Abstract
Vertebrate taste buds undergo continual cell turnover. To understand how the gustatory progenitor cells in the stratified lingual epithelium migrate and differentiate into different types of mature taste cells, we sought to identify genes that were selectively expressed in taste cells at different maturation stages. Here we report the expression of the voltage-gated potassium channel KCNQ1 in mammalian taste buds of mouse, rat and human. Immunohistochemistry and nuclear staining showed that nearly all rodent and human taste cells express this channel. Double immunostaining with antibodies against type II and III taste cell markers validated the presence of KCNQ1 in these two types of cells. Co-localization studies with cytokeratin 14 indicated that KCNQ1 is also expressed in type IV basal precursor cells. Null mutation of the kcnq1 gene in mouse, however, did not alter the gross structure of taste buds or the expression of taste signaling molecules. Behavioral assays showed that the mutant mice display reduced preference to some umami substances, but not to any other taste compounds tested. Gustatory nerve recordings, however, were unable to detect any significant change in the integrated nerve responses of the mutant mice to umami stimuli. These results suggest that although it is expressed in nearly all taste bud cells, the function of KCNQ1 is not required for gross taste bud development or peripheral taste transduction pathways, and the reduced preference of kcnq1-null mice in the behavioral assays may be attributable to the deficiency in the central nervous system or other organs.
Keywords: cell turnover, human biopsy, coexpression, gene knockout
INTRODUCTION
Mammalian taste buds are the major peripheral end organs of taste, where the initiation of taste perception occurs (Lindemann, 1996; Margolskee, 2002). The onion-shaped taste buds consist of 50–100 individual cells, which, based on their morphology and ultra-structure, can be classified into four cell types (Farbman, 1965; Kinnamon et al., 1985; Yee et al., 2001). Each cell type appears to play distinct roles in taste bud physiology. For example, type I cells are thought to be supporting cells as well as candidate salty taste receptor cells (Bartel et al., 2006; Lawton et al., 2000; Pumplin et al., 1997; Vandenbeuch et al., 2008). Type II cells are receptive cells for sweet, bitter and umami taste stimuli (DeFazio et al., 2006; Yang et al., 2000b). Type III cells are the only taste bud cells that form conventional synapses with afferent gustatory nerves; and these cells seem to be important to sour taste transduction as well (DeFazio et al., 2006; Huang et al., 2006; Kataoka et al., 2008; Yang et al., 2000a). Type IV cells are precursor cells, replacing aged and/or damaged cells during the rapid cell turnover in taste buds (Stone et al., 2002).
One of the characteristics common to many intragemmal taste bud cells is their electrical excitability. These cells can generate action potentials, spontaneously or in response to taste or electrical stimuli (Avenet and Lindemann, 1991; Chen et al., 1996; Cummings et al., 1993; Gilbertson et al., 1992; Kashiwayanagi et al., 1983; Roper, 1983; Yoshida et al., 2006). Electrophysiological studies have recorded various voltage-gated ion channels present in taste bud cells, including: tetrodotoxin (TTX)-sensitive Na+ channels, tetraethylammonium (TEA)-sensitive delayed rectifier K+ channels, inward rectifier K+ channels, outward rectifier Cl− channels, low-/high-voltage activated Ca2+ channels (Bigiani et al., 2003). Although some of these channels are detected only in certain types of taste bud cells, the voltage-gated K+ conductance seems to be the most ubiquitous among taste bud cells, and has been recorded even from basal cells (Mackay-Sim et al., 1996). It is largely unclear whether the intragemmal electrical activities such as the spontaneous action potentials or any particular voltage-gated ion channels play a role in taste bud development and taste cell differentiation.
In mature functional taste cells, stimulation of G protein-coupled taste receptors by bitter, sweet and umami substances or ion channels by sour and salty stimuli triggers intracellular signaling cascades, leading to the generation of receptor potentials. These tastant-evoked receptor potentials presumably regulate the activity of the voltage-sensitive hemichannels and their release of taste transmitters such as ATP or other bioactive molecules onto gustatory nerve fibers (Finger et al., 2005; Huang et al., 2007; Romanov et al., 2007; Zhao et al., 2005). However, it remains unknown which, if any, voltage-gated ion channels may contribute to the taste sensation by setting or resetting taste receptor cells’ resting and receptor potentials. Even less is known whether the taste signal transduction-induced electrical activity in taste bud cells sends the feedback signals to progenitor cells and controls these cells’ fate determination and differentiation.
Molecular identification and characterization of the voltage-gated ion channels expressed in taste bud cells is a prerequisite to elucidating their possible contribution to taste bud cell turnover and perhaps to taste signal transduction and transmission as well. So far a few of these channels have been molecularly characterized (Lin et al., 2004; Liu et al., 2005; Richter et al., 2004; Stevens et al., 2001). The messenger RNAs for two additional voltage-gated potassium channels, KCNQ1 and KCNH2, have been found in taste buds as well (Ohmoto et al., 2006). In our endeavor to identifying genes that are differentially expressed in taste bud cells vs. extragemmal cells, we independently discovered the expression of KCNQ1 in taste bud cells (Wang et al., 2006). Here we report the co-expression of the channel proteins in mouse, rat and human taste bud cells with other taste signaling molecules and the characterization of behavioral and nerve responses of kcnq1-null mutant mice to taste stimuli. Our results indicate that although it is expressed in nearly all taste bud cells, the function of KCNQ1 is not be required for gross taste bud development or peripheral taste transduction pathways, and the reduced preference of kcnq1-null mice to some umami stimuli may be attributable to the deficiency in the central nervous system, the digestive system or other organs. Part of the preliminary data was published in abstract form (Wang et al., 2006).
MATERIALS AND METHODS
Animals
All studies involving animals were performed according to protocols approved by the Monell Chemical Senses Center Institutional Animal Care and Use Committee. C57BL/6 and other genetically modified mice and Sprague-Dawley rats were housed in a climate-controlled environment at the Monell Chemical Senses Center’s Animal Care Facility.
The generation and characterization of the transgenic mice that carried the gene for green fluorescent protein (GFP) driven by an 8.4 kb α-gustducin promoter were previously described (Huang et al., 1999). The expression of GFP and α-gustducin in taste bud cells largely overlapped.
The generation and breeding of kcnq1-null mice were described previously (Casimiro et al., 2001). The mutant animals derived from 129/Sv × 129/Sv-CP embryonic stem cells were backcrossed with C57BL/6J mice for at least four generations. Heterozygous progeny were interbred to generate kcnq1+/+, kcnq1+/− and kcnq1−/− animals used for two bottle preference tests and gustatory nerve recordings.
Human tissue
Biopsies from fungiform and circumvallate papillae were obtained from human subjects undergoing tonsillectomy or uvulopalatopharyngoplasty surgery. Human subjects provided consent in accordance with an experimental protocol approved by the Thomas Jefferson University Institutional Review Board. All subjects were between 21 and 50 years of age and denied taste or smell difficulties. Subjects with a history of tobacco use, head and neck cancer or head irradiation were excluded from the study. All subjects exhibited normal gustatory function as assessed via forced-choice threshold testing using sucrose, sodium chloride, citric acid, quinine sulfate, and monosodium glutamate or denatonium benzoate solution, followed by suprathreshold testing to assess taste identification and intensity discrimination.
Biopsies were performed with subjects under general endotracheal anesthesia without infiltration of local anesthetic. Fungiform papillae biopsies were obtained from the dorsal surface of the anterior one third of the tongue using a curved spring micro scissors. Circumvallate papillae biopsies were obtained from the back of the tongue using a pair of cup forceps. All biopsies were briefly rinsed in PBS (pH 7.2) before fixation.
Isolation of kcnq1 cDNA from taste bud cells
Construction and screening of single taste bud cell cDNA libraries
Single taste bud cell cDNA libraries were prepared and screened as described previously (Huang et al., 2005; Huang et al., 1999; Perez et al., 2002). Briefly, tongues were removed from euthanized GFP transgenic mice and injected subepithelially with a mixture of dispase II and collagenase A; the lingual epithelium was peeled off from the rest of tongue; circumvallate and foliate papillae, and a piece of non-gustatory lingual epithelium devoid of taste buds were excised and dissociated into individual cells. Single green fluorescent cells, non-fluorescent taste cells from taste papillae and non-taste cells from non-gustatory epithelium were picked individually and their messenger RNAs were reverse transcribed, then amplified by the polymerase chain reaction (PCR). A portion of the amplified products from fluorescent or non-fluorescent taste cells was used to construct single cell cDNA libraries, which were then differentially screened with self or non-self probes that were prepared by radiolabeling the amplified products from the same taste bud cell or a non-gustatory lingual epithelial cell, respectively. The inserts of the differentially expressed clones were sequenced and analyzed by bioinformatics. One of these clones was identified by sequence as being a partial kcnq1 cDNA (Figure 1).
