Abstract
Cerebral volume loss has long been associated with normal aging but whether this is due to aging itself or to age-related diseases including incipient Alzheimer disease (AD) is uncertain. To understand the changes that occur in the aging brain, we examined the cerebral cortex of 27 normal individuals ranging in age from 56 to 103 years. None fulfilled the criteria for the neuropathological diagnosis of AD or other neurodegenerative disease. Seventeen of the elderly participants had cognitive testing an average of 6.7 months prior to death. We used quantitative approaches to analyze cortical thickness, neuronal number, and density. Frontal and temporal neocortical regions had clear evidence of cortical thinning with age but total neuronal numbers in frontal and temporal neocortical regions remained relatively constant over a 50-year age range. These data suggest that loss of neuronal and dendritic architecture, rather than loss of neurons, underlies neocortical volume loss with increasing age in the absence of AD.
Keywords: Aging, Cerebral cortex, Neurons, Stereology
INTRODUCTION
Cerebral volume loss has been observed in neuroradiologic studies as part of the spectrum of changes associated with normal aging, but whether this volume loss represents neuronal loss, incipient or asymptomatic neurodegenerative diseases or other pathological processes is not clear. Both longitudinal and cross-sectional imaging studies have examined volume loss in non-demented older individuals (1–5). The whole brain atrophy rate is −0.45% per year in adulthood (2). Volumetric magnetic resonance imaging (MRI) studies have found that both cortical and white matter volume decrease with age; gray matter volume declined in a linear fashion from early adulthood, whereas white matter volume loss appeared to start later in life and did not follow a linear pattern (3). Other studies have reported similar estimates of volume loss, as well as decreases in hippocampal volume in older adults (1, 4, 6). Some neuroimaging studies indicate that certain cortical regions, particularly the frontal lobes, are more vulnerable to volume loss with aging (4, 7–9). The extent to which these neuroimaging studies are influenced by the presence of asymptomatic AD-like neuropathologic changes or other age-related pathological processes such as vascular disease is uncertain. This is especially important since recent clinical-pathological correlation studies highlight the surprisingly frequent occurrence of substantial AD-related neuropathological changes (including atrophy) in cognitively intact elderly persons (10, 11). An alternative commonly cited hypothesis about brain changes with aging states that neuronal loss becomes prominent in the decades after middle age (12). Several neuropathological studies suggest, however, that the number of neurons in the cerebral cortex remains stable in the normal aging process (13–15). For example, Pakkenberg reported the number of neurons lost from early adulthood to the age of 90 to be less than 10% in a cohort the clinical status of which was unknown (16, 17). The number of glial cells also did not show a significant change (17).
The effect of aging on neuronal number within the human hippocampus has also been examined. Reduction in the number of neurons within the CA1 sector of the hippocampus was found with the development of Alzheimer disease (AD) but did not occur in normal aging (18–20), although some neuronal loss in subiculum and CA4 was noted.
To understand the effects of aging on the human cerebral cortex we examined the cortex of 27 individuals ranging in age from 56 to 103 years, none of whom had the neuropathological findings of AD or other neurodegenerative illness. Seventeen of these patients had had cognitive testing prior to their death. To examine the hypothesis that aging, even if not confounded by neurodegenerative disease, leads to structural changes in the brain, we analyzed changes within the cortical layering, overall thickness, neuronal number, and neuronal size distribution.
MATERIALS AND METHODS
Subjects
Twenty-seven subjects with no clinical or neuropathological evidence of AD and who were part of the Alzheimer Disease Research Center (ADRC) at Massachusetts General Hospital from 1991–2006 were selected. Consent was given for brain donation by next-of-kin in all cases and a postmortem examination was conducted by the ADRC according to standardized protocols. The pathological material and all available clinical history were reviewed on all cases. Seventeen of the subjects were elderly residents at Hebrew Rehabilitation Center, a long-term care facility in Boston, Massachusetts, and had agreed to annual cognitive testing and a brain autopsy. These cognitively tested subjects from the long-term care facility were considered eligible for inclusion if they had no or mild cognitive impairment based on chart review and interviews with nursing and medical staff conducted by a trained research nurse and geriatrician. CERAD based neuropsychological testing was performed on an annual basis on these 17 participants.
