Abstract
Integrin-linked kinase (ILK) facilitates signal transduction between extracellular events and important intracellular survival pathways involving protein kinase B/Akt. We examined the role of ILK in determining pancreatic adenocarcinoma cellular chemoresistance to the nucleoside analogue gemcitabine. Cellular ILK expression was quantified by Western blot analysis. We examined the effects of overexpression of active ILK and of ILK knockdown induced by RNA interference on gemcitabine chemoresistance. We also examined the effects of modulating ILK expression on gemcitabine-induced caspase 3– mediated apoptosis, phosphorylation status of Akt (Ser473) and glycogen synthase kinase. Overexpression of ILK increased cellular gemcitabine chemoresistance, whereas ILK knockdown induced chemosensitization via increased caspase 3– mediated apoptosis. ILK knockdown attenuated Akt Ser473 and glycogen synthase kinase phosphorylation, whereas overexpression of constitutively active myristoylated Akt was sufficient to induce significant recovery in gemcitabine chemoresistance in the presence of ILK knockdown. Levels of ILK expression affect gemcitabine chemoresistance in pancreatic adenocarcinoma cells. This novel finding suggests that therapies directed against ILK and its downstream signaling targets may have the potential to enhance the efficacy of gemcitabine-based chemotherapy.
Integrin-linked kinase (ILK) is an intracellular serine/threonine kinase that interacts with the cytoplasmic domains of integrin β1 and β3 subunits (1). This 59-kDa protein contains a phosphoinositide phospholipid–binding domain, which is flanked by NH2-terminal ankyrin repeat and COOH-terminal kinase domains. ILK kinase activity is stimulated by cellular attachment to extracellular matrix components and by growth factors. These stimuli result in suppression of apoptosis and promotion of cell survival via protein kinase B/Akt signaling events. ILK seems to couple integrin and growth factor receptors to this important cell survival pathway. ILK is also able to induce inhibitory phosphorylation of glycogen synthase kinase 3 (GSK-3), which modulates cyclin D1–mediated cell cycle regulation as well as activities of transcription factors, including activator protein and β-catenin-Lef-1/Tcf (2, 3). Cellular adhesion plays an important role in determining cellular resistance to chemotherapeutic agents in a range of malignancies (4–8), and evidence strongly implicates integrin signaling in adhesion-dependent chemoresistance (9–11). Inhibition of ILK using small-molecule inhibitors and dominant-negative ILK expression constructs has been shown to attenuate the invasiveness of human tumor cells (12), suggesting that this molecule may be therapeutically relevant. However, despite substantial evidence linking downstream targets of ILK to tumor cell chemoresistance, the role of ILK in this aspect of malignant cellular behavior has not been studied.
Pancreatic adenocarcinoma is among the most chemoresistant of malignancies. Even treatment with the current first line chemotherapeutic agent, the nucleoside analogue gemcitabine (2′,2′-difluorodeoxycytidine), offers minimal benefit in terms of survival and quality of life (13). Akt plays a central role in the resistance of pancreatic adenocarcinoma cells to apoptotic stimuli, including exposure to gemcitabine (14–16). Increased Akt activity protects cells from apoptosis in a variety of ways, including direct suppression of caspase activation (17, 18). Given the importance of ILK in protein kinase B/Akt signaling, we hypothesized that targeting ILK might represent a novel approach to modulate the chemoresistance of pancreatic adenocarcinoma cells to gemcitabine. In this study, we present the first evidence indicating a novel role for ILK in determining pancreatic adenocarcinoma cellular chemoresistance to gemcitabine.
Materials and Methods
Cells and cell culture
PANC1, MIAPaCa2, and Capan2 pancreatic ductal adenocarcinoma cells were obtained from American Type Culture Collection (Rockville, MD). Cells were maintained in DMEM containing 10% fetal bovine serum (Life Technologies, Gaithersburg, MD). Cells were incubated in a humidified (37°C, 5% CO2) incubator and passaged upon reaching 80% confluence.
Expression vectors and transfection
Constitutively active ILK [ILK(S343D)], constitutively active myristoylated Akt, and dominant-negative Akt (K179M) expression constructs were obtained from Upstate (Waltham, MA). Cells were transfected with the appropriate expression construct, or control empty vector (pUSE), using Lipofect-AMINE 2000 (Invitrogen, Carlsbad, CA) in accordance with the manufacturer’s protocol. Stable clones were selected by continuous treatment with G418 (Life Technologies, 0.8 mg/mL) over 4 weeks.