Figure 1.
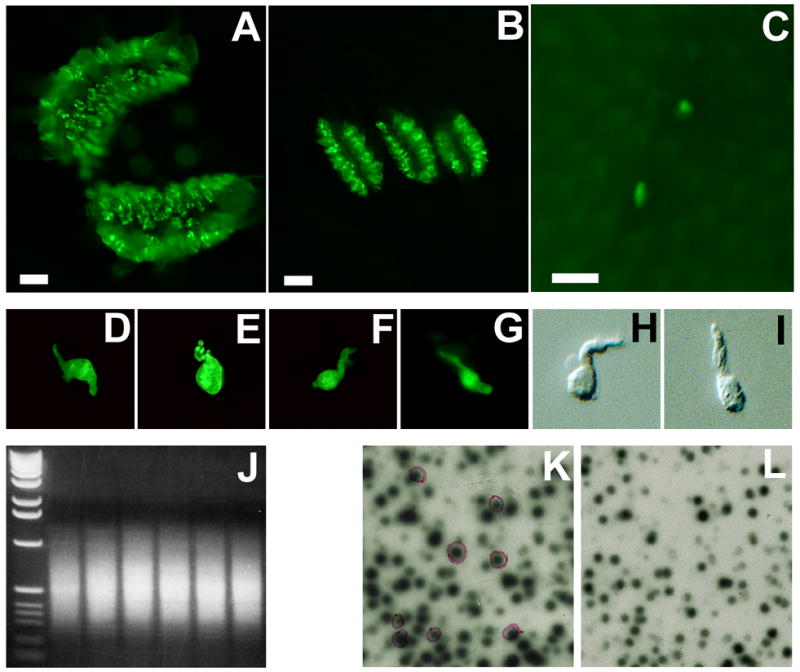
Isolation of taste cell-selective genes. GFP-labeled taste papillae (A: circumvallate; B: foliate; and C: fungiform. Scale bar: 250 μm) were isolated from GFP transgenic mice and dissociated into individual taste bud cells (D to G: four green fluorescent cells; H and I: two non-fluorescent cells). Transcripts from each cell were amplified by PCR with an average size of 600 bp (J: an electrophoretic gel image of amplified products from the six single cells; left lane: 1 kb DNA molecular weight marker). A portion of the amplified products was used to construct single cell cDNA libraries, which were subtractively screened: λ bacteriophage double lift was screened with self-probe (K) and non-taste-probe (L). Differentially expressed clones with stronger signals on the left lift (K) and weaker or no signals on the right lift (L), shown in purple circles, were identified and sequenced.
Isolation of full-length kcnq1 sequence
Circumvallate and foliate papillae from C57BL/6 mice were excised from the peeled-off lingual epithelium as described above. Total RNA was extracted using Absolutely RNA Microprep kit (Strategene, Cedar Creek, TX) from the excised taste papillae and used to synthesize 1st strand cDNA. PCR amplification was carried out with the synthesized cDNA and the PCR primers that were designed based on the reference sequence (GenBank accession: NM_008434) to cover the entire coding region (Sense primer: CTGCCTTCACCTCAGCTCCGAG; antisense primer: TGAGAACCAGGTGGGTGTG). PCR products were subcloned into pGEM T-easy vector (Promega, Madison, WI) and confirmed using DNA sequencing analysis.
Immunocytochemistry
Tissue Preparation
Both rodent tongues and human biopsies were fixed in 4% paraformaldehyde in PBS for 1 hour, cryoprotected in 20% sucrose overnight and sliced into sections with a cryostat.
Single labeling with one antibody
Sections of 10 microns were blocked in the blocking buffer (3% BSA, 0.3% Triton X-100, 2% horse serum and 0.1% sodium azide in PBS) for 1 hr at room temperature and then incubated overnight at 4 oC with diluted primary antibody in the blocking buffer. After washing four times in PBS containing 0.3% Triton X-100, the sections were incubated with Cy3- or Alexa 488-conjugated secondary antibody at room temperature for 30 minutes. To preserve the fluorescence, the tissue sections were covered with Vectashield Mounting Medium (Vector Laboratories, H-1000) and fluorescence micrographs were taken using a fluorescence or confocal microscope.
To visualize the nuclei, 4',6-diamidino-2-phenylindole (DAPI)-containing mounting medium (Vector Laboratories, H-1200) was used to cover the tissue sections.
Double labeling with two antibodies
After the incubation with the first primary antibody and the first secondary antibody as described above for the single labeling, sections were blocked again in the blocking buffer, followed by incubation with a second primary antibody, and with Cy3- or Alexa 633-conjugated second secondary antibodies. Fluorescence micrographs were taken using a confocal microscope.
Leica TCS SP2 Spectral Confocal Microscope and Leica Scanware software (Leica Microsystems Inc.) were used to acquire confocal images. These images were then arranged and adjusted for contrast and brightness using Photoshop v8 (Adobe Systems Inc.).
Antibodies
Source (manufacturer, catalog number, host species and immunizing antigen), characterization and dilutions of the antibodies used are as follows: Two polyclonal antibodies against KCNQ1 were used: one is an affinity-purified rabbit antibody (Chemicon, AB5932, raised with a synthetic peptide corresponding to amino acids 585 to 604 of human KCNQ1 C-terminal sequence: SNTIGARLNRVEDKVTQLDQ, and cross-reacting with rat and mouse KCNQ1, used in immunohistochemistry at 1:1,000 dilution); its specificity has been confirmed by the manufacturer via Western blotting recognizing a protein of approximately 72 kD from adult mouse ventricles, and verified by previous reports (Liao et al., 2005); and the other is an affinity-purified goat antibody (Santa Cruz, SC-10646, also raised against a synthetic peptide of human KCNQ1 C-terminal 20 amino acid residues: NTLPTYEQLTVPRRGPDEGS; cross-reacting with rat and mouse KCNQ1, used in immunohistochemistry at 1:1,000 dilution), its specificity has been confirmed by the manufacturer via Western blotting recognizing a human KCNQ1-fusion protein, and verified by other researchers via immunofluorescent staining of the COS-1 cells transiently transfected with the KCNQ1 cDNA constructs, and via Western blotting of the transfected COS-1 cell extracts recognizing a single band of the expected size (Rasmussen et al., 2004); and the specificity of the above two KCNQ1 antibodies was also validated by this study with taste tissue sections from kcnq1-null mice, which showed non-staining by the antibodies (Figure 2).
Figure 2.
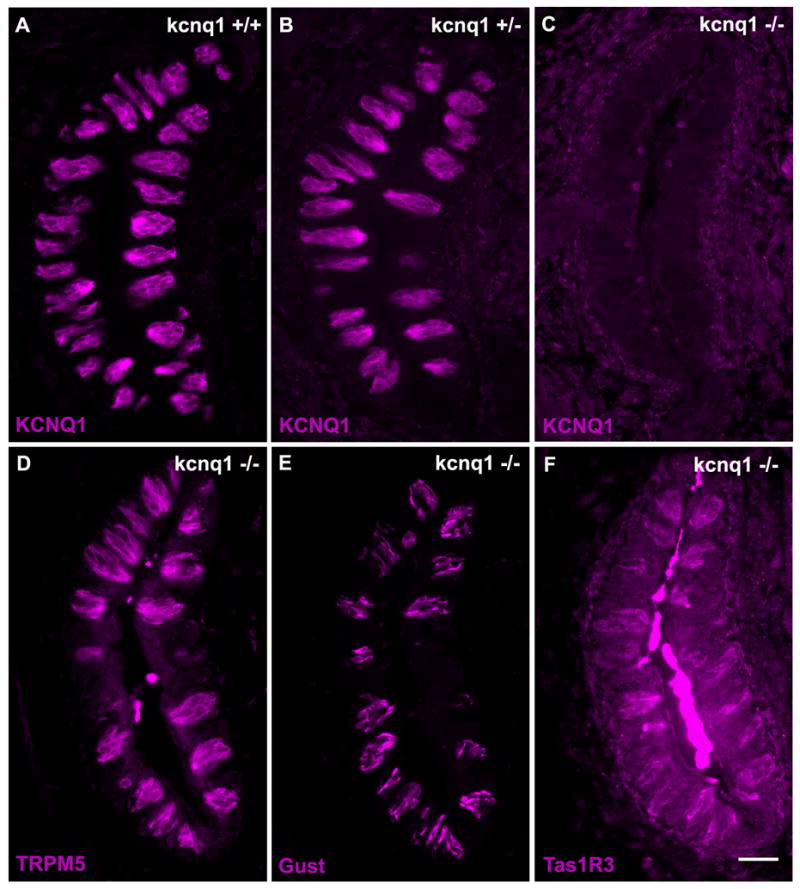
Expression of KCNQ1 and taste signaling molecules in the kcnq1-deficient and wild-type mice. Immunostaining of taste sections confirmed the expression of KCNQ1 in wild-type (A, kcnq1+/+) and heterozygous (B, kcnq1+/−) mice but the absence of the protein in the kcnq1-null mutant (C, kcnq1−/−). The gross structure of taste buds and the expression of TRPM5 (D), α-gustducin (E, Gust) and Tas1R3 (F) appeared normal in the knockout animals. Scale bar: 100 μm.