Tissue Collection and Diagnosis
At autopsy, coronal slices from one hemisphere were snap-frozen between dry ice-cooled aluminum plates; the opposite hemisphere was fixed in 10% buffered formalin. The fixed hemisphere was cut in the coronal plane and blocks were selected from 17 to 19 brain areas for a comprehensive evaluation of the neurodegenerative process, as previously described (21). The formalin fixed blocks were exposed to 90% formic acid for 1 hour prior to dehydration and paraffin embedding. Eight-μm histologic sections were stained with Luxol fast blue-hematoxylin and eosin (LFB-H&E) stain. Selected blocks were also evaluated with Bielschowsky silver impregnation, Congo red stain or β-amyloid immunostain (Dako, Carpinteria, CA) and anti-NeuN (Chemicon, Temecula, CA) immunostain. Neuropathological diagnoses were made according to established diagnostic criteria (22, 23).
Microscopy and Quantitative Analysis
We applied stereological approaches, emphasizing systematic random sampling of the microscope fields throughout multiple cortical regions, to assess neuronal number, density, and percent of cortex occupied by each cortical lamina using commercial image analysis/stereology packages.
LFB-H&E-stained sections from the superior frontal gyrus, temporal pole, and hippocampus were analyzed using unbiased stereologic counting methods to determine neuron density and number. Measurements of the full cortical thickness and separate measures of the molecular layer thickness were made over 20 points distributed over the entire cortical section. Areas without complete cortical layers were excluded. The mean full cortical thickness and mean thickness of the molecular layer were calculated for each case. An image analysis system (CAST; Olympus, Melville, NY) mounted on an uprightBX51 Olympus microscope with an integrated motorized stage (Prior Scientific, Rockland, MA) was used to outline regions, sample, and count neurons. The neurons were counted manually in LFB-H&E-stained sections. Results of these counts were confirmed using anti-Neu-N immunostained sections of the frontal cortex for selected cases. A probe was then used with a meander sampling paradigm to sample an area of cortex at the crest of a gyrus in the sections. Approximately 100 points per case were sampled and the cortical layer in which these points were distributed was recorded. This provided a percentage of the cortex making up each cortical layer. Each cortical layer was outlined and a stereologic counting frame was used to determine neuronal density for each cortical layer. Neurons were countedin a 28.1- × 28.1- × 8-μm counting frame placed usinga meander sampling paradigm with step lengths needed tosample 100 to 250 neurons per region. Typical step lengths were 125 μm in layer I, 125 to 150 μmin layer II, 200 μm in layer III, 150 to 200 μm in layer IV, and 175 to 250 μm in layers V and VI. Layers V and VI were counted as a single region. Neuronal densities were calculated for each cortical layer as well as for the entire cortical thickness. Neuronal number was calculated from the estimated neuronal density and then standardized based on a 1 mm width of cortex and the previously measured average cortical thickness for each case.
Statistical Analysis
Pearson correlations, partial correlations, multiple regression, and analysis of covariance (ANCOVA) were used to test the relationships between the neuropathologic variables, age, gender, and Mini-Mental State Examination (MMSE) score. Confidence intervals (CIs) for regression coefficients were calculated with 95% confidence limits. Significance tests were two-tailed with alpha level set at 0.05. SAS version 9.1.3 statistical/graphical software was used to carry out the analyses.
RESULTS
Demographics
All patients were Caucasian and ranged in age from 56 to 103 years old; 16 were female and 11 male. Seventeen of the patients had had cognitive testing 0.5 to 16 months prior to death (mean = 6.7) (Tables 1, 2). Based on clinical judgment and chart review none of the patients met the National Institute of Neurological Disorders and Stroke (NINDS) criteria for dementia.
Table 1.
Demographics Data and Cognitive Testing Descriptive Statistics
| Frontal Cortex | Temporal Cortex | Hippocampus CA1 | |
|---|---|---|---|
| n | 27 (F = 16, M = 11) | 17 (F = 10, M = 7) | 21 (F = 12, M = 9) |
| Age range | 56–103 | 62–103 | 62–103 |
| Mean Age ± SD | 82.67 ± 12.38 | 87.29 ± 9.43 | 85.57 ± 10.28 |
| MMSE (n) Mean score ± SD | (17) 25.0 ± 2.65 | (15) 25.0 ± 2.83 | (16) 25.0 ± 2.73 |
| Last visit ± SD(mean, months prior to death) | 6.74 ± 5.23 | 6.63 ± 5.17 | 6.34 ± 5.13 |
n = sample size; SD = standard deviation; F = female; M = male.
Table 2.