Small interfering RNA
Small interfering RNA (siRNA) targeting the following sequences were synthesized and purified by Qiagen-Xeragon (Germantown, MD): ILK sense 5′-CCUGACGAAGCUCAACGAGAAd(TT)-3′, ILK antisense 5′-UUCUCGUUGAGCUUCGUCAGGd(TT)-3′; control sense 5′-CCUGACGAAGCUCAACGAAAAd(TT)-3′, control antisense 5′-UUUUCGUUGAGCUUCGUCAGGd(TT)-3′. The target specificity of these sequences was confirmed by BLAST search (http://www.ncbi.nih.gov/BLAST). Homologous siRNAs were dissolved in buffer [100 mmol/L potassium acetate, 30 nmol/L HEPES-KOH, 2 nmol/L magnesium acetate (pH 7.4)] to a final concentration of 20 µmol/L, heated to 90°C for 60 seconds and incubated at 37°C for 60 minutes before use to disrupt any higher order aggregates formed during synthesis. Cells were plated into 35-mm 6-well trays and allowed to adhere for 24 hours. In all, 8 µL siPORT Amine transfection reagent (Ambion, Inc., Austin, TX) per well were added to serum-free medium for a final complexing volume of 200 µL, vortexed, and incubated at room temperature for 15 minutes. The transfection reagent/siRNA complexes were added to the wells containing 800 µL medium with 10% fetal bovine serum and incubated in normal cell culture conditions for 6 hours, after which 1 mL DMEM containing 10% fetal bovine serum was added. Assays were done 48 hours post-transfection.
Cytotoxicity assay
Cytotoxicity was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT, Trevigen, Inc., Gaithersburg, MD) in accordance with the manufacturer’s instructions. Results of the MTT assay have been shown to correlate well with [3H]-thymidine incorporation in pancreatic cancer cell lines (19). Logarithmically growing cells were plated at 5 × 103 cells per well in 96-well plates, allowed to adhere overnight, and were cultured in the presence or absence of 0 to 10 µmol/L gemcitabine. Gemcitabine-induced cytotoxicity was determined after 24 hours of exposure. Plates were read using a Vmax microplate spectrophotometer (Molecular Devices, Sunnyvale, CA) at a wavelength of 570 nm corrected to 650 nm and normalized to controls. Each independent experiment was done thrice, with 10 determinations for each condition tested. The concentration of gemcitabine required to inhibit proliferation by 50% (IC50) was calculated from these results. At identical time points, cells were trypsinized to form a single cell suspension. Intact cells, determined by trypan blue exclusion, were counted using a Neubauer hemocytometer (Hausser Scientific, Horsham, PA). Cell counts were used to confirm MTT results.
Apoptosis staining
Following gemcitabine treatment, cells were washed, resuspended in 0.5 mL of PBS, and 1 µL/mL YO-PRO-1, and propidium iodide were added (Vybrant Apoptosis Assay Kit #4, Molecular Probes, Eugene, OR). Cells were incubated for 30 minutes on ice and analyzed by flow cytometry (FACScan, Becton Dickinson, Franklin Lakes, NJ), measuring fluorescence emission at 530 and 575 nm. Cells stained with the green fluorescent dye YO-PRO-1 were counted as apoptotic; necrotic cells stained with propidium iodide. The number of apoptotic cells was divided by the total number of cells (minimum of 104 cells), giving the apoptotic fraction. Data were analyzed using CellQuest software (Becton Dickinson). All observations were reproduced at least thrice in independent experiments.
Fluorometric caspase 3 activity assay
Lysates were assayed for caspase 3 activity using the BD ApoAlert fluorometric Caspase Assay Plate (BD Biosciences Clontech, Palo Alto, CA) in accordance with the manufacturer’s instructions. Plates were read (excitation, 360 nm; emission, 480 nm) using a CytoFluor 4000 multiwell fluorescence plate reader (Applied Biosystems, Foster City, CA). A minimum of three determinations was done for each sample.
Western blot analysis
Cells were washed with ice-cold PBS and whole cell extracts were prepared using cell lysis buffer [20 mmol/L Tris (pH 7.5), 0.1% Triton X, 0.5% deoxycholate, 1 mmol/L phenyl-methylsulfonyl fluoride, 10 µg/mL aprotinin, and 10 µg/mL leupeptin] and cleared by centrifugation at 12,000 × g, 4°C. Total protein concentration was measured using the bicinchoninic acid assay kit (Sigma, St Louis, MO) with bovine serum albumin as a standard. Cell lysates containing 30 µg total protein were analyzed by immunoblotting. Anti-ILK, anti-Akt, and anti-p-Akt (Ser473) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-GSK-3α/β (Ser21/9) antibody was obtained from Cell Signaling Technology (Beverly, MA). Anti-actin antibodies were obtained from LabVision (Freemont, CA). Chemoluminescent detection (Upstate, Lake Placid, NY) was done in accordance with the manufacturer’s instructions. The ILK signal was quantified using ImagePro Plus software version 4.0 and normalized to that of actin.