Anti-synaptobrevin-2 (Wako, 018-15791) was raised in rabbit with a KLH-conjugated synthetic peptide of N-terminal 20 amino acid residues of synaptobrevin-2 of rat origin: MSATAATVPPAAPAGEGGPP, and used in immunohistochemistry at 1:200 dilution, and its specificity has been confirmed in previous studies demonstrating its immunoreactivity within synaptic vesicles and colocalization with other taste signaling molecules in subsets of taste bud cells (Yang et al., 2004).
Anti-α-gustducin antibody (Santa Cruz, SC-395) is an affinity-purified rabbit polyclonal antibody, raised against a synthetic peptide corresponding to amino acid residues 93-112 of α-gustducin of rat origin, used in immunohistochemistry at 1:1,000 dilution, and its specificity has been confirmed by pre-absorption with its immunizing peptide (SC-395p) and by other studies (Yang et al., 2007).
Anti-TRPM5 antibody is a polyclonal antibody, raised in rabbit with a KLH-conjugated synthetic peptide of amino acid residues 1028–1049 of mouse TRPM5, used in immunohistochemistry at 1:1,000 dilution, and its specificity has been confirmed by: 1) the abolition of its immunostaining on rodent taste tissue sections following pre-incubation with the immunizing peptide (Perez et al., 2002); 2) the absence of its immunoreactivity with taste tissue sections from Trpm5-null mice (Damak et al., 2006).
The affinity-purified anti-Tas1R3 antibody was raised in rabbit with a hemocyanin-conjugated synthetic peptide of amino acid residues 45–62 of mouse Tas1R3, used in immunohistochemistry at 1:1,000 dilution, and its specificity was confirmed by blocking its immunoreactivity with the immunizing peptide and by the absence of immunoreactivity on taste tissue sections from T1R3-knockout mice (Damak et al., 2003; Reed et al., 2004).
Anti-NCAM (Millipore, AB5032) is an affinity purified antibody raised in rabbit with highly purified chicken NCAM protein, cross-reactive with NCAM from human, mouse and rat, used in immunohistochemistry at 1:500 dilution, and its specificity was confirmed by Western blotting of mouse brain homogenates recognizing proteins of 200–250 kD, or 140–180 kD after treatment with neurminidase.
Anti-SNAP-25 antibody (Sternberger Monoclonals, SMI81) is a mouse monoclonal IgG, raised with the whole SNAP-25 proteins, used in immunohistochemistry at 1:500 dilution, and its specificity has been confirmed by Western blotting of bovine brain extracts recognizing proteins of 25-kDa and its epitope has been determined to lie within the C-terminal peptide (Keller and Neale, 2001; Keller et al., 1999; Mehta et al., 1996).
Anti-cytokeratin 14 antibody (Chemicon, MAB3232) is a mouse monoclonal IgG raised by immunizing purified proteins from epithelial cells, and its specificity has been confirmed by Western blotting of human buccal epithelial cell extracts recognizing 50 kD proteins (Wetzels et al., 1991).
The following secondary antibodies were used at 1:500 dilution: FITC-conjugated donkey-anti-goat, Cy3-conjugated goat-anti-rabbit, FITC-conjugated donkey-anti-mouse and Tetramethyl Rhodamine-conjugated donkey-anti-goat antibodies (Jackson ImmunoResearch, West Grove, PA), Alexa fluor 488-conjugated donkey-anti-rabbit, Alexa fluor 488-conjugated donkey-anti-goat and Alexa fluor 633 goat-anti-mouse antibodies (Invitrogen).
Control experiments were carried out with the omission of one or two primary antibodies, preincubation of primary antibodies with antigenic peptides, or taste tissue sections from gene knockout mice. Unspecific staining of primary antibodies or cross-reactivity between two secondary antibodies was not found.
Two bottle preference tests
Two bottle preference tests were conducted as described previously (Bachmanov et al., 2001; Bachmanov et al., 1998; Wong et al., 1996). Three groups of age- and sex-matched young adult animals (3–12 weeks of age) with 9–10 mice per group representing three genotypes were used: wild-type (kcnq1+/+), heterozygous mutant (kcnq1+/−) and homozygous mutant (kcnq1−/−). Individually caged animals were presented with two bottles: one containing deionized water, the other taste solution. The bottle positions were switched after 24 hours to eliminate any positional effect. The volume of consumed liquid in each bottle was recorded and preference scores were calculated for each animal by dividing the consumption of the test solution by the total intake of fluid.
Five test solutions representing the five basic taste qualities were used initially: SC45647 (sweet), denatonium benzoate (bitter), citric acid (sour), inosine-5’-monophosphate (IMP, umami) and NaCl (salty). To confirm animals’ response to umami taste, two additional umami substances were used: monopotassium L-glutamate (MPG), and a mixture of IMP and monosodium L-glutamate (MSG). To determine concentration-dependent preferences, a range of concentrations for each compound was presented in ascending order. To minimize any carryover effects from a previous test, animals were given only distilled water for 4–7 days between tests of two different taste substances.
Preference ratios for each concentration of the test solutions over a 2-day period were calculated by dividing the intake of test solution by the total fluid consumption, i.e. the sum of solution intake and water intake. Means and standard errors were obtained by averaging the preference ratios from individual animals of the same genotype group. A two-way analysis of variance (ANOVA) was performed with genotype as a between-group factor and concentration as a within-group factor using the Statistica software package to assess the effect of genotypes on taste preferences for each test compound. If there was a significant effect, then post hoc pair-wise comparisons of means at each test solution concentration were conducted with student t-tests between two genotype groups, i.e., kcnq1−/− vs. kcnq1+/+ and kcnq1−/− vs. kcnq1+/−. The criterion for significance was set to a P value <0.05.
Gustatory Nerve Recordings
The procedures for the mouse chorda tympani nerve recordings were described previously (Inoue et al., 2007; Inoue et al., 2001). Briefly, two kcnq1-null mice and three wild-type littermates were anesthetized with an intraperitoneal injection of a mixture of ketamine (10.7 mg/kg), xylazine (2.2 mg/kg) and acepromazine (0.35 mg/kg). A cannula was inserted in the trachea, and the animal was placed supine in a non-traumatic headholder. The right chorda tympani nerve at the jaw was exposed at its exit from the lingual nerve by removal of the internal pterygoid muscle. The chorda tympani nerve was then dissected free from surrounding tissues and placed on a platinum wire electrode. A few drops of mineral oil were placed in the wound site to prevent desiccation of the nerve. An indifferent electrode was positioned in nearby muscle tissue.
For chemical stimulation of the fungiform taste papillae, the anterior part of the animal’s tongue was enclosed in a flow chamber. Taste substances (NaCl: 100 mM; HCl: 10 mM; Denatonium: 20 mM; IMP: 10 mM; MPG: 100 mM; a mixture of 100 mM MSG + 0.5 mM IMP) were delivered at room temperature into the flow chamber by gravity flow at a rate of 0.5 ml/sec for 30 sec. Between applications of the taste stimuli, the tongue was rinsed with distilled water for at least 1 min. Ammonium chloride (NH4Cl) at 100 mM was presented frequently throughout the recordings to serve as a reference stimulus. The whole nerve responses to the lingual application of taste substances were amplified, integrated at a time constant of 1 second, converted to digital signals and analyzed offline. The magnitudes of the responses to tastants were determined as the areas below the curves and normalized by the response to 100 mM NH4Cl from the same animal, and then averaged for animals from the same genotype group. Paired comparisons of the averaged values were analyzed between the wild-type control and kcnq1-null mice using student t-tests. P value <0.05 was the criterion for statistical significance.
