Abstract
The extent to which neurons proceed into the cell cycle and the mechanisms whereby cell cycle re-entry leads to apoptosis vary in response to agonists. We previously showed upregulation of early G1 regulators in thrombin-treated neurons yet neurons did not proceed to S phase but to apoptosis. The objective of this study is to explore mechanisms which might prevent S phase entry and promote apoptosis in thrombin-treated neurons. Cultured rat brain neurons are exposed to thrombin (200 nM) for 30 min to 4.5 h and the expression of cyclin C, cyclin dependent kinases (cdk1, 2 ,3, 8) and the cell cycle inhibitor p27 assessed. Our data show a simultaneous decrease of both cyclin C and cdk3 proteins soon after thrombin treatment. The decrease in cyclin C also correlates with decreases in cdk1 and cdk2, at both mRNA and protein levels. There is no change in expression of cdk8 or the cell cycle inhibitor p27 in response to thrombin treatment. These results suggest that decreases in G1-S regulators cyclin C and cdks 3, cdk2 and cdk1 in response to thrombin could make conditions unfavorable for S phase entry and favor neuronal apoptosis.
Keywords: Cyclin C, cell cycle, neuronal apoptosis, cdk1, cdk2, cdk3, thrombin, S phase
Introduction
The multifunctional protease thrombin causes neuronal cell death both in vitro and in vivo [17,20,24]. Thrombin causes rapid tau aggregation and has been found in the senile plaques characteristic of Alzheimer's disease (AD) [1,3,15,24]. We have documented the presence of thrombin in AD-derived microvessels and have shown that intracerebroventricular administration of thrombin results in apoE fragmentation and cognitive impairment, suggesting that thrombin may contribute to neuronal cell death in AD. [9,17]. The pathway involved in thrombin-induced neurotoxicity involves activation of the protease activated receptor (PAR-1), followed by RhoA activation, and concludes with cell cycle re-entry [6,19]. Our previous work demonstrates that in thrombin-treated neurons cyclin D1 and E (early G1 cyclins) and the cyclin dependent kinase, cdk4, are activated and that these events lead to upregulation of the pro-apoptotic protein Bim and apoptosis [19]. However, cdk4 activation-induced cell cycle progression does not proceed beyond the S phase, despite expression of cyclin E. Determining the specific proteins that regulate decisions concerning unscheduled DNA replication and apoptosis is important for developing therapeutic interventions to preserve neuronal function. The cell cycle events that prevent S phase entry in thrombin-treated neurons have not been defined.
Therapeutic interventions aimed at inhibiting cell cycle have been shown to prevent neuronal cell death in animal models of CNS injury [5,26,28]. Each phase of the cell cycle is tightly regulated by cyclins and cyclin-dependent kinases. Cyclin C has recently been implicated in the cell cycle, both in G0 to G1 and G1 to S phase regulation [21,22]. Cdk3 and cdk8 are the cyclin dependent kinases that partner with cyclin C. Cdk1 and Cdk2 are E2F target genes that are also affected by several cyclins [18,27]. Cell cycle progression is also regulated by the CIP/KIP family (including p27KIP1) which inhibit the cyclin and cdk complexes, preventing activation of downstream targets and thus contributing to the decision whether or not the cell cycle continues to progress [7,23]. This study focuses on the expression patterns of these relevant cell cycle regulatory molecules (cdk 1, cdk2, cdk3, cdk8, cyclin C, p27KIP1) in cortical neurons treated with thrombin, and how their expression might explain the failure of thrombin-treated neurons to enter S phase.
Materials and Methods
Primary neuronal cultures
Rat cerebral cortical cultures were prepared from 17-day gestation rat fetuses, as previously described [8,20]. The cells were plated at a density of 500,000/ml on multi-well plates coated with poly-L-lysine, in media containing Dulbecco's modified Eagle's medium supplemented with 2 mM L-glutamine, 10% heat-activated horse serum, and antibiotic/antimycotic. On day 3, the medium was changed to Neurobasal medium containing B-27 supplement, antibiotic/antimycotic, 0.5 mM glutamine, and 20 μg/ml 5-fluoro-2′-deoxyuridine to inhibit proliferation of non-neuronal cells. On day 6, cells were changed to fresh medium without 5-fluoro-2′-deoxyuridine. Experimental treatments were carried out on day 8 in Neurobasal medium containing N-2 supplement, 0.5 mM L-glutamine and antibiotic/antimycotic (treatment medium).