Statistical analysis
Differences between groups were analyzed using Student’s t test, multifactorial ANOVA of initial measurements and Mann Whitney U test, for nonparametric data, as appropriate, using Statistica 5.5 software (StatSoft, Inc., Tulsa, OK). In cases in which averages were normalized to controls, the SDs of each nominator and denominator were taken into account in calculating the final SD. P < 0.05 was considered statistically significant.
Results
Overexpression of active integrin-linked kinase increases pancreatic adenocarcinoma cell gemcitabine chemoresistance
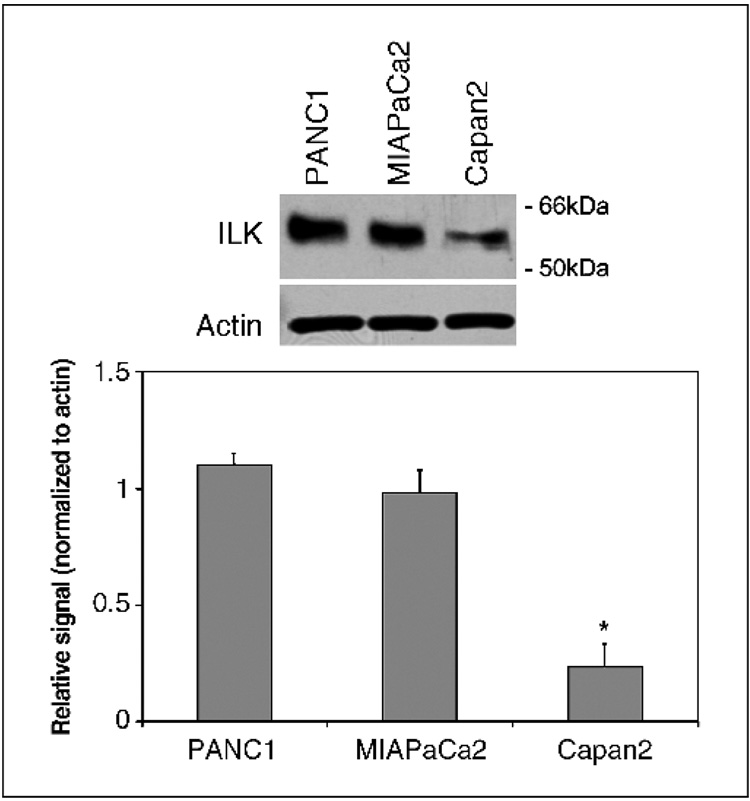
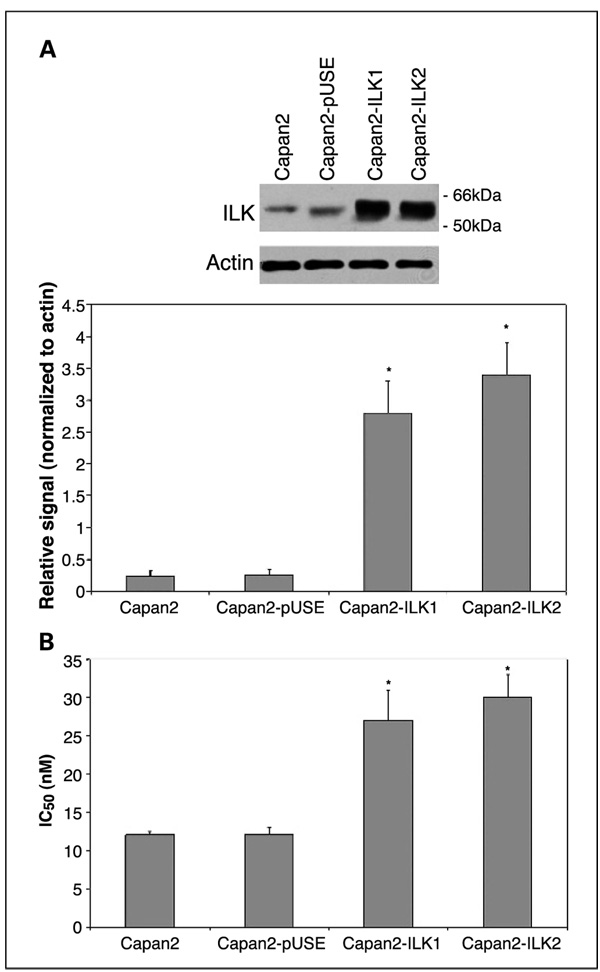
The gemcitabine IC50 values (concentration of gemcitabine required to inhibit cellular proliferation by 50%) of the pancreatic adenocarcinoma cell lines PANC1, MIAPaCa2, and Capan2 have been determined previously and we confirmed these results (20). The IC50 of gemcitabine for PANC1, MIAPaCa2, and Capan2 are 50, 40, and 12 nmol/L, respectively. Levels of ILK expression in PANC1, MIAPaCa2, and Capan2 cells were compared by Western blotting (Fig. 1). PANC1 and MIAPaCa2, the most chemoresistant cell lines, exhibited greater levels of ILK expression than Capan2, which has lower chemoresistance to gemcitabine. To define the role of ILK in gemcitabine chemosensitivity, we established clones of Capan2 that were stably transfected with a constitutively active ILK (S343D) expression construct. Overexpression of ILK by two transfectant clones (Capan2-ILK1 and Capan2-ILK2) was confirmed by Western blot analysis (Fig. 2A). The gemcitabine IC50 values for the parental cell line (Capan2), empty vector transfectants (Capan2-pUSE), Capan2-ILK1, and Capan2-ILK were quantified by MTT cytotoxicity assay. Whereas empty vector transfection had no effect on gemcitabine IC50, overexpression of active ILK resulted in a significant increase in chemoresistance to gemcitabine (Fig. 2B).