RESULTS
1) Isolation of KCNQ1
To isolate genes that are specifically or more abundantly expressed in intragemmal taste bud cells than in non-gustatory lingual epithelial cells, we modified a single cell procedure that had been employed to successfully identify several taste signaling molecules, including the G protein subunits, Gβ3γ13, the transient receptor potential ion channel TrpM5 and the voltage-gated chloride channel ClC-4 and its variant ClC-4A (Huang et al., 2005; Huang et al., 1999; Perez et al., 2002). We utilized a transgenic mouse line that labeled the α-gustducin-expressing taste bud cells with the green fluorescent proteins (GFP). Green fluorescent, non-fluorescent taste bud cells and non-gustatory lingual epithelial cells were isolated from the circumvallate and foliate papillae, and from a piece of lingual epithelium devoid of taste buds. cDNAs were synthesized from mRNAs of individual cells, amplified by polymerase chain reactions (PCR), and used to construct single taste bud cell cDNA libraries. These libraries were subtractively screened with probes from the same taste bud cell as well as with probes from a non-taste epithelial cell to isolate differentially expressed clones, which were sequenced and searched using the Blast program against GenBank databases (Figure 1). Blast search results indicated that one of these clones matched the 3’-end cDNA sequence of the voltage-gated potassium channel, KCNQ1. To isolate the full-length coding cDNA sequence, PCR reactions were carried out with cDNAs from taste papillae and a set of PCR primers covering the full coding sequence. Sequencing analysis of the sole PCR product confirmed that the kcnq1 cDNA expressed in taste buds is identical to the reference sequence (GenBank Accession number: NM_008434).
2.) Expression of KCNQ1 in taste bud cells
To determine the localization of the KCNQ1 channel protein in taste buds, we performed immunocytochemistry with two anti-KCNQ1 antibodies from rabbit and from goat, respectively. The specificity of these two antibodies was proven in previous studies (Liao et al., 2005; Rasmussen et al., 2004). In this paper we again validated with 4 taste tissue sections from each of 6 animals from three genotype groups: 2 wild-type (kcnq1+/+), 2 heterozygote (kcnq1+/−) and 2 homozygote (kcnq1−/−). It seemed that the expression of KCNQ1 proteins was normal in both wild-type and heterozygous mice, but undetectable in homozygous mice, whereas the expression of the taste signaling molecules Tas1R3 receptor, α-gustducin and TRPM5 appeared to be normal (Figure 2).
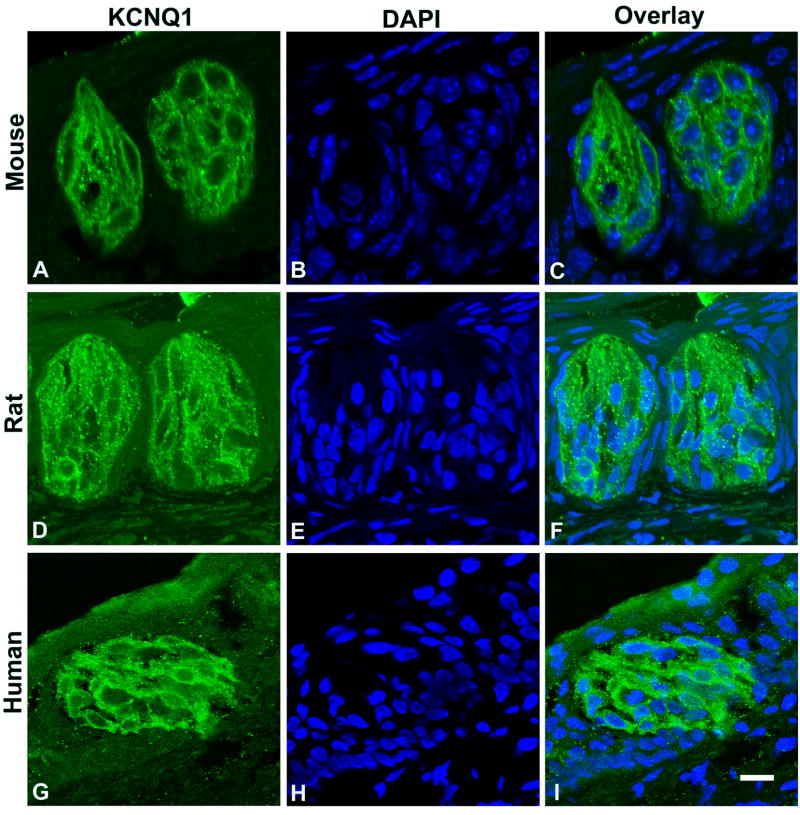
The immunostaining pattern in Figure 2 indicated that the expression of KCNQ1 proteins was restricted to taste buds. To determine how widely the KCNQ1 expression is in taste bud cells, we double stained 10 taste sections from 2 human subjects, 30 more taste sections from 3 rats and another 30 from 3 mice with both anti-KCNQ1 antibody and 4’,6’-diamidino-2-phenylindole (DAPI) to visualize both KCNQ1-expressing cells and all nuclei present on each section. Examination of the confocal laser scanning microscope images indicated that nuclei were largely absent near the taste pores (Figure 3), which was consistent with our previous observation (Wang et al., 2007). The KCNQ1 staining covered the entire taste bud, which was surrounded by cells with long nuclei orientated along the sides. All cells in a taste bud, including those near the serosal bottom, displayed KCNQ1 antibody staining, indicating that all human and rodent mature taste bud cells and possibly some precursor cells express KCNQ1 (Figure 3).
Figure 3.
Single plane confocal images of double stained taste tissue sections with anti-KCNQ1 (green) antibody and DAPI (blue). Longitudinal sections of mouse (top panels: A, B and C) and rat (middle panels: D, E and F) circumvallate taste buds, and an oblique section of a human circumvallate taste bud (bottom panels: G, H and I) showed that nearly all intragemmal nuclei were enveloped in a KCNQ1-stained cell membrane. Scale bar: 20 μm.
However, since DAPI stained the nuclei while the vast majority of KCNQ1 channels were localized to the cytoplasmic membrane, the two fluorescent signals did not overlap. Therefore it was possible that the KCNQ1 staining might be from cell membranes of wrapping type I cells instead of those of the same cell. To rule out that possibility, we carried out double immunostaining with antibodies against type II, III and IV taste cell markers.
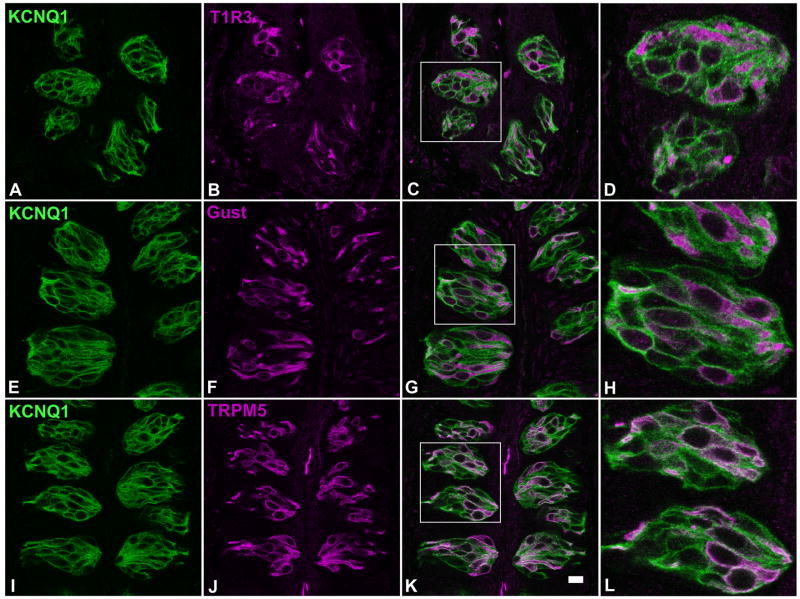
Specific antibodies against the following three type II cell markers were utilized: 1) Tas1R3, one of the two subunits of the dimeric receptors for sweet and umami tastes (Kitagawa et al., 2001; Li et al., 2002; Max et al., 2001; Montmayeur et al., 2001; Nelson et al., 2002; Nelson et al., 2001; Sainz et al., 2001); 2) α-gustducin, a G protein subunit that is involved in bitter, sweet and umami taste transduction (McLaughlin et al., 1992; Wong et al., 1996); 3) TRPM5, a transient receptor potential ion channel that is a common denominator for bitter, sweet and umami signaling cascades (Hofmann et al., 2003; Liu and Liman, 2003; Perez et al., 2002; Prawitt et al., 2003; Zhang et al., 2003). Confocal laser scanning microscopy images of 60 taste tissue sections from 5 mice indicated that nearly all Tas1R3-, α-gustducin- or TRPM5-expressing taste bud cells also express the KCNQ1 channel (Figure 4). This result demonstrates that almost all type II taste receptor cells express this channel.
Figure 4.
Single plane confocal images showed that a large number of mouse circumvallate taste bud cells were immunoreactive to an anti-KCNQ1 antibody (left panels A, E and I). Double immunostaining with antibodies against Tas1R3 (T1R3, panel B), α-gustducin (Gust, panel F) and TRPM5 (panel J), indicated that KCNQ1 was present in nearly all cells that were immunoreactive to these three antibodies (superimposed images: C, G and K, and their high magnification images: D, H and L). Scale bar: 20 μm.