Neuropathologic Findings
| Age | Sex | MMSE (Last visit-months prior to death) | Braak and Braak Score | Neuritic Plaques | Neuropathologic Findings |
|---|---|---|---|---|---|
| 56 | M | - | 0/VI | None | Diffuse amyloid plaques |
| 60 | F | - | I/VI | None | Mild CAA, diffuse amyloid plaques |
| 63 | F | - | 0/VI | None | Rare diffuse plaques in temporal lobe |
| 66 | M | - | 0/VI | None | Diffuse amyloid plaques |
| 68 | M | - | I/VI | Sparse | Rare diffuse plaques |
| 74 | F | 28/30 (1) | I/VI | None | Hemorrhagic infarct right thalamus, remote |
| 74 | F | - | 0/VI | None | Hypertensive changes of cortical vessels |
| 75 | M | - | II/VI | None | Hypertensive cerebrovascular disease, multiple microinfarcts involving white matter. |
| 80 | F | - | I/VI | Sparse | Rare diffuse plaques in temporal cortex |
| 82 | M | 28/30 (10) | II/VI | None | Rare Lewy bodies in the substantia nigra, atherosclerosis |
| 85 | M | 23/30 (0.5) | II/VI | None | Hypertensive cerebrovascular disease, arteriolar- and arteriolosclerosis |
| 85 | F | - | II/VI | Sparse | Mild CAA, focal diffuse plaques in temporal cortex |
| 86 | F | 27/30 (10) | I/VI | Sparse/focally moderate | Infarct right orbitofrontal cortex and putamen, remote; arteriolar- and arteriolosclerosis |
| 86 | M | 26/30 (12) | II/VI | Sparse/focally moderate | Mild to moderate diffuse plaques in the neocortex, arteriolar and arteriolosclerosis, micro-infarcts, remote |
| 86 | F | - | I/VI | None | Arteriolar- and arteriolosclerosis intracerebral blood vessel |
| 88 | F | 22/30 (3) | I/VI | Sparse | Subacute hemorrhage, left thalamus; Mild CAA; remote infarct left cerebellar hemisphere and microinfarct cerebral cortex; Hypertensive cerebrovascular disease |
| 88 | F | 20/30 (5) | II/VI | Sparse | Recent infarct left MCA territory, small infarct basis pontis, remote; arteriolar- and arteriolosclerosis in cerebral arteries |
| 89 | F | 20/27 (10) | II/VI | None | Old pontine infarct, arteriolar- and arteriolosclerosis |
| 89 | F | 24/30 (1.5) | II/VI | Sparse | Hypertensive cerebrovascular disease, arteriolar- and arteriolosclerosis; micro-infarcts, remote |
| >90 | M | 25/30 (11.5) | III/VI | Sparse | Infarct in the periventricular white matter in the occipital lobe, remote |
| >90 | M | 25/30 (2) | I/VI | None | Lewy bodies in substantia nigra and locus ceruleus with neuronal loss; hippocampal sclerosis, arteriolar and arteriolosclerosis, micro-infarcts, remote |
| >90 | M | 27/30 (12) | III/VI | Moderate | Microscopic infarct basal ganglia, and thalamus, remote; rare amyloid plaques, arteriolar- and arteriolosclerosis |
| >90 | F | 27/28 (0.5) | II/VI | Sparse | Infarct right posterior temporal lobe/calcarine cortex, remote; arteriolar and arteriolosclerosis |
| >90 | F | 25/30 (16) | III/VI | Moderate | Cerebrovascular arteriolar- and arteriolosclerosis, atherosclerosis, micro- infarcts, remote |
| >90 | M | 24/30 (3.5) | II/VI | Moderate | Hypertensive cerebrovascular disease with lacunar infarcts and infarct in cerebellar hemisphere |
| >90 | F | 29/30 (3) | I/VI | Sparse | Hypertensive cerebrovascular disease, multiple microinfarcts in globus pallidus, thalamus, cerebellum |
| >90 | F | 25/30 (13) | II/VI | Sparse | Hypertensive cerebrovascular disease, severe; multiple microinfarcts, involving basal ganglia and thalamus |
Mini-Mental State Examination (MMSE) is last exam prior to death; - indicates no MMSE score available (no cognitive testing performed); B&B = Braak and Braak score (I–VI); CAA = cerebral amyloid angiopathy.