RT-PCR
Neurons were rinsed with Hank's balanced salt solution and total RNA was extracted using the Trizol method (Invitrogen, Carlsbad, CA). 1 μg of RNA was reverse transcribed using random primers (Roche Applied Science, Indianapolis, IN). 2 μg of cDNA were amplified by PCR (5 min-95° C, 40 cycles of 45 sec- 94° C and 1 min-60° C). The 1 min at 60° C serves for both annealing and elongation, and this improves product specificity. Since there is no separate elongation time, 40 cycles do not reach the plateau range, as concluded from initial experiments under same cycling conditions, for varying numbers of cycles. Primers used for PCR are shown in Table 1. The PCR products were visualized on a 1.6% agarose gel using UV transillumination.
Table 1.
Primers used for the polymerase chain reaction (PCR)
| Gene | GI number | Primers | Amplicon size |
|---|---|---|---|
| β actin | 42475926 | Fwd 5'TGTCACCAACTGGGACGATA 3' Rev 5'TCTCAGCTGTGGTGGTGAAG 3' |
392 bp |
| Cyclin C | 109476214 | Fwd 5' CATGTGTGTTTCTGGCATCC 3' Rev 5' TCCGTCCTGTAGGTGTCATTC 3' |
295 bp |
| Cdk2 | 41054835 | Fwd 5' CATTCCTCTTCCCCTCATCA 3' Fwd 5' GTCACCATTTCGGCAAAGAT 3' |
300 bp |
| Cdk1 | 9506474 | Fwd 5' TTGAAAGCGAGGAAGAAGGA 3' Fwd 5' CCGAAATCTGCCAGTTTGAT 3' |
334 bp |
| p27 | 50054215 | Fwd 5' CAGAATCATAAGCCCCTGGA 3' Fwd 5' TCTGTTCTGTTGGCCCTTTT 3' |
320bp |
Western blotting
Neurons were rinsed with HBSS and total protein was extracted from the neurons using lysis buffer containing 0.1% SDS, 1% Triton X-100 and 0.5% phenylmethyl sulfonylfluoride. Protein was determined by the Bradford method. Equal amounts of protein were run on a 10% polyacrylamide gel, transferred on to a PVDF membrane, blocked with 5 % milk solution (non-fat dry milk in TBST) and immuno-blotted with cyclin C (1:100) cdk3 (1:100), cdk5 (1:500), cdk2 (1:500), cdk1 (1:500), P27KIPI (1:500), and GAPDH (1:500) primary antibodies and peroxidase-conjugated secondary antibodies. Membranes were developed with chemiluminescence reagents and band intensities quantified using the Quantity-One software (BioRad, Hercules, CA).
Statistical analysis
Results were expressed as means ± SD. Pairwise comparisons between controls and treatment groups were conducted using the one-way ANOVA followed by the Tukey test for multiple comparisons. P<0.05 was considered significant.
Results
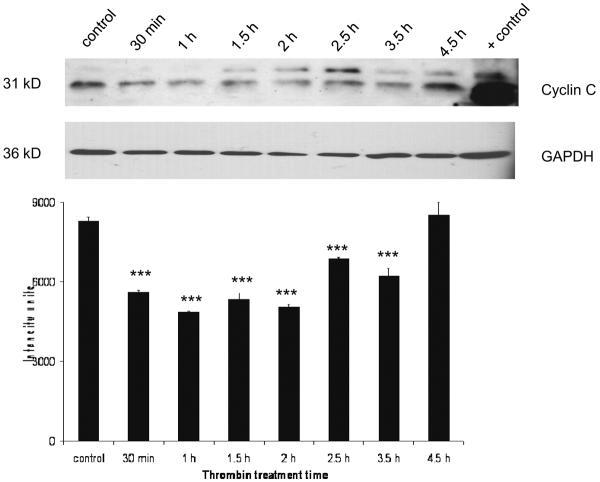
Expression of cyclin C protein, but not mRNA, level decreased in response to thrombin
There was a basal level of cyclin C expression detectable in untreated cultures. Expression of cyclin C significantly decreased compared to control (p<0.001) within 30 min of thrombin treatment and remained lower until 3.5 h. At 4.5 h cyclin C returned to control levels (Figure 1). At the mRNA level, there was no detectable change in cyclin C expression following thrombin treatment (data not shown).