Fig. 1.
ILK expression among the pancreatic adenocarcinoma cell lines PANC1, MIAPaCa2, and Capan2 was quantified by Western blot analysis. PANC1 and MIAPaCa2, which have the greatest degree of gemcitabine chemoresistance (20), exhibited higher levels of ILK expression than Capan2. Representative example with mean densitometric values from triplicate blots.*, P < 0.05 versus Capan2 cells.
Fig. 2.
A, stable overexpression of active ILK was confirmed by western blotting in Capan2-ILK1and Capan2-ILK2 cells. Representative example with mean densitometric values from triplicate blots.*, P < 0.05 versus Capan2-pUSE transfectants. B, gemcitabine IC50 was determined by MTT cytotoxicity assay. Capan2-ILK1 and Capan2-ILK2 transfectants exhibited significantly increased gemcitabine chemoresistance, relative to both parental cells (Capan2) and empty vector transfectants (Capan2-pUSE).*, P < 0.05 versus Capan2-pUSE.
Integrin-linked kinase overexpression suppresses gemcitabine-induced caspase-mediated apoptosis
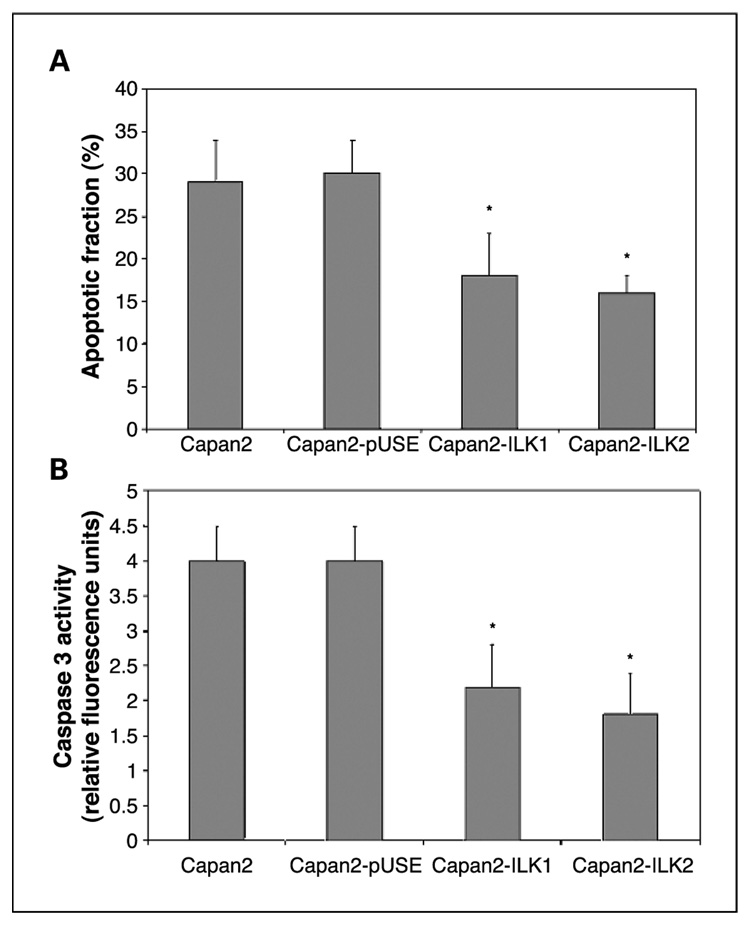
Apoptosis was quantified by flow cytometric analysis. Following exposure of ILK transfectant cells to 1 µmol/L gemcitabine for 24 hours, the apoptotic fraction of Capan2-ILK1 and Capan2-ILK2 were, respectively, 40% and 46% less than that of Capan2-pUSE and Capan2 cells, which showed no significant difference in their apoptotic response to gemcitabine (Fig. 3A).