Neuropathological Evaluation
Most of the brains had evidence of cerebrovascular disease and many had gross and microscopic infarcts. One patient had a recent hemorrhage. Most of the brains also contained neurofibrillary tangles but no case scored higher than a Braak and Braak score of III/VI. The majority of brains had scattered diffuse amyloid plaques, but only rare, sparse or focally moderate neuritic plaques. Mild cerebral amyloid angiopathy was present in 3 cases. Lewy Bodies were present in the substantia nigra of 2 patients, but neither patient had clinical symptoms of Parkinson disease.
Cortical Thinning Occurs with Increasing Age
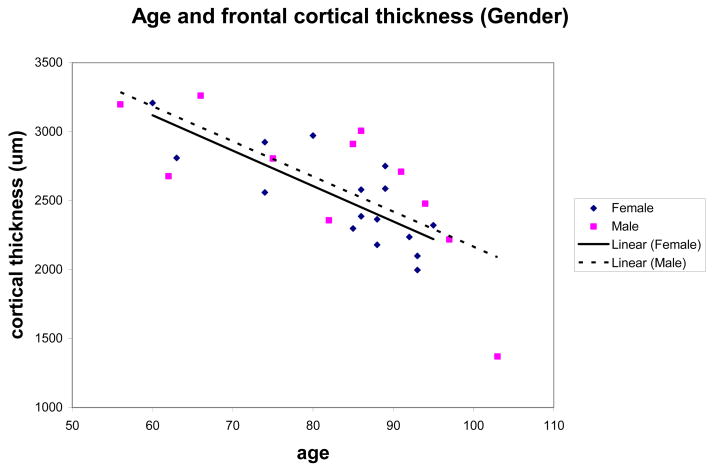
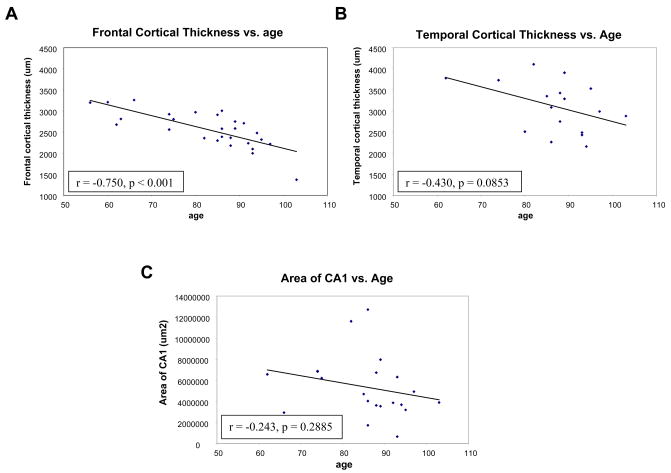
The cortical thickness decreased by 0.8 percent per year with increasing age in the frontal cortex at a rate of −25 microns per year (n = 27; r = −0.75; p < 0.0001; 95% CI: −35.09 to −16.4 microns/year). Within the temporal cortex a trend of decreasing cortical thickness was evident with a 0.67 percent decrease per year at a rate of −27 microns per year (n = 17; r −0.43; p = 0.085; 95% CI: −59.4 to 4.32 microns/year) (Fig. 1A, B). The hippocampal CA1 region in 21 cases demonstrated similar volume loss of 0.8 percent and a rate of −69,081 μm2 per year but the correlation of CA1 sector size with age did not reach statistical significance (n = 21; r = −0.24; p = 0.2885; 95% CI: −201487 to 63325 μm2/year) (Fig. 1C). An ANCOVA of frontal and of temporal thickness, by gender, covarying age, showed volume loss, density and number of neurons were not influenced by gender, nor did males and females differ in the slope of the linear relationship of thickness to age (Fig. 2).
Figure 1.
Scatterplots of cortical thickness versus age. Frontal cortical thickness in 27 cases demonstrated a significant correlation of decreasing cortical thickness with age (A). Temporal cortical thickness in 17 cases (B) and hippocampal CA1 sector area in 21 cases (C) also demonstrated decreasing cortical thickness or decreasing area of CA1 with increasing age.
Figure 2.
Scatterplot of age and frontal cortical thickness of male and females demonstrating no differences based on gender.