Figure 1. Effect of thrombin on expression of Cyclin C protein.
Neurons were incubated 30 min to 4.5 h with 200 nM thrombin, lysed and protein separated by SDS-PAGE, transferred to a PVDF membrane and immunoblotted for cyclin C (25–30 μg/lane). Lysate from brain endothelial cells was used as a positive control. GAPDH was measured to confirm loading equivalency among lanes (n = 3) ***p < 0.001 vs. control.
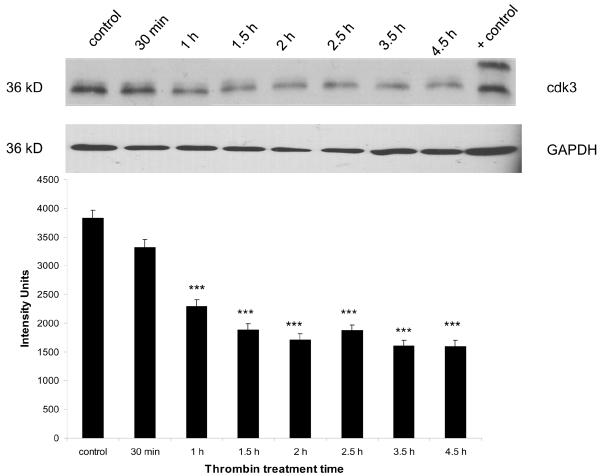
Thrombin decreased cdk3, but not cdk8, expression in cultured neurons
There was a basal level of expression detectable in untreated cultures. Expression of cdk3 significantly decreased compared to control (p<0.001) within 1 h of thrombin treatment and remained lower throughout the experiment (4.5 h) (Figure 2). Cdk8 expression was unchanged following thrombin treatment (data not shown).
Figure 2. Effect of thrombin on expression of cdk3 protein.
Neurons were incubated 30 min to 4.5 h with 200 nM thrombin, lysed and protein separated by SDS-PAGE, transferred to a PVDF membrane and immunoblotted for cdk3 (25–30 μg/lane). Lysate from brain endothelial cells was used as a positive control. GAPDH was measured to confirm loading equivalency among lanes (n = 3). ***p < 0.001 vs. control
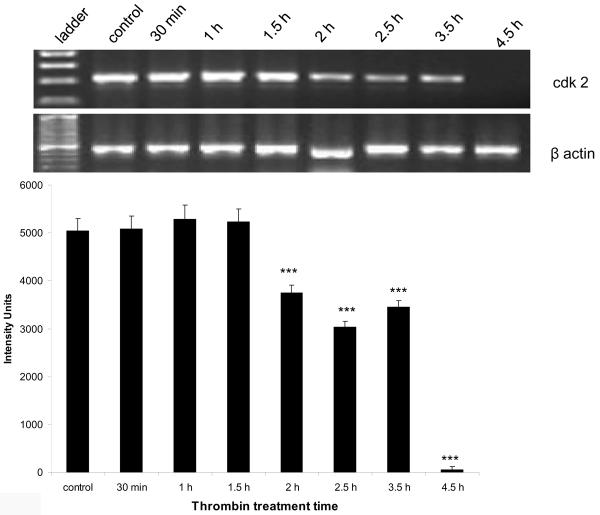
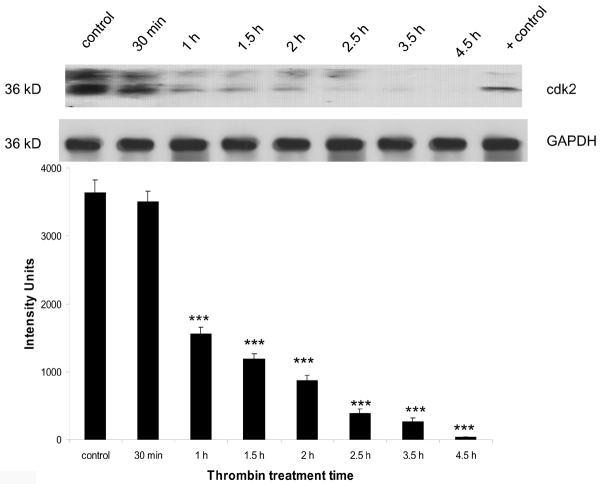
Expression of the cyclin dependent kinases cdk2 and cdk1 was diminished by thrombin
Cdk2 expression
At the mRNA level, there was a significant decrease (p<0.001) in the expression of cdk2 beginning at 2 h post-thrombin treatment, that continued to decrease and eventually disappear by 4.5 h (Figure 3a). Expression of cdk2 at the protein level also decreased significantly (p<0.001) beginning at 1.5 h post-thrombin treatment and continued to decrease and eventually disappear at 4.5 h (Figure 3b).