Fig. 3.
A, apoptotic fraction of cells was determined by flow cytometric analysis of at least 10,000 cells following exposure to1 µmol/L gemcitabine for 24 hours. The apoptotic fractions of both ILK transfectants were significantly lower than those of Capan2 and Capan2-pUSE. Columns, mean values from triplicate experiments; bars,± SD.*, P < 0.05 versus Capan2-pUSE. B, cellular caspase 3 activity following exposure to 1 µmol/L gemcitabine for 24 hours was significantly lower in Capan2-ILK1 and Capan2-ILK2 transfectants. Columns, mean values from triplicate experiments; bars, ±SD. *, P < 0.05 versus Capan2-pUSE.
Caspase 3 activities of cell lysates, obtained following exposure to 1 µmol/L gemcitabine for 24 hours, were quantified by fluorometric assay. Capan2-ILK1 and Capan2-ILK2 transfectants exhibited a 45% and 55% lower level of caspase 3 activation than Capan2-pUSE transfectants, respectively (Fig. 3B).
Integrin-linked kinase knockdown potentiates gemcitabine-induced cytotoxicity
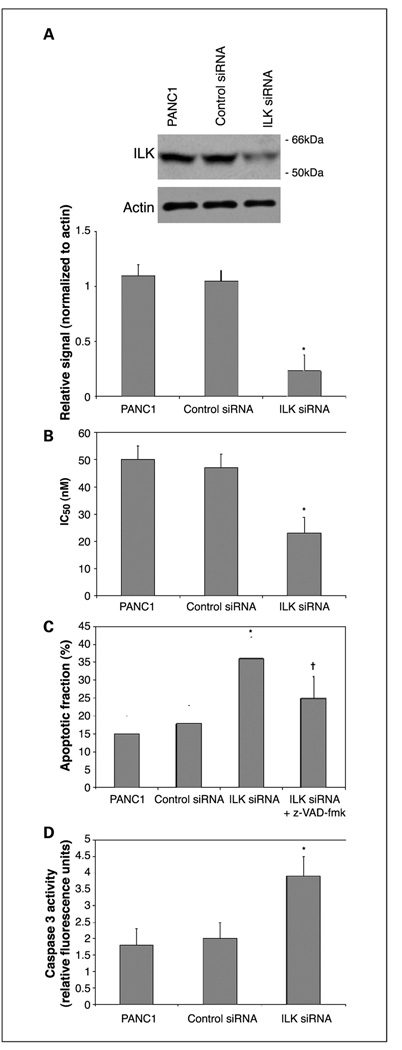
PANC1 cells were chosen to study the effects of ILK knockdown as these cells inherently express a relatively high level of ILK. ILK expression was suppressed by transfection of ILK-specific siRNA. Forty-eight hours following transfection of siRNA, cells were subjected to gemcitabine cytotoxicity assay as described previously. Continued suppression of ILK expression of up to 75% was confirmed by Western blotting, 72 hours following siRNA transfection (Fig. 4A). ILK knockdown resulted in a 51% decrease in the gemcitabine IC50, as determined by MTT assay, relative to control siRNA transfectants (Fig. 4B). Both the apoptotic fraction and caspase 3 activation induced by exposure to gemcitabine were significantly increased following transfection of ILK-specific siRNA but not control siRNA (Fig. 4C and D). Furthermore, when the apoptotic fraction was determined following exposure to gemcitabine in the presence of the caspase inhibitor z-VAD-fmk, the increase in apoptotic fraction induced by ILK knockdown was significantly reduced, signifying that increased gemcitabine-induced apoptosis in cells exposed to ILK siRNA is caspase dependent.
Fig. 4.
A, knockdown of ILK expression in PANC1 cells was confirmed by Western blot 48 hours following transfection of ILK-specific but not control siRNA. Representative example with mean densitometric values from triplicate blots. *, P < 0.05 versus control siRNA. B, gemcitabine-induced cytotoxicity was significantly enhanced following ILK knockdown. *, P < 0.05 versus control siRNA. Both the apoptotic fraction (C) and caspase 3 activation (D) in response to gemcitabine were significantly increase by exposure to ILK-specific siRNA. Apoptotic fraction of cells treated with ILK-specific siRNA was significantly reduced by exposure to the caspase inhibitor z-VAD-fmk. Columns, mean values from triplicate experiments; bars, ±SD. *, P < 0.05 versus control siRNA. †, P < 0.05 versus ILK siRNA.