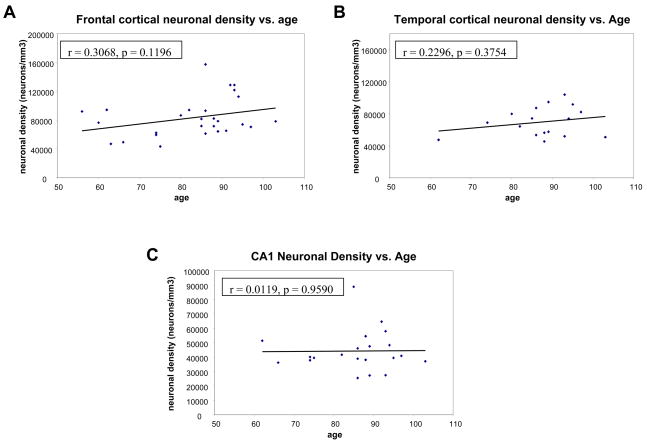
We next evaluated whether neuronal density changes with age. The neuronal density calculated for the entire cortical thickness (all layers), within the frontal and temporal cortex did not change with increasing age (mean = 83,155.13 neurons/mm3; n = 27; r = 0.31; p = 0.1196; 95% CI for rate = −187.08 to 1533.7 neurons/mm3 for frontal cortex, and mean = 69,493.81 neurons/mm3; n = 17; r = 0.23; p = 0.3754; 95% for rate = CI −588.36 to 1470.91 neurons/mm3 for temporal cortex) (Fig. 3A, B). Lamina-specific neuronal densities also showed no differential laminar vulnerability. Similar results were obtained when neuron density was calculated using Neu-N immunostaining. The neuronal density in the CA1 sector of the hippocampus also stayed constant with increasing age (mean = 44,199 neurons/mm3; n = 21; r = 0.01; NS; 95% CI for rate = −643.73 to 676.57 neurons/mm3; Fig. 3C).
Figure 3.
Scatterplots of neuronal density versus age. Frontal cortical neuronal density (A) and temporal cortical neuronal density (B) show a small but not statistically significant increase with increasing age. Hippocampal neuronal density stays constant (C).
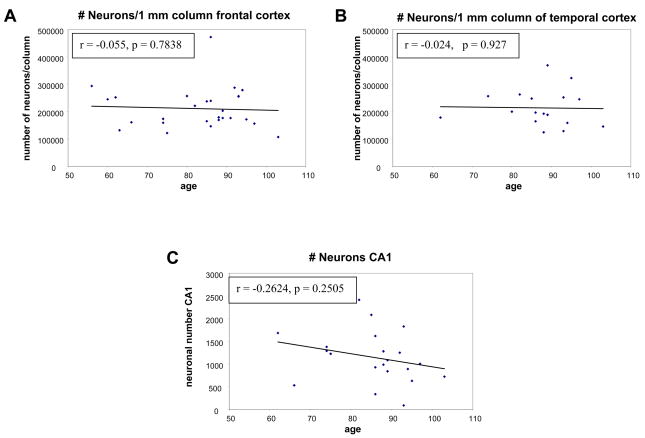
Since the cortical thickness decreases with age, and the neuronal density does not, we asked if the total number of neurons in a column of cortex diminished with age. To estimate the number of neurons in a 1-mm-wide full thickness cortical strip, we multiplied the measured neuronal density by the average measured section cortical thickness. Thus the neuronal number was calculated for a region of the same width, but varied based on the average cortical thickness measurement for each case. The result indicated that the number of neurons stays constant with age for both frontal and temporal regions, that is, the loss of cortical volume seen with age is offset by the small increase in neuronal density (r for age vs. number of neurons was virtually zero in each case) (Fig. 4A, B). On the other hand, the CA1 sector of the hippocampus had a decreased area with age (Fig. 1C) but no change in neuronal density (Fig. 3C). The decline in CA1 neuron number with age did not, however, reach statistical significance (r = −0.26, p = 0.25) (Fig. 4C).
Figure 4.
Scatterplots of neuronal number for frontal (A), and temporal regions (B) for estimated number of neurons in a cortical strip of 1 mm, by 1 mm, by calculated cortical thickness showing the neuronal number remaining constant with increasing age. Scatterplot of neuronal number in hippocampal CA1 region versus age (C) demonstrating loss of neurons with increasing age.