Figure 3a. Thrombin-induced downregulation of cdk2 mRNA.
Neurons were incubated with 30 min to 4.5 h 200 nM thrombin, total RNA extracted, reverse transcribed and amplified using primers specific for cdk2, and the housekeeping gene β-actin (n = 3). Upper panel: cdk2 300 bp amplicon, lower panel: β-actin 392 bp amplicon. ***p < 0.001 vs. control
Figure 3b. Downregulation of cdk2 protein by thrombin.
Cortical neurons were incubated 30 min to 4.5 h with 200 nM thrombin, lysed and protein separated by SDS-PAGE, transferred to a PVDF membrane and immunoblotted for cdk2 (25–30 μg/lane). Lysate from brain endothelial cells was used as a positive control. GAPDH was measured to confirm loading equivalency among lanes (n = 3). ***p < 0.001 vs. control
Cdk1 expression
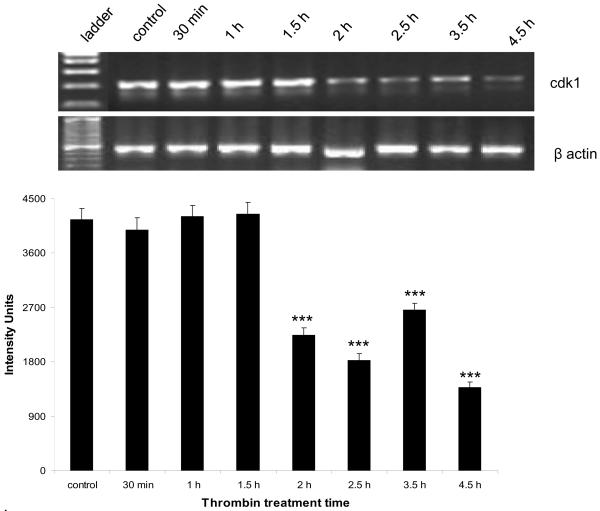
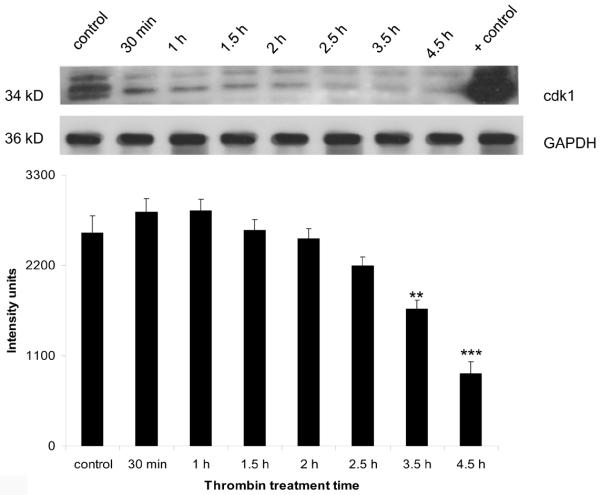
The expression of both cdk1 mRNA and protein followed a pattern similar to that of cdk2. At the mRNA level, there was a significant (p < 0.001) decrease beginning at 2 h post-thrombin treatment that was still significant at 4.5 h (Figure 4a). The decrease in protein followed a slightly delayed course with levels significantly lower than control (p<0.01) at 3.5 h post-thrombin treatment and remaining depressed until 4.5 h (Figure 4b).