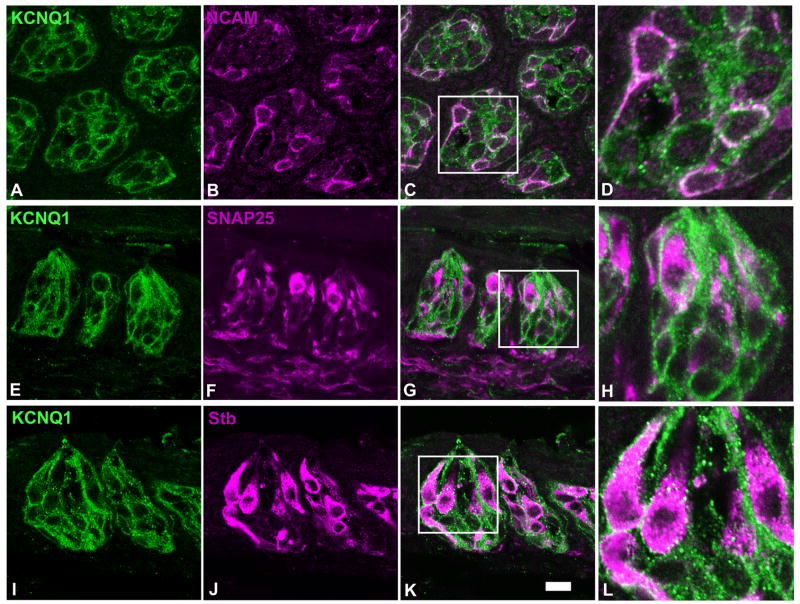
To confirm that KCNQ1 is also present in synapse-forming taste bud cells, double immunostaining was performed with antibodies against the type III cell marker neuronal cell adhesion molecule (NCAM) (Nelson and Finger, 1993), and synaptic proteins: SNAP-25 and synaptobrevin-2; the former, like NCAM, is found only in type III cells whereas the latter is present in both type II and III taste bud cells (Yang et al., 2000a; Yang et al., 2004). Since the primary antibody against SNAP-25 is a mouse monoclonal antibody, 60 taste tissue sections from 5 rats were used in this double-staining experiment to minimize any intrinsic background signals. Confocal laser scanning microscopy images showed that NCAM, like KCNQ1 channels, seemed to be present only on the cytoplasmic membranes of taste bud cells whereas SNAP-25 and synaptobrevin-2 were found largely in the cytosol with some proteins on the cytoplasmic membranes (Figure 5). The imaging results indicated that nearly all taste bud cells immunoreactive to the antibodies against NCAM, SNAP-25 or synaptobrevin-2 were immunoreactive to the anti-KCNQ1 antibody whereas many more cells exhibited the KCNQ1-like immunoreactivity (Figure 5). The results also showed that these two synaptic proteins, especially SNAP-25, occur in intragemmal and perigemmal nerve fibers, while KCNQ1 is absent in these fibers, suggesting that KCNQ1 channels only function within the taste bud cells, and do not participate in the activity of these innervating and surrounding nerve fibers.
Figure 5.
Single plane confocal images of double immunostaining of rat taste sections with antibodies against KCNQ1 (panels A, E and I), NCAM (panel B), SNAP-25 (panel F) and synaptobrevin-2 (Stb, panel J). To clearly display subcellular colocalization of the immunostaining, one transverse (top row) and two longitudinal (middle and bottom rows) sections were used. Overlays (panels C, G and K) and their high magnification images (panels D, H and L) indicated that the vast majority of SNAP-25 or synaptobrevin-2-expressing taste bud cells also expressed KCNQ1. Note: SNAP-25 and synaptobrevin-2 antibodies also stained extragemmal nerve fibers whereas KCNQ1 staining was largely restricted to the intragemmal cells. Scale bar: 20 μm.
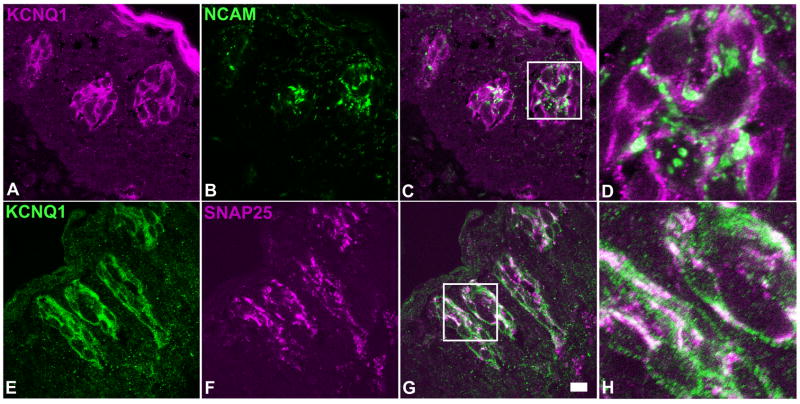
To determine whether the co-expression patterns of KCNQ1 with other taste cell markers also exist in human taste buds, we performed similar double immunolabeling experiments with 10 taste tissue sections of 2 human subjects, and antibodies against NCAM and SNAP-25. Confocal laser scanning microscopy images indicated that KCNQ1 was co-localized with NCAM and SNAP-25 to human taste bud cells (Figure 6).
Figure 6.
Single plane confocal images of double immunostaining of human circumvallate sections with antibodies against KCNQ1 and two type III cell markers: NCAM and SNAP-25. Upper panels: a transverse section stained with anti-KCNQ1 (A) and anti-NCAM (B) antibodies; Lower panels: a longitudinal section stained with anti-KCNQ1 (panel E) and anti-SNAP-25 (panel F) antibodies. Overlay of the images (panels C and G) and their high magnification images (panels D and H) showed that all NCAM or SNAP-25 -immunoreactive human taste bud cells displayed KCNQ1 antibody immunoreactivity. Note: To rule out any possible fluorophore effect on imaging, the secondary antibodies conjugated with different fluorophores were used to visualize the KCNQ1 staining on the sections. Scale bar: 20 μm.
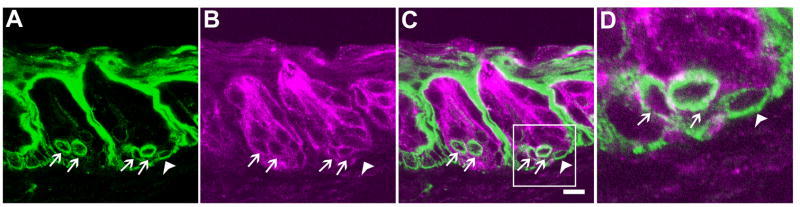
To verify the expression of KCNQ1 in taste bud precursor cells, we carried out the double immunostaining on 30 taste tissue sections from 3 rats with cytokeratin 14 antibodies. Cytokeratin 14 is a member of the keratin family that is a group of intermediate filaments. Cytokeratin 14 is known to be present in immature taste bud cells (Asano-Miyoshi et al., 2008). Confocal laser scanning microscopy images showed that cytokeratin 14 is mostly detected in the intragemmal epithelial cells, but also in some basal cells in taste buds (Figure 7). Most cytokeratin 14-expressing taste bud cells also expressed KCNQ1 (arrows in Figure 7). But occasionally, we observed that in a few cytokeratin-14 positive basal cells the KCNQ1 signal was undetectable (arrowhead in Figure 7).
Figure 7.
Single plane confocal images of double immunostaining of rat circumvallate sections with antibodies against cytokeratin 14 (A) and KCNQ1 (B). The overlay (panel C) and its high magnification image (panel D) showed that most of cytokeratin 14-immunoreactive intragemmal cells were also KCNQ1-immunoreactive (arrows). And one cell at the very bottom of a taste bud was not labeled by KCNQ1 antibody (arrowhead). Note the prolonged exposure of panel B to visualize any residual KCNQ1 antibody staining in the section. Scale bar: 20 μm.
3) Characterization of responses of kcnq1-null animals to taste stimuli
The widespread expression of the KCNQ1 channel among taste bud cells across different species suggests that this channel may play a fundamental role in taste bud development and physiology. Immunostaining of taste tissue sections from the kcnq1 null mutant mice with antibodies against taste signal transduction components indicated that the gross structure of the taste buds seemed normal and no noticeable changes in the expression patterns of these components were detected (Figure 2).