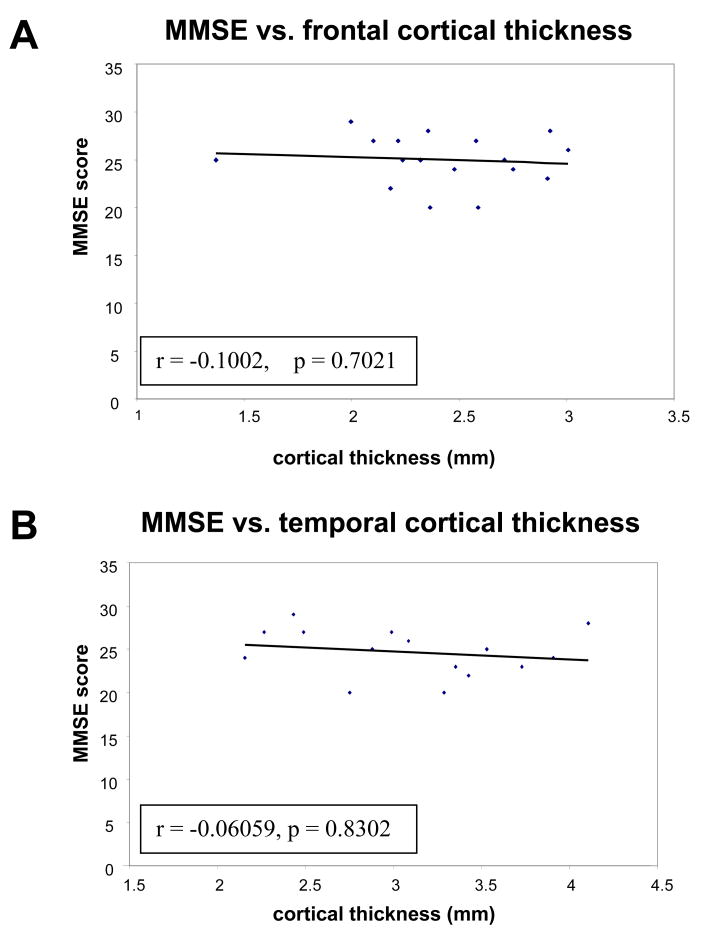
We next examined whether the other neuropathologic findings observed were incidental or correlated with cognitive alteration as assessed by last known MMSE prior to death. Neuritic plaque burdens ranged from sparse to moderate and the Braak and Braak score in all cases was III or less. In AD patients there is extensive plaque and tangle burden with prominent cortical atrophy and neuronal loss. In our patients who did not have AD, neuritic plaques (a rank ordinal scale of density) and tangle burden (the Braak and Braak stage) were positively correlated with age (r = 0.55, p < 0.003 vs. plaques and r = 0.75, p < 0.0001 vs. Braak and Braak). Thus it was important to assess the relations of age, plaque burden, and Braak and Braak score to the other brain measures, where the former are statistically adjusted for (i.e. partialed from) each other via multiple regression to estimate non-spurious, unconfounded relations for each. Partialed relations of age vs. thickness were found to be negative, but stronger and more significant than in the case of the ordinary unadjusted Pearson correlations reported above (Frontal: partial r = −0.60, p = 0.0014; Temporal: partial r = −0.63, p = 0.0125; Hippocampus: partial r = −0.38, p = 0.1). Across all the 3 brain regions, however, the partialed relations of plaques and tangles to cortical thickness were not significant (except for a positive relation for Braak score in the temporal lobe, partial r = 0.6, p = 0.02). Thus age appears to have a true negative relation to cortical thickness, which becomes stronger when the obscuring, confounding effects of plaques and tangles are statistically held constant. This also suggests that there was no direct influence of the small numbers of plaques and tangles on the decrease in cortical thickness and, therefore, that the cortical changes seen in aging are not simply the result of early AD changes but are related to aging itself. The presence of diffuse plaques, vascular lesions (i.e. atherosclerosis or microinfarcts), or Lewy bodies in the substantia nigra did not correlate with cortical atrophy. Cortical atrophy also did not correlate with MMSE score (Fig. 5A, B).
Figure 5.
Scatterplots of last available Mini Mental State Examination (MMSE) score versus frontal cortical thickness in 17 cases (A) and temporal cortical thickness in 15 cases (B) demonstrating no correlation between cortical thickness and last available MMSE score (mean = 6.7 months prior to death).
DISCUSSION
Volumetric imaging studies of the human brain have demonstrated volume loss of both the cortex and white matter with aging (2–4, 6). Our results indicate that the cortex becomes thinner with age corresponding to these volumetric studies and suggest that the observed neuropathologic changes reflect this same pathologic process. With aging there are also functional changes in the brain that are not necessarily associated with AD (24) and may indicate that some regions are differentially affected. The frontal cortex is one area that consistently shows volume loss with age (7, 8, 25) as well as functional alterations (26).