Figure 4a. Thrombin-induced downregulation of cdk1 mRNA.
Neurons were incubated 30 min to 4.5 h with 200 nM thrombin, total RNA extracted, reverse transcribed and amplified using primers specific for cdk1, and the housekeeping gene β-actin (n = 3). Upper panel: cdk1 334 bp amplicon, lower panel: β-actin 392 bp amplicon. ***p < 0.001 vs. control
Figure 4b. Downregulation of cdk1 protein by thrombin.
Neurons were incubated 30 min to 4.5 h with 200 nM thrombin, lysed and protein separated by SDS-PAGE, transferred to a PVDF membrane and immunoblotted for cdk2 (25–30 μg/lane). Lysate from brain endothelial cells was used as a positive control. GAPDH was measured to confirm loading equivalency among lanes (n = 3). **p < 0.01,***p < 0.001 vs. control
Discussion
Control of the cell cycle involves the dynamic interplay between proteins that favor vs. those that oppose cell cycle progression. We have previously shown that thrombin exposure causes cell cycle reentry, a significant increase in cyclins D1 and E and an absence of cyclin A expression [19]. In the current study, we find that cdk3, cdk2 and cdk1, all of which are associated with G1-S transition, decrease in response to thrombin stimulation of neurons, despite activation of the G1 cyclins (D1 and E).
Here we document that expression of cyclin C protein in neurons decreases significantly within 30 min after treatment with thrombin. Until recently cyclin C was not thought to play a part in cell cycle regulation [14,22]. However, cyclin C is abundantly expressed in the G0 phase, and has been shown to bind and activate both cdk3 and cdk8 [2,16,21,22]. In nonneuronal cells, cyclin C promotes G0 to G1 entry and with cdk3 to inactivates Rb leading to G1 entry. Therefore, our data that thrombin decreases expression of both cyclin C and cdk3 suggests that the absence of these proteins prevents inactivation of Rb, despite the upregulation of other cell cycle regulators that promote cell cycle progression (cyclins D1 and E).
Thrombin treatment results in a decrease in cyclin C protein, despite constant mRNA levels, suggesting that thrombin regulates cyclin C at the protein level. A regulation of this type has been described in the yeast S. cereviseae [12]. In that study the authors demonstrate cyclin C protein is targeted to the mitochondria for destruction following oxidative stress, and this process is required for apoptosis. Therefore, our observations of decreased cyclin C protein expression but not mRNA expression are suggestive of protein degradation in response to thrombin-induced stress.
Here we show significant decreases in both mRNA and protein levels of both cdk2 and cdk1 in thrombin-treated neurons. Cyclin C has been implicated in G1/S and G2/M transitions, by being able to induce transcription of both cdk2 and cdk1 [14]. Therefore, the decrease in cdk1 and cdk2 we demonstrate could be a consequence of the degradation of cyclin C. The simultaneous decrease of both cdk1 and 2 may trigger initiation of apoptosis in neurons. Findings in other cell types demonstrate that combined depletions of cdk1 and cdk2 lead to apoptosis [4]. Also, downregulation of cdk2 leads to activation of the pro-apoptotic forkhead transcription factor FOXO1, which is normally phosphorylated and inactivated by cdk2 [11]. Hence, several lines of evidence indicate that downregulation of cdk2 itself could be an apoptotic trigger.
