Transmission Collaborative Study Group. AIDS. 1997;11:437–444. doi: 10.1097/00002030-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 44.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 45.Effros RB. Replicative senescence: the final stage of memory T cell differentiation? Curr HIV Res. 2003;1:153–165. doi: 10.2174/1570162033485348. [DOI] [PubMed] [Google Scholar]
- 46.Panel on Antiretroviral Guidelines for Adult and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. November 3, 2008. pp. 1–139. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 20 July, 2009.
- 47.Kaufmann GR, Bloch M, Zaunders JJ, Smith D, Cooper DA. Long-term immunological response in HIV-1-infected subjects receiving potent antiretroviral therapy. AIDS. 2000;14:959–969. doi: 10.1097/00002030-200005260-00007. [DOI] [PubMed] [Google Scholar]
- 48.Lucas GM GK, Chaisson RE, et al. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 49.Dronda F, Zamora J, Moreno S, Moreno A, Casado JL, et al. CD4 cell recovery during successful antiretroviral therapy in naive HIV-infected patients: the role of intravenous drug use. AIDS. 2004;18:2210–2212. doi: 10.1097/00002030-200411050-00018. [DOI] [PubMed] [Google Scholar]
- 50.Cheng DM, Nunes D, Libman H, Vidaver J, Alperen JK, et al. Impact of hepatitis C on HIV progression in adults with alcohol problems. Alcohol Clin Exp Res. 2007;31:829–836. doi: 10.1111/j.1530-0277.2007.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kakehasi FM, Tupinambas U, Cleto S, Aleixo A, Lin E, et al. Persistence of genotypic resistance to nelfinavir among women exposed to prophylactic antiretroviral therapy during pregnancy. AIDS Res Hum Retroviruses. 2007;23:1515–1520. doi: 10.1089/aid.2007.0025. [DOI] [PubMed] [Google Scholar]
- 52.Eastman PS, Shapiro DE, Coombs RW, Frenkel LM, McSherry GD, et al. Maternal viral genotypic zidovudine resistance and infrequent failure of zidovudine therapy to prevent perinatal transmission of human immunodeficiency virus type 1 in pediatric AIDS Clinical Trials Group Protocol 076. J Infect Dis. 1998;177:557–564. doi: 10.1086/514228. [DOI] [PubMed] [Google Scholar]
- 53.Kully C, Yerly S, Erb P, Kind C, Krautheim A, et al. Codon 215 mutations in human immunodeficiency virus-infected pregnant women. Swiss Collaborative ‘HIV and Pregnancy’ Study. J Infect Dis. 1999;179:705–708. doi: 10.1086/314615. [DOI] [PubMed] [Google Scholar]
- 54.Welles SL, Pitt J, Colgrove R, McIntosh K, Chung PH, et al. HIV-1 genotypic zidovudine drug resistance and the risk of maternal–infant transmission in the women and infants transmission study. The Women and Infants Transmission Study Group. AIDS. 2000;14:263–271. doi: 10.1097/00002030-200002180-00008. [DOI] [PubMed] [Google Scholar]
- 55.Ekpini RA, Nkengasong JN, Sibailly T, Maurice C, Adje C, et al. Changes in plasma HIV-1-RNA viral load and CD4 cell counts, and lack of zidovudine resistance among pregnant women receiving short-course zidovudine. AIDS. 2002;16:625–630. doi: 10.1097/00002030-200203080-00015. [DOI] [PubMed] [Google Scholar]
- 56.Bardeguez AD, Shapiro DE, Mofenson LM, Coombs R, Frenkel LM, et al. Effect of cessation of zidovudine prophylaxis to reduce vertical transmission on maternal HIV disease progression and survival. J Acquir Immune Defic Syndr. 2003;32:170–181. doi: 10.1097/00126334-200302010-00009. [DOI] [PubMed] [Google Scholar]