Through stereologic methods our results indicate that while the frontal and temporal regions of cortex are undergoing thinning, the total neuronal number remains relatively constant over the 47-year age range (i.e. from 56 to 103). The absence of neuronal loss with increasing age is comparable and consistent with data from Pakkenberg et al for whole brain neuron counts (17) and for previous studies of entorhinal cortex (15) and superior temporal sulcus (27). What accounts for the cortical thinning? The cerebral cortex is comprised of a set number of elements including neurons, glia, microglia, neuropil, and blood vessels. If neurons are not lost, one is left to question what is leading to the clear decrease in cortical volume. Previous studies have suggested an age-related decrease in neuronal size (13) and loss of presynaptic terminals (28). Loss of complexity of dendritic arborizations has also been described in Golgi studies of “normal” aging (29). Although the present study did not examine the white matter in these cases, the reported observation of volume loss in this compartment also suggests that some of the decrease in cortical thickness could be attributed to loss of ascending projections to the cortex. Together these data suggest that loss of neuropil (i.e. neuronal structural complexity) might contribute to the dramatic thinning that occurs with increasing age.
The aging process has also been examined in the non-human primate neocortex. While some structural changes including thinning of layer I, reduction of synapses, and changes in strength of microcolumns have been identified, the cortical neuronal numbers have appeared to stay constant with age (14, 30–33). Therefore, in nonhuman primates, age-related changes also appear to involve cortical thinning and remodeling of layer I and neuropil rather than neuronal loss per se. Defining the cortical changes may contribute to understanding the cognitive alterations associated with aging and help distinguish this process from age-related neurodegenerative diseases.
Many of the elderly subjects in this study had evidence of vascular disease and infarcts but even in these patients neuronal loss was not present and the decrease in cortical volume still correlated with age. The vascular disease observed indicates the importance of understanding its effects on changes in cognition as well as white matter volume loss in aging.
The sample size in this study was limited, particularly in the temporal and hippocampal regions. A power analysis indicated that with an N of 27, we had good power (approximately 0.8) to detect a population correlation of r = 0.5 or higher. Smaller effects may have escaped detection (34). Therefore, negative results must be interpreted with caution, pointing to the need for further study in a large sample to clarify these issues.
Volume loss was also demonstrated in the CA1 sector of the hippocampus, a finding that has been seen in non-demented aged individuals by MRI examination (35). The neuronal density in this region remained constant with age and the actual number of neurons were decreased, but not to a significant extent. This observation is in agreement with prior stereologic studies of the CA1 sector which found that the neuronal number stayed constant with age as well as in patients with mild cognitive impairment (19, 20). It should be noted, however, that our definition of the CA1 region was more restricted than that described by West (18) and many of the individuals in our study had other age-related systemic diseases including hypertension, cerebral atherosclerosis, microinfarcts, lacunes, white matter pallor, and hippocampal sclerosis (1 case, excluded from analysis).
All of the cognitively tested patients in the present study were not demented from a neuropsychological perspective when they were initially tested several years prior to their deaths. However, several individuals developed decrements in the MMSE and impairments in verbal delayed recall within the year prior to death. None of these patients had neuropathologic features of AD at autopsy. These individuals did not have more severe changes in the neuropil than other similarly aged individuals and we attribute their mild impairments to vascular lesions in the setting of age-related diminished “reserve” due to loss of neuropil integrity (Table 2). Interestingly, we did not identify any specific factor, including diffuse amyloid deposits, Lewy bodies in the substantia nigra, or atherosclerosis as contributing to the decline in MMSE. Indeed, what is striking is that cognition is relatively well preserved in these elderly individuals despite a loss of cortical neuropil that is sufficient to cause readily detectable changes in cortical volume. Thus, age-related brain atrophy, well documented in numerous neuroimaging studies, likely reflects atrophy of dendritic fields in neuropil rather than loss of neurons. This is consistent with the hypothesis that there is little or no cortical neuronal loss simply as a consequence of aging.
In summary, we have examined the brains of a cohort of non-demented individuals, aged 56 to 103, who by clinical and neuropathological evaluation were free of AD and other age-related neurodegeneration, for age-related brain changes that may be attributable to non-AD causes, including aging per se. We confirmed neuroradiological observations of cortical shrinkage and found rates of −0.8% per year in the frontal cortex and −0.67% per year in the temporal cortex. In contrast to classical observations, we did not detect evidence for neuronal loss even in extreme old age. Our results also support the neuroimaging data of greater volume loss within the frontal cortex over temporal cortex in aging. We suggest that there may be changes in either neuronal size or a simplification in the complexity of dendritic and axonal interactions in the neuropil (as suggested by earlier Golgi staining evaluation of the aging cortex) (29). We speculate that this change in cortical structure, rather than neuronal loss, provides the substrate for age-related volume loss and age-related cognitive changes, and dramatically differentiates the effects of aging from those of AD in which both neuronal number and neuronal density are diminished.