The functions of cdk2 and cdk3 are both known to be necessary for S-phase entry [25]. Stable E2F-1 binding to functional cyclin A/cdk2 is essential for avoiding S-phase arrest and death [13]. It has been suggested that cdk3 function might be required for the activation of E2F as a transcription modulation element, and that cdk3 possibly contributes to additional steps required for S-phase entry beyond the activation of certain members of the E2F family of transcription factors [10]. Thus, the decrease in cdk3 evoked by thrombin in the current study could be another mechanism that prevents S phase entry. Finally, it is unlikely that S phase progression is arrested by p27/KIP1-mediated inactivation of cdk1 and cdk2 in thrombin-induced neuronal apoptosis because expression of this cell cycle inhibitor does not change in response to thrombin.
Taken together, our data suggest that the neuronal response to thrombin is varied and complex. On the one hand, thrombin induces expression of G1 cyclins (D1 and E) that promote cell cycle re-entry and progression [19]. On the other hand, thrombin causes a significant decrease in cyclin C protein levels that in conjunction with downstream decreases in cdk1, cdk2 and cdk3 may suppress cell cycle progression. These observations suggest a multiplicity of often conflicting signals regulate cell cycle progression and ultimately control cell cycle-driven apoptosis in neurons. These data demonstrate, for the first time, the involvement of cyclin C in neuronal apoptosis, and relate this cyclin temporally to other cell cycle mediators. The downregulation of cyclin C and cdk1, 2 and 3 could possibly lead to apoptosis independent of the cyclin D1/cdk4 pathway.
Acknowledgements
Sources of support: This work was supported in part by grants from the National Institutes of Health (AG15964, AG020569 and AG028367). Dr. Grammas is the recipient of the Shirley and Mildred Garrison Chair in Aging. The authors gratefully acknowledge the secretarial assistance of Terri Stahl.
References
- [1].Akiyama H, Ikeda K, Kondo H, McGeer PL. Thrombin accumulation in brains of patients with Alzheimer's disease. Neurosci Lett. 1992;146:152–4. doi: 10.1016/0304-3940(92)90065-f. [DOI] [PubMed] [Google Scholar]
- [2].Akoulitchev S, Chuikov S, Reinberg D. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature. 2000;407:102–106. doi: 10.1038/35024111. [DOI] [PubMed] [Google Scholar]
- [3].Arai T, Miklossy J, Klegeris A, Guo JP, McGeer PL. Thrombin and prothrombin are expressed by neurons and glial cells and accumulate in neurofibrillary tangles in Alzheimer disease brain. J. Neuropathol. Exp. Neurol. 2006;65:19–25. doi: 10.1097/01.jnen.0000196133.74087.cb. [DOI] [PubMed] [Google Scholar]
- [4].Cai, Latham VM, Jr, Zhang X, Shapiro GI. Combined depletion of cell cycle and transcriptional cyclin-dependent kinase activities induces apoptosis in cancer cells. Cancer Res. 2006;66:9270–80. doi: 10.1158/0008-5472.CAN-06-1758. [DOI] [PubMed] [Google Scholar]
- [5].Di Giovanni S, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B, Faden AI. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc. Natl. Acad. Sci. USA. 2005;102:8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J. Neurosci. 1997;17:5316–26. doi: 10.1523/JNEUROSCI.17-14-05316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Elledge SJ, Harper JW. Cdk inhibitors: on the threshold of checkpoints and development. Curr. Opin. Cell Biol. 1994;6:847–52. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- [8].Grammas P, Moore P, Weigel PH. Microvessels from Alzheimer's disease brains kill neurons in vitro. Am. J. Pathol. 1999;154:337–42. doi: 10.1016/S0002-9440(10)65280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Grammas P, Samany PG, Thirumangalakudi L. Thrombin and inflammatory proteins are elevated in Alzheimer's disease microvessels: implications for disease pathogenesis. J. Alzheimers Dis. 2006;9:51–8. doi: 10.3233/jad-2006-9105. [DOI] [PubMed] [Google Scholar]