Integrin-linked kinase knockdown modulates Akt Ser473 and glycogen synthase kinase phosphorylation
ILK facilitates the phosphorylation of Akt at Ser473, which is required for Akt activation (21–23). In addition to modulating activity of Akt, ILK also induces phosphorylation of downstream targets such as glycogen synthase kinase and myosin light chain (22). In view of the important role of Akt in mediating pancreatic adenocarcinoma cellular resistance to gemcitabine (14–16, 24), we examined the effect of ILK knockdown on Akt Ser473 phosphorylation, as well as phosphorylation of its downstream target, GSK. We observed a decrease in both Akt Ser473 phosphorylation (Fig. 5A) and phosphorylation of GSK (Fig. 5B) in cells transfected with ILK siRNA, which is consistent with a model in which ILK facilitates phosphorylation of Akt and GSK.
Fig. 5.
A, phosphospecific immunoblotting showed that knockdown of ILK expression suppressed Akt Ser473 phosphorylation. B, inhibitory phosphorylation of glycogen synthase kinase (p-GSK) was suppressed by ILK knockdown. Control siRNA had no effect on the phosphorylation status of either Akt or GSK. Representative example with mean densitometric values from triplicate blots. *, P < 0.05 versus control siRNA.
Activated Akt rescues cells from chemosensitization to gemcitabine induced by integrin-linked kinase knockdown
Given the effects of ILK knockdown on Akt phosphorylation status, we sought to determine the effects of concomitant overexpression of constitutively active Akt, and ILK knockdown, on gemcitabine-induced cytotoxicity. Cotransfection of a constitutively active myristoylated Akt expression construct at the time of ILK siRNA transfection was sufficient to maintain the gemcitabine IC50 of PANC1 cells at levels significantly higher than those of cells in which ILK knockdown was done in isolation (Fig. 6).
Fig. 6.

Transfection of a constitutively active myristoylated Akt expression construct (myr-Akt) induced significant recovery of the chemoresistant phenotype in PANC1 cells transfected with ILK-specific siRNA. Gemcitabine IC50 values were quantified by MTT cytotoxicity assay. Columns, mean values from triplicate experiments; bars, ±SD. *, P < 0.05 versus empty vector (pUSE).
Discussion
Cellular adhesion to substrate is protective against a range of chemotherapeutic agents (7–11). ILK plays a critical role in coupling extracellular signaling events to intracellular cell survival pathways. In this study, we have shown for the first time that overexpression of active ILK increases gemcitabine chemoresistance in pancreatic adenocarcinoma cells. This chemoprotective effect occurs in association with suppression of caspase 3 activity. Furthermore, posttranscriptional knockdown of ILK expression by RNAi enhances gemcitabine-induced caspase 3–mediated apoptosis, decreases Akt Ser473 phosphorylation, and suppresses levels of GSK phosphorylation. In addition to its recognized roles in transducing signals resulting from growth factor receptor ligation and extracellular matrix interaction, ILK can act as a determinant of pancreatic adenocarcinoma cellular resistance to gemcitabine.
Increased expression and activity of ILK protects epithelial cells from apoptotic events such as anoikis, permitting anchorage-independent cell cycle progression and conferring greater tumorigenic ability and cellular invasiveness (3, 12, 21, 25). ILK interacts with β1 and β3 integrin subunits and is activated by contact with extracellular matrix components (1, 21). Overexpression of the ILK has been shown to modulate β-catenin subcellular localization and function (3). ILK also facilitates phosphorylation of Akt at Ser473, which is a requirement for Akt activation (21–23). Serine phosphorylation of Akt by ILK results in inhibitory phosphorylation of the downstream target GSK (22), which is associated with greater resistance to cellular insults such as exposure to ionizing irradiation (26, 27). Akt has been recognized to play an important role in pancreatic adenocarcinoma cellular chemoresistance (14–16) and the ability of ILK knockdown to enhance gemcitabine-induced cytotoxicity is consistent with the role of ILK as a regulator of Akt activity. We confirmed that Akt plays a significant role in the changes in gemcitabine chemoresistance induced by ILK knockdown, as cotransfection of a constitutively active myristoylated Akt expression construct was sufficient to preserve gemcitabine chemoresistance significantly above that of cells in which ILK knockdown was done in isolation. Whereas ILK-deficient chondrocytes have been shown to exhibit unaltered Akt and GSK phosphorylation (28, 29), others have shown Akt phosphorylation at Ser473 to be ILK dependent (30). Our results also suggest with an important role for ILK as a regulator of Akt activity.
ILK is implicated in the genesis and progression of a variety of malignancies. However, ILK expression has not been previously studied in pancreatic adenocarcinoma nor has its role in cellular chemoresistance. ILK protein hyperexpression is an early event in colonic polyposis (31) and carcinogenesis (32). ILK biochemical activity and protein expression is increased in polyps from familial adenomatous polyposis patients. Sulindac and aspirin, two agents that have been shown to have therapeutic and chemopreventive effects in colorectal carcinogenesis, target ILK and ILK-mediated signaling events (31). Inhibition of ILK has been reported to result in the transcriptional stimulation of E-cadherin expression and is correlated with inhibition of transcription of snail, a repressor of E-cadherin gene expression (33). ILK also plays a role in vascular endothelial growth factor–mediated tumor angiogenesis via protein kinase B/Akt–dependent signaling (34).