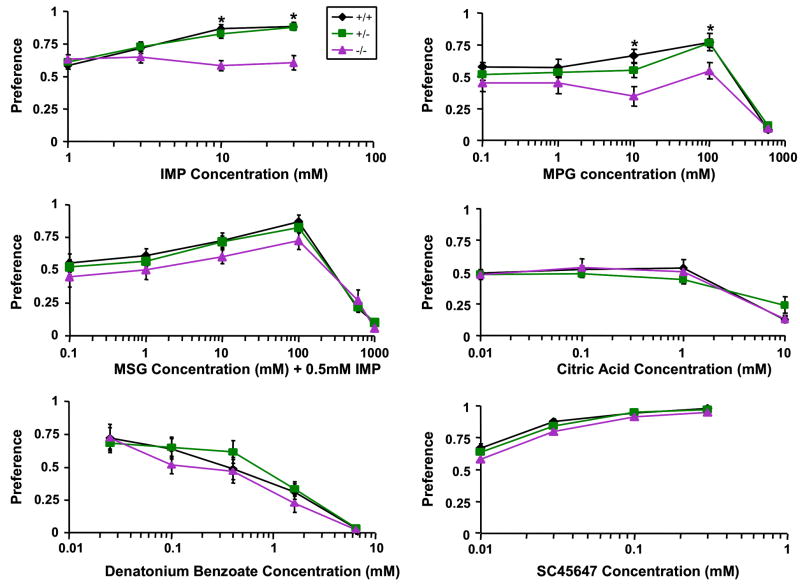
To characterize the possible roles of KCNQ1 channel in taste sensation, we carried out two experiments: 48-hour two bottle preference tests and chorda tympani nerve recordings. We used young adult knockout animals at age from 3 to 12 weeks. Older knockout animals tended to exhibit circling symptoms resulting from a deficient vestibular system, which could exacerbate bottle-position effect in the 48-hour two bottle preference tests. The behavioral data from individually caged mice showed that the kcnq1-knockout animals had a significantly reduced preference for IMP than did their heterozygous or wild-type littermates at the concentrations of 10 and 30 mM (p<0.05)(Figure 8). To confirm this diminished preference is true for other umami substances, additional tests were performed with MPG and with a mixture of MSG with 0.5 mM IMP. MPG at 10 and 100 mM were significantly less preferred by the knockout mice than by the heterozygous or wild-type littermates. Interestingly, the mixtures of MSG and IMP tended to be less preferred as well, but the reduction in preference was not statistically significant. However, the preferences of the mutant, heterozygous and wild-type mice for solutions of other taste qualities: SC45647 (sweet), sour (citric acid) and denatonium (bitter) were similar (Figure 8). The preference tests for NaCl were also performed at three representative concentrations (37.5, 75, 150 mM) before the animals started to display circling behavior and no significant differences were found among these three groups of mice (data not shown).
Figure 8.
Preferences of kcnq1-null, heterozygous and wild-type mice for taste solutions. *Significant difference between the knockouts and heterozygotes or wild-types.
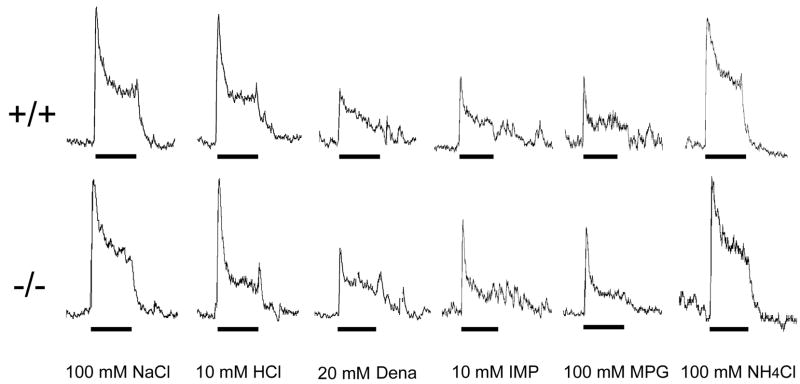
To examine whether the diminished preference of umami substances in kcnq1-null animals was attributable to the deficiency in the peripheral taste transduction, we performed gustatory nerve recordings from two kcnq1-knockout mice and three wild-type littermates. The integrated responses of chorda tympani nerves from the knockout animals seemed to be as robust as those from their wild-type littermates and no significant difference was observed in these responses (Figure 9).
Figure 9.
Integrated responses of the chorda tympani nerve of wild-type (+/+) and kcnq1-null (−/−) mice to tastants. Both wild-type and kcnq1-knockout mice showed robust responses to 100 mM NaCl, 10 mM HCl, 20 mM denatonium (Dena), 10 mM IMP, 100 mM MPG and the reference stimulus: 100 mM NH4Cl, and no significant differences in these responses between the two genotypes were found. Horizontal bars under nerve recordings show 30-second periods of taste stimulus application to the tongue.
DISCUSSION
To better understand taste bud cell turnover and taste signal transduction, particularly the involvement of voltage-gated ion channels in these processes, it is necessary to identify genes that are selectively expressed in taste bud cells at various maturation stages. The single cell approach has been effective in isolating cell type-specific genes such as pheromone receptor genes (Dulac and Axel, 1995; Herrada and Dulac, 1997; Matsunami and Buck, 1997; Pantages and Dulac, 2000). We have utilized this approach previously to isolate several taste signaling components, including the G protein subunits Gβ3, Gγ13, the transient receptor potential ion channel TRPM5, and the voltage-gated chloride channels ClC4 and ClC-4A (Huang et al., 2005; Huang et al., 1999; Perez et al., 2002). In this study, we identified the expression of the voltage-gated potassium channel KCNQ1 (Figure 1). The occurrence of the KCNQ1 transcripts and some preliminary studies were reported previously (Ohmoto et al., 2006; Wang et al., 2006). Here we found that KCNQ1 is present in nearly all cells throughout taste buds, including basal precursor cells, from both rodents and humans, that knockout of this gene seemed to impact only on rodent preference for umami substances in 48-hour two bottle preference tests, and that electrical recordings failed to detect significant change in the rodent chorda tympani nerve responses to all taste compounds tested, including umami substances. To our knowledge, this is the first study to demonstrate the expression of a voltage-gated ion channel in human taste papillae, and KCNQ1 is the only voltage-gated channel so far found in precursor taste bud cells.
1) Wide expression of KCNQ1 in taste buds
Immunocytochemistry with KCNQ1 antibody showed that the ion channel was present in many taste bud cells (Figure 2). Double staining with DAPI showed that nearly all DAPI-stained nuclei in taste buds were surrounded by KCNQ1 antibody-stained cytoplasmic membranes and the two fluorescent signals hardly overlapped (Figure 3). Since it is known that type I taste bud cells have sheetlike cytoplasmic processes that envelope nerve fibers and other taste cells (Pumplin et al., 1997), the non-overlapping pattern made it difficult to determine whether the KCNQ1 staining enveloping type II and III cells’ nuclei was from their own plasma membranes or from those of type I cells. Double immunostaining with antibodies against type II and III cell markers showed that KCNQ1 is co-localized to subsets of human and rodent taste bud cells with transmembrane or membrane-bound proteins: TRPM5, α-gustducin, Tas1R3, SNAP-25, synaptobrevin-2 and NCAM (Figures 4, 5 and 6), demonstrating that the KCNQ1-immunoreactivity encompassing type II and III cells’ nuclei was from their own cell membranes. On the other hand, since no any other cells are known to wrap type I cells, we can basically conclude from the double staining pattern of KCNQ1 antibody and DAPI (Figure 3) that type I cells also expressed this channel.
Cytokeratin 14 is a cytoskeleton protein that is present only in undifferentiated precursor cells in taste buds (Asano-Miyoshi et al., 2008). Double immunostaining showed that the majority of cytokeratin 14-expressing basal cells also express KCNQ1 channels. However, a few cytokeratin 14-immunoreactive cells that situated at the very bottom of taste buds were not labeled by KCNQ1 antibody (Figure 7). The apparent explanation for this observation is that these rare cells belong to an even younger group that has not started to transcribe this gene yet. But they are likely to synthesize this channel protein when they mature since this study has shown that all mature taste bud cells carry this channel.
KCNQ1 seems to be the most widely expressed voltage-gated ion channel found so far in taste bud cells. In situ hybridization data, which indicated the wide presence of KCNQ1 transcripts in taste bud cells, supported this notion (Ohmoto et al., 2006; Wang et al., 2006). A large body of electrophysiological data accumulated over the last two decades showed that almost all taste bud cells exhibited voltage-gated potassium currents (Bigiani et al., 2003; Chen and Herness, 1997). It is possible that KCNQ1 is the channel that renders all taste bud cells the voltage-gated potassium currents, although it is possible that additional voltage-gated potassium channels may contribute to these currents as well.