- [10].Hofmann F, Livingston DM. Differential effects of cdk2 and cdk3 on the control of pRb and E2F function during G1 exit. Genes Dev. 1996;10:851–61. doi: 10.1101/gad.10.7.851. [DOI] [PubMed] [Google Scholar]
- [11].Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–7. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- [12].Kawauchi K, Chihama Y, Nabeshima M. Hoshino, Cdk5 phosphorylates and stabilizes p27(kip1) contributing to actin organization and cortical neuronal migration. Nat. Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- [13].Krek W, Xu G, Livingston DM. Cyclin A-kinase regulation of E2F-1 DNA binding function underlies suppression of an S phase checkpoint. Cell. 1995;83:1149–58. doi: 10.1016/0092-8674(95)90141-8. [DOI] [PubMed] [Google Scholar]
- [14].Liu ZJ, Ueda T, Miyazaki T, Tanaka N, Mine S, Tanaka Y, Taniguchi T, Yamamura H, Minami Y. A critical role for cyclin C in promotion of the hematopoietic cell cycle by cooperation with c-Myc. Mol. Cell Biol. 1998;18:3445–54. doi: 10.1128/mcb.18.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McGeer PL, Klegeris A, Walker DG, Yasuhara O, McGeer EG. Pathological proteins in senile plaques. Tohoku J. Exp. Med. 1994;174:269–77. doi: 10.1620/tjem.174.269. [DOI] [PubMed] [Google Scholar]
- [16].Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH. A family of human cdc2-related protein kinases. EMBO J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mhatre M, Nguyen A, Kashani S, Pham T, Adesina A, Grammas P. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol. Aging. 2004;25:783–93. doi: 10.1016/j.neurobiolaging.2003.07.007. [DOI] [PubMed] [Google Scholar]
- [18].Murray AW. Recycling the cell cycle; cyclins revisited. Cell. 2004;116:221–34. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- [19].Rao HV, Thirumangalakudi L, Desmond P, Grammas P. Cyclin D1, cdk4, and Bim are involved in thrombin-induced apoptosis in cultured cortical neurons. J. Neurochem. 2007;101:498–505. doi: 10.1111/j.1471-4159.2006.04389.x. [DOI] [PubMed] [Google Scholar]
- [20].Reimann-Philipp U, Ovase R, Weigel PH, Grammas P. Mechanisms of cell death in primary cortical neurons and PC12 cells. J. Neurosci. Res. 2001;64:654–60. doi: 10.1002/jnr.1119. [DOI] [PubMed] [Google Scholar]
- [21].Ren S, Rollins BJ. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell. 2004;117:239–51. doi: 10.1016/s0092-8674(04)00300-9. [DOI] [PubMed] [Google Scholar]
- [22].Rickert P, Seghezzi W, Shanahan F, Cho H, Lees E. Cyclin C/CDK8 is a novel CTD kinase associated with RNA polymerase II. Oncogene. 1996;12:2631–40. [PubMed] [Google Scholar]
- [23].Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- [24].Suo Z, Wu M, Citron BA, Palazzo RE, Festoff BW. Rapid tau aggregation and delayed hippocampal neuronal death induced by persistent thrombin signaling. J. Biol. Chem. 2003;278:37681–9. doi: 10.1074/jbc.M301406200. [DOI] [PubMed] [Google Scholar]
- [25].van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–4. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- [26].Verdaguer E E, Jimenez A A, Canudas AM AM, ordà EG JEG, Sureda FX FX, Pallàs M M, Camins A A. Inhibition of cell cycle pathway by flavopiridol promotes survival of cerebellar granule cells after an excitotoxic treatment. J. Pharmacol. Exp. Ther. 2004;308:609–616. doi: 10.1124/jpet.103.057497. [DOI] [PubMed] [Google Scholar]
- [27].Verdaguer E, Susana Gde A, Clemmens A, Pallá M, Camins A. Implication of the transcription factor E2F-1 in the modulation of neuronal apoptosis. Biomed Pharmacother. 2007;61:390–9. doi: 10.1016/j.biopha.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [28].Wang F, Corbett D, Osuga H, Osuga S, Ikeda JE, Slack RS, Hogan MJ, Hakim AM, Park DS. Inhibition of cyclin dependent kinases improves CA1 neuronal survival and behavioral performance after global ischemia in the rat. J. Cereb. Blood Flow Metab. 2002;22:171–182. doi: 10.1097/00004647-200202000-00005. [DOI] [PubMed] [Google Scholar]