Considerable evidence derived from work done in a variety of malignancies, including pancreatic adenocarcinoma, indicates that Akt is a key regulator of cellular apoptosis (14–16, 18, 35). Activated Akt protects cells from a variety of apoptotic stimuli, including exposure to gemcitabine (14, 15, 18, 35). The caspase cascade of proteolytic enzymes comprises initiator and executioner elements. Following activation, these proteases degrade intracellular targets, resulting in apoptotic cell death. Active Akt has been shown to phosphorylate initiator caspase 9 directly, preventing its activation (17). Inhibition of this initiator caspase may interfere with apoptosome function and impair activation of effector caspase 3. Akt has also been reported to inhibit caspases by post-translational modification of an as yet unidentified cytosolic factor located downstream of cytochrome c release and upstream of caspase 9 activation (18). The results of this study indicate that modulation of Akt activity through alteration of ILK expression can influence pancreatic adenocarcinoma cellular gemcitabine chemoresistance.
In summary, our study is the first to characterize the role of ILK in mediating pancreatic adenocarcinoma cellular chemoresistance. We have shown that whereas overexpression of ILK protects cells against gemcitabine-induced apoptosis, posttranscriptional silencing of ILK expression induces a significant increase in caspase-mediated apoptosis following exposure to gemcitabine. Our findings suggest that strategies directed against ILK and its downstream signaling targets may represent a novel approach to enhance the efficacy of gemcitabine in pancreatic cancer.
Acknowledgments
We thank the technical assistance of Jan Rounds.
Grant support: National Pancreas Foundation and Department of Surgery, Brigham and Women’s Hospital departmental funds.
References
- 1.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, et al. Regulation of cell adhesion and anchorage-dependent growth by a new β 1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 2.Troussard AA, Tan C, Yoganathan TN, Dedhar S. Cell-extracellular matrix interactions stimulate the AP-1 transcription factor in an integrin-linked kinase- and glycogen synthase kinase 3-dependent manner. Mol Cell Biol. 1999;19:7420–7427. doi: 10.1128/mcb.19.11.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novak A, Hsu SC, Leung-Hagesteijn C, et al. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signaling pathways. Proc Natl Acad Sci U S A. 1998;95:4374–4379. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura T, Kato Y, Fuji H, Horiuchi T, Chiba Y, Tanaka K. E-cadherin-dependent intercellular adhesion enhances chemoresistance. Int J Mol Med. 2003;12:693–700. [PubMed] [Google Scholar]
- 5.Weekes CD, Kuszynski CA, Sharp JG. VLA-4 mediated adhesion to bone marrow stromal cells confers chemoresistance to adherent lymphoma cells. Leuk Lymphoma. 2001;40:631–645. doi: 10.3109/10428190109097661. [DOI] [PubMed] [Google Scholar]
- 6.Uhm JH, Dooley NP, Kyritsis AP, Rao JS, Gladson CL. Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin Cancer Res. 1999;5:1587–1594. [PubMed] [Google Scholar]
- 7.St Croix B, Kerbel RS. Cell adhesion and drug resistance in cancer. Curr Opin Oncol. 1997;9:549–556. doi: 10.1097/00001622-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28:38–44. doi: 10.1097/00006676-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Damiano JS, Hazlehurst LA, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) protects the K562 chronic myelogenous leukemia cell line from apoptosis induced by BCR/ABL inhibition, cytotoxic drugs, and γ-irradiation. Leukemia. 2001;15:1232–1239. doi: 10.1038/sj.leu.2402179. [DOI] [PubMed] [Google Scholar]
- 10.Hazlehurst LA, Damiano JS, Buyuksal I, Pledger WJ, Dalton WS. Adhesion to fibronectin via β1 integrins regulates p27kip1 levels and contributes to cell adhesion mediated drug resistance (CAM-DR) Oncogene. 2000;19:4319–4327. doi: 10.1038/sj.onc.1203782. [DOI] [PubMed] [Google Scholar]
- 11.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 12.Troussard AA, Costello P, Yoganathan TN, Kumagai S, Roskelley CD, Dedhar S. The integrin linked kinase (ILK) induces an invasive phenotype via AP-1 transcription factor-dependent upregulation of matrix metalloproteinase 9 (MMP-9) Oncogene. 2000;19:5444–5452. doi: 10.1038/sj.onc.1203928. [DOI] [PubMed] [Google Scholar]