2) Possible physiological roles of KCNQ1 in gustation
The Shaker-type K+ channel KCNQ1 is known to be critical to the normal function of cardiac and auditory systems. Mutations in KCNQ1 have resulted in deafness (Jervell and Lange-Nielsen (JLN) syndrome) and cardiac arrhythmia (long QT syndrome) (Neyroud et al., 1997; Schwartz et al., 1975). Kcnq1-null animal models also display similar symptoms of the long QT and JLN syndromes (Casimiro et al., 2001). The widespread expression of KCNQ1 in taste buds suggests that as in cardiac and auditory systems, KCNQ1 may play a fundamental role in taste bud development and function. However, two bottle behavioral tests indicated that the sole altered taste preference by the kcnq1-null mutation was the diminished liking for umami substances. Yet the gustatory nerve recordings suggested that even this change may not stem from the taste transduction in taste buds. One explanation for this apparently striking difference in the KCNQ1’s role between gustatory system and cardiac and auditory systems is that neither the long-term 48-hour two-bottle preference tests nor the whole gustatory nerve recordings were sensitive enough to detect subtle effects of the channel on taste bud cells’ physiology, for example, cytoplasmic membrane potential repolarization, which can be discerned using taste bud cell recordings. Another possible explanation is that the role of KCNQ1 in taste bud cells has been compensated by other potassium channels. Indeed other members of the KCNQ channel subfamily as well as the KCNQ modulators--the KCNE proteins are also expressed in taste bud cells (Wang, Zhou and Huang, unpublished data). Multiple gene knockout, such as that for ATP receptors (Finger et al., 2005), may be needed to reveal the contribution of KCNQ channels or other potassium channels to taste bud cells’ electrical activity.
3) Reduced preference for umami substances in the kcnq1-knockout mice
The Kcnq1 knockout animals displayed reduced preference for the umami substances IMP and MPG (Figure 8). Their preference for MSG in the presence of IMP tended to be reduced as well although the reduction did not seem statistically significant. These knockout animals did not differ from the wild-type mice in preferences for substances of other taste qualities. The reduction in preference for the umami substances did not appear attributable to the dysfunction of anterior taste system since the chorda tympani nerve recordings showed unaltered responses to all orally applied umami compounds as well as other taste stimuli (Figure 9). Interestingly, similar discrepancy between behavioral and nerve recording data in response to umami stimuli have been reported in other mutant mice: animals with null mutations in α-gustducin or IP3 receptor IP3R3 showed a nearly abolished behavioral preference but displayed considerable nerve responses to umami compounds (He et al., 2004; Hisatsune et al., 2007). It is not certain what causes this discrepancy. However, in the case of kcnq1 knockout, it is possible that the reduction in behavioral response may result from postingestive effect in the gut as explained below.
KCNQ1 is known to be prominently expressed in the mouse kidney and gastrointestinal tract (Dedek and Waldegger, 2001). Disruption of the kcnq1 gene resulted in gastric hyperplasia, impaired Cl− secretion and Na+/K+ reabsorption (Lee et al., 2000; Vallon et al., 2005). And these symptoms may have led to reduced preference or increased aversion to some umami substances. Interestingly, umami, sweet and bitter receptors are also found in the GI tract (Dyer et al., 2005; Wu et al., 2002). The taste receptor Tas1R3, which is the common subunit of both umami and sweet receptors, plays an important role in the regulation of expression of the sodium-dependent glucose transporter isoform 1 in the absorption of dietary sugars (Margolskee et al., 2007). It is tempting to postulate that Tas1R receptors and KCNQ1 channels directly or indirectly act together regulating amino acid absorption whereas the kcnq1 knockout may diminish a rewarding postingestive effect, resulting in reduced preference for umami compounds. Further studies are needed to characterize the role of KCNQ1 in postingestive effect.
In summary, we have identified a voltage-gated potassium channel, KCNQ1, which is expressed in almost all taste bud cells, including the immature basal cells. Initial characterization of kcnq1-null mice indicated that the mutant mice had normal gross taste bud structure and peripheral taste transduction. Further studies using finer methods such as electron microscope and single fiber recordings may reveal subtle impact of the kcnq1 mutation on taste bud structure and physiology. Its wide expression in taste bud cells suggests that this channel is responsible for the previously recorded potassium currents in taste bud cells. Identifying additional genes that are preferentially expressed in taste bud cells can facilitate our understanding of taste bud cell differentiation and physiology.
Acknowledgments
We thank Maria Theodorides and Lynn A. Vo for technical support.
Grant Sponsor: National Institutes of Health: R01 DC007487 (LH), R03 DC007974 (HW), R01 DC00882 (AAB), R01 DC003155 (RFM); National Science Foundation: DBJ-0216310 (N. Rawson).
LITERATURE CITED
- Asano-Miyoshi M, Hamamichi R, Emori Y. Cytokeratin 14 is expressed in immature cells in rat taste buds. J Mol Histol. 2008;39(2):193–199. doi: 10.1007/s10735-007-9151-0. [DOI] [PubMed] [Google Scholar]
- Avenet P, Lindemann B. Noninvasive recording of receptor cell action potentials and sustained currents from single taste buds maintained in the tongue: the response to mucosal NaCl and amiloride. J Membr Biol. 1991;124(1):33–41. doi: 10.1007/BF01871362. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Li X, Li S, Neira M, Beauchamp GK, Azen EA. High-resolution genetic mapping of the sucrose octaacetate taste aversion (Soa) locus on mouse Chromosome 6. Mamm Genome. 2001;12(9):695–699. doi: 10.1007/s00335-001-2061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Voluntary sodium chloride consumption by mice: differences among five inbred strains. Behav Genet. 1998;28(2):117–124. doi: 10.1023/a:1021471924143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497(1):1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigiani A, Ghiaroni V, Fieni F. Channels as taste receptors in vertebrates. Prog Biophys Mol Biol. 2003;83(3):193–225. doi: 10.1016/s0079-6107(03)00058-0. [DOI] [PubMed] [Google Scholar]
- Casimiro MC, Knollmann BC, Ebert SN, Vary JC, Jr, Greene AE, Franz MR, Grinberg A, Huang SP, Pfeifer K. Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc Natl Acad Sci U S A. 2001;98(5):2526–2531. doi: 10.1073/pnas.041398998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Herness MS. Electrophysiological actions of quinine on voltage-dependent currents in dissociated rat taste cells. Pflugers Arch. 1997;434(3):215–226. doi: 10.1007/s004240050388. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sun XD, Herness S. Characteristics of action potentials and their underlying outward currents in rat taste receptor cells. J Neurophysiol. 1996;75(2):820–831. doi: 10.1152/jn.1996.75.2.820. [DOI] [PubMed] [Google Scholar]
- Cummings TA, Powell J, Kinnamon SC. Sweet taste transduction in hamster taste cells: evidence for the role of cyclic nucleotides. J Neurophysiol. 1993;70(6):2326–2336. doi: 10.1152/jn.1993.70.6.2326. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31(3):253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301(5634):850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Arch. 2001;442(6):896–902. doi: 10.1007/s004240100609. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26(15):3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83(2):195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33(Pt 1):302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Fine Structure of the Taste Bud. J Ultrastruct Res. 1965;12:328–350. doi: 10.1016/s0022-5320(65)80103-4. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310(5753):1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Avenet P, Kinnamon SC, Roper SD. Proton currents through amiloride-sensitive Na channels in hamster taste cells. Role in acid transduction. J Gen Physiol. 1992;100(5):803–824. doi: 10.1085/jgp.100.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci. 2004;24(35):7674–7680. doi: 10.1523/JNEUROSCI.2441-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90(4):763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K. Abnormal taste perception in mice lacking the type 3 inositol 1, 4, 5-trisphosphate receptor. J Biol Chem. 2007 doi: 10.1074/jbc.M705641200. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442(7105):934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Cao J, Wang H, Vo LA, Brand JG. Identification and functional characterization of a voltage-gated chloride channel and its novel splice variant in taste bud cells. J Biol Chem. 2005;280(43):36150–36157. doi: 10.1074/jbc.M507706200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2(12):1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104(15):6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiol Genomics. 2007 doi: 10.1152/physiolgenomics.00161.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK. Whole nerve chorda tympani responses to sweeteners in C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26(7):915–923. doi: 10.1093/chemse/26.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwayanagi M, Miyake M, Kurihara K. Voltage-dependent Ca2+ channel and Na+ channel in frog taste cells. Am J Physiol. 1983;244(1):C82–88. doi: 10.1152/ajpcell.1983.244.1.C82. [DOI] [PubMed] [Google Scholar]
- Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sevigny J, Kinnamon JC, Finger TE. The Candidate Sour Taste Receptor, PKD2L1, Is Expressed by Type III Taste Cells in the Mouse. Chem Senses. 2008;33(3):243–254. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JE, Neale EA. The role of the synaptic protein snap-25 in the potency of botulinum neurotoxin type A. J Biol Chem. 2001;276(16):13476–13482. doi: 10.1074/jbc.M010992200. [DOI] [PubMed] [Google Scholar]
- Keller JE, Neale EA, Oyler G, Adler M. Persistence of botulinum neurotoxin action in cultured spinal cord cells. FEBS Lett. 1999;456(1):137–142. doi: 10.1016/s0014-5793(99)00948-5. [DOI] [PubMed] [Google Scholar]
- Kinnamon JC, Taylor BJ, Delay RJ, Roper SD. Ultrastructure of mouse vallate taste buds. I. Taste cells and their associated synapses. J Comp Neurol. 1985;235(1):48–60. doi: 10.1002/cne.902350105. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun. 2001;283(1):236–242. doi: 10.1006/bbrc.2001.4760. [DOI] [PubMed] [Google Scholar]
- Lawton DM, Furness DN, Lindemann B, Hackney CM. Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci. 2000;12(9):3163–3171. doi: 10.1046/j.1460-9568.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- Lee MP, Ravenel JD, Hu RJ, Lustig LR, Tomaselli G, Berger RD, Brandenburg SA, Litzi TJ, Bunton TE, Limb C, Francis H, Gorelikow M, Gu H, Washington K, Argani P, Goldenring JR, Coffey RJ, Feinberg AP. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest. 2000;106(12):1447–1455. doi: 10.1172/JCI10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99(7):4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao T, Wang L, Halm ST, Lu L, Fyffe RE, Halm DR. K+ channel KVLQT1 located in the basolateral membrane of distal colonic epithelium is not essential for activating Cl− secretion. Am J Physiol Cell Physiol. 2005;289(3):C564–575. doi: 10.1152/ajpcell.00561.2004. [DOI] [PubMed] [Google Scholar]
- Lin W, Burks CA, Hansen DR, Kinnamon SC, Gilbertson TA. Taste receptor cells express pH-sensitive leak K+ channels. J Neurophysiol. 2004;92(5):2909–2919. doi: 10.1152/jn.01198.2003. [DOI] [PubMed] [Google Scholar]
- Lindemann B. Taste reception. Physiol Rev. 1996;76:719–766. doi: 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003;100(25):15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Hansen DR, Kim I, Gilbertson TA. Expression and characterization of delayed rectifying K+ channels in anterior rat taste buds. Am J Physiol Cell Physiol. 2005;289(4):C868–880. doi: 10.1152/ajpcell.00115.2005. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, Delay RJ, Roper SD, Kinnamon SC. Development of voltage-dependent currents in taste receptor cells. J Comp Neurol. 1996;365(2):278–288. doi: 10.1002/(SICI)1096-9861(19960205)365:2<278::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002;277(1):1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104(38):15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell. 1997;90(4):775–784. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- Mehta PP, Battenberg E, Wilson MC. SNAP-25 and synaptotagmin involvement in the final Ca(2+)-dependent triggering of neurotransmitter exocytosis. Proc Natl Acad Sci U S A. 1996;93(19):10471–10476. doi: 10.1073/pnas.93.19.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4(5):492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416(6877):199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJP, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106(3):381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nelson GM, Finger TE. Immunolocalization of different forms of neural cell adhesion molecule (NCAM) in rat taste buds. J Comp Neurol. 1993;336(4):507–516. doi: 10.1002/cne.903360404. [DOI] [PubMed] [Google Scholar]
- Neyroud N, Tesson F, Denjoy I, Leibovici M, Donger C, Barhanin J, Faure S, Gary F, Coumel P, Petit C, Schwartz K, Guicheney P. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet. 1997;15(2):186–189. doi: 10.1038/ng0297-186. [DOI] [PubMed] [Google Scholar]
- Ohmoto M, Matsumoto I, Misaka T, Abe K. Taste receptor cells express voltage-dependent potassium channels in a cell age-specific manner. Chem Senses. 2006;31(8):739–746. doi: 10.1093/chemse/bjl016. [DOI] [PubMed] [Google Scholar]
- Pantages E, Dulac C. A novel family of candidate pheromone receptors in mammals. Neuron. 2000;28(3):835–845. doi: 10.1016/s0896-6273(00)00157-4. [DOI] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci U S A. 2003;100(25):15166–15171. doi: 10.1073/pnas.2334624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin DW, Yu C, Smith DV. Light and dark cells of rat vallate taste buds are morphologically distinct cell types. J Comp Neurol. 1997;378(3):389–410. doi: 10.1002/(sici)1096-9861(19970217)378:3<389::aid-cne7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rasmussen HB, Moller M, Knaus HG, Jensen BS, Olesen SP, Jorgensen NK. Subcellular localization of the delayed rectifier K(+) channels KCNQ1 and ERG1 in the rat heart. Am J Physiol Heart Circ Physiol. 2004;286(4):H1300–1309. doi: 10.1152/ajpheart.00344.2003. [DOI] [PubMed] [Google Scholar]
- Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24(4):938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter TA, Dvoryanchikov GA, Chaudhari N, Roper SD. Acid-sensitive two-pore domain potassium (K2P) channels in mouse taste buds. J Neurophysiol. 2004 doi: 10.1152/jn.00273.2004. [DOI] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. Embo J. 2007;26(3):657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper S. Regenerative impulses in taste cells. Science. 1983;220(4603):1311–1312. doi: 10.1126/science.6857254. [DOI] [PubMed] [Google Scholar]
- Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem. 2001;77(3):896–903. doi: 10.1046/j.1471-4159.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Periti M, Malliani A. The long Q-T syndrome. Am Heart J. 1975;89(3):378–390. doi: 10.1016/0002-8703(75)90089-7. [DOI] [PubMed] [Google Scholar]
- Stevens DR, Seifert R, Bufe B, Muller F, Kremmer E, Gauss R, Meyerhof W, Kaupp UB, Lindemann B. Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature. 2001;413(6856):631–635. doi: 10.1038/35098087. [DOI] [PubMed] [Google Scholar]
- Stone LM, Tan SS, Tam PP, Finger TE. Analysis of cell lineage relationships in taste buds. J Neurosci. 2002;22(11):4522–4529. doi: 10.1523/JNEUROSCI.22-11-04522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Grahammer F, Volkl H, Sandu CD, Richter K, Rexhepaj R, Gerlach U, Rong Q, Pfeifer K, Lang F. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci U S A. 2005;102(49):17864–17869. doi: 10.1073/pnas.0505860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou M, Brand J, Huang L. Inflammation activates the interferon signaling pathways in taste bud cells. J Neurosci. 2007;27(40):10703–10713. doi: 10.1523/JNEUROSCI.3102-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou M, Rong Q, Inoue M, Bachmanov AA, Margolskee RF, Pfeifer KE, Huang L. Expression of a Voltage-Gated Potassium Channel KCNQ1 in Taste Bud Cells. Chem Senses. 2006;31(AChemS Abstracts):A18. doi: 10.1002/cne.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzels RH, Kuijpers HJ, Lane EB, Leigh IM, Troyanovsky SM, Holland R, van Haelst UJ, Ramaekers FC. Basal cell-specific and hyperproliferation-related keratins in human breast cancer. Am J Pathol. 1991;138(3):751–763. [PMC free article] [PubMed] [Google Scholar]
- Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A. 2002;99(4):2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol. 2000a;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yang R, Ma H, Thomas SM, Kinnamon JC. Immunocytochemical analysis of syntaxin-1 in rat circumvallate taste buds. J Comp Neurol. 2007;502(6):883–893. doi: 10.1002/cne.21317. [DOI] [PubMed] [Google Scholar]
- Yang R, Stoick CL, Kinnamon JC. Synaptobrevin-2-like immunoreactivity is associated with vesicles at synapses in rat circumvallate taste buds. J Comp Neurol. 2004;471(1):59–71. doi: 10.1002/cne.20021. [DOI] [PubMed] [Google Scholar]
- Yang R, Tabata S, Crowley HH, Margolskee RF, Kinnamon JC. Ultrastructural localization of gustducin immunoreactivity in microvilli of type II taste cells in the rat. J Comp Neurol. 2000b;425(1):139–151. doi: 10.1002/1096-9861(20000911)425:1<139::aid-cne12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. "Type III" cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440(1):97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Shigemura N, Sanematsu K, Yasumatsu K, Ishizuka S, Ninomiya Y. Taste responsiveness of fungiform taste cells with action potentials. J Neurophysiol. 2006;96(6):3088–3095. doi: 10.1152/jn.00409.2006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112(3):293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci U S A. 2005;102(31):11100–11105. doi: 10.1073/pnas.0501988102. [DOI] [PMC free article] [PubMed] [Google Scholar]