Acknowledgments
Supported by AG05134 (Massachusetts Alzheimer Disease Research Center), AG08487, AG004390, R01-AG021133, R21-AG023661 and R03-AG024633. We thank Brigita Urbanc and H.E. Stanley for critical reading and helpful comments on the manuscript.
References
- 1.Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–94. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 2.Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–39. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- 3.Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part II: Quantitative magnetization transfer ratio histogram analysis. AJNR Am J Neuroradiol. 2002;23:1334–41. [PMC free article] [PubMed] [Google Scholar]
- 4.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci. 2003;23:3295–301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Toledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer’s disease: In vivo detection of entorhinal cortex atrophy. Ann N Y Acad Sci. 2000;911:240–53. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu RS, Lemieux L, Bell GS, Sisodiya SM, Shorvon SD, Sander JW, et al. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage. 2003;20:22–33. doi: 10.1016/s1053-8119(03)00219-2. [DOI] [PubMed] [Google Scholar]
- 7.Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, et al. Regional frontal cortical volumes decrease differentially in aging: An MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–69. [PubMed] [Google Scholar]
- 8.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 9.Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–82. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 10.Bennett DA. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Dis Assoc Disord. 2006;20:S63–68. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]
- 11.Silver MH, Newell K, Brady C, Hedley-White ET, Perls TT. Distinguishing between neurodegenerative disease and disease-free aging: correlating neuropsychological evaluations and neuropathological studies in centenarians. Psychosom Med. 2002;64:493–501. doi: 10.1097/00006842-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Brody H. Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol. 1955;102:511–16. doi: 10.1002/cne.901020206. [DOI] [PubMed] [Google Scholar]
- 13.Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987;21:530–39. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- 14.Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: Are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: Effect of sex and age. J Comp Neurol. 1997;384:312–20. [PubMed] [Google Scholar]
- 17.Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJ, Nyengaard JR, et al. Aging and the human neocortex. Exp Gerontol. 2003;38:95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- 18.West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- 19.West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–72. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- 20.West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol Aging. 2004;25:1205–12. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Vonsattel JP, Aizawa H, Ge P, DiFiglia M, McKee AC, MacDonald M, et al. An improved approach to prepare human brains for research. J Neuropathol Exp Neurol. 1995;54:42–56. doi: 10.1097/00005072-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–97. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 24.Minati L, Grisoli M, Bruzzone MG. MR spectroscopy, functional MRI, and diffusion-tensor imaging in the aging brain: A conceptual review. J Geriatr Psychiatry Neurol. 2007;20:3–21. doi: 10.1177/0891988706297089. [DOI] [PubMed] [Google Scholar]
- 25.Pardo JV, Lee JT, Sheikh SA, Surerus-Johnson C, Shah H, Munch KR, et al. Where the brain grows old: Decline in anterior cingulate and medial prefrontal function with normal aging. Neuroimage. 2007;35:1231–37. doi: 10.1016/j.neuroimage.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–71. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 28.Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43:192–97. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- 29.Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in aging human cortex. Exp Neurol. 1975;47:392–403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- 30.Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998;8:671–84. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- 31.Peters A, Moss MB, Sethares C. The effects of aging on layer 1 of primary visual cortex in the rhesus monkey. Cereb Cortex. 2001;11:93–103. doi: 10.1093/cercor/11.2.93. [DOI] [PubMed] [Google Scholar]
- 32.Peters A. Structural changes in the normally aging cerebral cortex of primates. Prog Brain Res. 2002;136:455–65. doi: 10.1016/s0079-6123(02)36038-2. [DOI] [PubMed] [Google Scholar]
- 33.Cruz L, Roe DL, Urbanc B, Cabral H, Stanley HE, Rosene DL. Age-related reduction in microcolumnar structure in area 46 of the rhesus monkey correlates with behavioral decline. Proc Natl Acad Sci U S A. 2004;101:15846–51. doi: 10.1073/pnas.0407002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 35.Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: A study of a five-year change. Neurology. 2004;62:433–38. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]