- 13.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 14.Ng SSW, Tsao MS, Chow S, Hedley DW. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000;60:5451–5455. [PubMed] [Google Scholar]
- 15.NgS S, Tsao MS, Nicklee T, Hedley DW. Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clin Cancer Res. 2001;7:3269–3275. [PubMed] [Google Scholar]
- 16.Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. Inhibition of the phosphatidylinositol 3′-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2002;1:989–997. [PubMed] [Google Scholar]
- 17.Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 18.Zhou H, Li XM, Meinkoth J, Pittman RN. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol. 2000;151:483–494. doi: 10.1083/jcb.151.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raitano AB, Scuderi P, Korc M. Binding and biological effects of tumor necrosis factor and γ interferon in human pancreatic carcinoma cells. Pancreas. 1990;5:267–277. doi: 10.1097/00006676-199005000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. RNA interference targeting the M2 subunit of ribonucleotide reductase enhances pancreatic adenocarcinoma chemosensitivity to gemcitabine. Oncogene. 2003;23:1539–1548. doi: 10.1038/sj.onc.1207272. [DOI] [PubMed] [Google Scholar]
- 21.Dedhar S. Cell-substrate interactions and signaling through ILK. Curr Opin Cell Biol. 2000;12:250–256. doi: 10.1016/s0955-0674(99)00083-6. [DOI] [PubMed] [Google Scholar]
- 22.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch DK, Ellis CA, Edwards PA, Hiles ID. Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene. 1999;18:8024–8032. doi: 10.1038/sj.onc.1203258. [DOI] [PubMed] [Google Scholar]
- 24.Duxbury MS, Ito H, Benoit E, Zinner MJ, Ashley SW, Whang EE. RNA interference targeting focal adhesion kinase enhances pancreatic adenocarcinoma gemcitabine chemosensitivity. Biochem Biophys Res Commun. 2003;311:786–792. doi: 10.1016/j.bbrc.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 25.Attwell S, Roskelley C, Dedhar S. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene. 2000;19:3811–3815. doi: 10.1038/sj.onc.1203711. [DOI] [PubMed] [Google Scholar]
- 26.Cordes N, Van Beuningen D. Arrest of human lung fibroblasts in G2 phase after irradiation is regulated by converging phosphatidylinositol-3 kinase and β1-integrin signaling in vitro. Int J Radiat Oncol Biol Phys. 2004;58:453–462. doi: 10.1016/j.ijrobp.2003.09.069. [DOI] [PubMed] [Google Scholar]
- 27.Cordes N, Van Beuningen D. Cell adhesion to the extracellular matrix protein fibronectin modulates radiation-dependent G2 phase arrest involving integrin-linked kinase (ILK) and glycogen synthase kinase-3β (GSK-3β) in vitro. Br J Cancer. 2003;88:1470–1479. doi: 10.1038/sj.bjc.6600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grashoff C, Aszodi A, Sakai T, Hunziker EB, Fassler R. Integrin-linked kinase regulates chondrocyte shape and proliferation. EMBO Rep. 2003;4:432–438. doi: 10.1038/sj.embor.embor801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai T, Li S, Docheva D, et al. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troussard AA, Mawji NM, Ong C, Mui A, Arnaud R, Dedhar S. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J Biol Chem. 2003;278:22374–22378. doi: 10.1074/jbc.M303083200. [DOI] [PubMed] [Google Scholar]
- 31.Marotta A, Tan C, Gray V, et al. Dysregulation of integrin-linked kinase (ILK) signaling in colonic polyposis. Oncogene. 2001;20:6250–6257. doi: 10.1038/sj.onc.1204791. [DOI] [PubMed] [Google Scholar]
- 32.Marotta A, Parhar K, Owen D, Dedhar S, Salh B. Characterisation of integrin-linked kinase signalling in sporadic human colon cancer. Br J Cancer. 2003;88:1755–1762. doi: 10.1038/sj.bjc.6600939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan C, Costello P, Sanghera J, et al. Inhibition of integrin linked kinase (ILK) suppresses β-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC−/− human colon carcinoma cells. Oncogene. 2001;20:133–140. doi: 10.1038/sj.onc.1204052. [DOI] [PubMed] [Google Scholar]
- 34.Tan C, Cruet-Hennequart S, Troussard A, et al. Regulation of tumor angiogenesis by integrin-linked kinase (ILK) Cancer Cell. 2004;5:79–90. doi: 10.1016/s1535-6108(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 35.Fahy BN, Schlieman M, Virudachalam S, Bold RJ. AKT inhibition is associated with chemosensitisation in the pancreatic cancer cell line MIA-PaCa-2. Br J Cancer. 2003;89:391–397. doi: 10.1038/sj.bjc.6601037. [DOI] [PMC free article] [PubMed] [Google Scholar]