Abstract
Androgen levels decline with aging. Some androgens may exert anti-anxiety and cognitive-enhancing effects; however, determining which androgens have anxiolytic-like and/or mnemonic effects is of interest given the different mechanisms that may underlie some of their effects. For example, the 5α-reduced metabolite of testosterone (T), dihydrotesterone, can be further converted to 5α-androstane,17β-diol-3α-diol (3α-diol) and 5α-androstane,17β-diol-3β-diol (3β-diol), both of which bind with high affinity to the beta isomer of the intracellular estrogen receptor beta (ERβ). However, androsterone, another metabolite of T, does not bind well to ERβ. To investigate the effects of T metabolites, male rats were subjected to gonadectomy then implanted with silastic capsules of 3α-diol, 3β-diol, androsterone, or oil control. After recovery, the rats were tested in elevated plus maze (EPM), light/dark transition (LD), and Morris water maze (MWM). 3α-diol both decreased anxiety-like behavior in the EPM and LD, and increased cognition in MWM, while 3β-diol improved cognition in MWM, but had no effects on anxiety behavior, compared to vehicle or androsterone. These data suggest that the actions of 3α-diol and 3β-diol at ERβ may be responsible for some of testosterone’s anti-anxiety and cognitive-enhancing effects.
Keywords: Aging, Androsterone, Anxiety, Cognition, Testosterone, 3alpha-diol, 3beta-diol
Introduction
Aging in men is accompanied by a gradual decline in endogenous androgen levels, which can have negative effects on anxiety and cognition. In support, waning testosterone (T) levels in men are associated with increased anxiety and decreased visuospatial abilities (Janowsky et al. 1994; Li et al. 2002; Janowsky 2006). Additionally, young hypogonadal men, with low endogenous T and dihydrotestosterone (DHT) levels, are more susceptible to anxiety and/or depressive disorders, and exhibit decreased performance in cognitive tasks (Howell and Shalet 2001; Kaminetsky 2005). This is additionally observed in men prescribed T-lowering drugs like leuprolide acetate (Lupron) for treatment of prostate cancer. Lupron decreases endogenous T levels to those of castration, and results in decreased mood, anti-anxiety, and cognition (Heyns et al. 2003; Palomba et al. 2008). However, administration of T to aging men reinstates their affective and cognitive performance (Alexander et al. 1998; Delhez et al. 2003; Janowsky 2006). Conversely, men with higher levels of T, and its metabolites, have reported elevated moods, lower levels of depression, and increased cognitive performance (Earls 1987; Gouchie and Kimura 1991). Therefore, in men, T and its metabolites may have anti-anxiety and cognitive-enhancing effects.
Animal studies of androgen extirpation and replacement have demonstrated that androgens can have anti-anxiety and cognitive-enhancing effects. Removal of an animal’s testes—their primary source of endogenous androgens—through gonadectomy (GDX) results in increased anxiety-like and decreased cognitive behavior (Frye and Seliga 2001; Edinger and Frye 2004); however, replacement of extirpated androgens to GDX animals can reverse anxiety-like and cognitive detriments (Edinger and Frye 2004, 2005; Walf et al. 2004). This is similarly observed in rodents administered androgens, with decreased anxiety-like behavior and enhanced learning and memory (Ceccarelli et al. 2001; Frye and Seliga 2001; Edinger and Frye 2004; Edinger et al. 2004). Thus, androgens can mediate both anxiety-like and cognitive processes of male rats.
Testosterone’s mediation of anxiety-like and cognitive processes may be through the actions of its metabolites (Handa et al. 2007). T is metabolized by 5α-reductase to DHT, which is then converted to 5α-androstane-3α,17β-diol (3α-diol) and 5α-androstane-3β,17β-diol (3β-diol; Brown et al. 1994; Frye et al. 2007). While T and DHT bind with high affinity to androgen receptors (ARs; Roselli et al. 1987), 3α-diol and 3β-diol bind with greater affinity to estrogen receptor beta (ERβ; Roselli et al. 1987), while 3α-diol may also bind to GABA/benzodiazepine receptors (GBRs; Gee 1988). Studies implicate activation of ERβ as a mediator of cell proliferation, learning and memory (Rissman et al. 2002; Zhang et al. 2002), as well as reducing anxiety-like behavior. In animals administered diarylpropionitrile—a selective estrogen receptor modulator specific to ERβ and not the alpha-isoform of the receptor—there is a reduction in anxiety-like behavior for the open field, elevated plus maze (EPM), elevated zero maze, social interaction tasks (Walf et al. 2008, 2009). Additionally, during the proestrous phase, when endogenous E2 levels are high, wildtype, but not ERβ knockout mice, exhibit decreased anxiety-like behavior and increased cognition in hippocampally mediated tasks (Walf et al. 2008, 2009). These findings indicate that the anti-anxiety and cognitive-enhancing effects of T are likely mediated by its metabolites that adhere to the ERβ substrate.
To delineate ERβ’s role in mediating anti-anxiety and cognitive-enhancing effects, the following experiment was conducted. In order to isolate androgenic actions at ERβ, as opposed to other ligands that androgens may bind to, we utilized GDX male rats, which were then chronically administered 3α-diol (ERβ/GBR agonist), 3β-diol (ERβ agonist), androsterone (GBR agonist), or vehicle. These androgens were chosen because they are readily metabolized from T, and their binding affinities are known (Frye et al. 2007; Roselli et al. 1987; Gee 1988). Anxiety-like and cognitive behavior was assessed in the EPM, light/dark transition (LD), and the Morris water maze (MWM). We hypothesized that if ERβ activation is responsible for T’s anti-anxiety and cognitive effects, then animals given implants of the ERβ agonists 3α-diol and 3β-diol will display similar cognitive and anxiolytic-like improvements seen with T administration; however, those that receive androsterone will exhibit behavior that is not statistically different from vehicle administration in both anxiety-like and cognitive tasks.
Methods
These methods were pre-approved by the Institutional Animal Care and Use Committee at the University at Albany- SUNY.
Animals and housing
Subjects (N = 99) were male Long-Evans rats, approximately 55 days old, obtained from our in-house breeding colony (original stock from Taconic Farms, Germantown, NY). Rats were group-housed (3–4 per cage) in polycarbonate cages (45 × 24 × 21 cm) in the Laboratory Animal Care Facility of The Life Sciences Research Building at The University at Albany-SUNY in a temperature-controlled room (21 ± 1°C) that was maintained on a 12:12 reversed light cycle (lights off at 0800 hours). Rats had continuous access to Purina Rat Chow and tap water in their home cages.
Surgery
Young adult rats were GDX under xylazine (12 mg/kg; Bayer, Shawnee Mission, KS) and ketamine (60 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) anesthesia at least 3–6 weeks before behavioral testing.
Androgen administration
Rats were randomly assigned to receive a single silastic implant (1.57 mm inner diameter, 3.18 mm outer diameter) of crystalline 3α-diol, 3β-diol, androsterone, or cholesterol vehicle (Sigma, St. Louis, MO; 10 mm/animal). Drugs were chosen for their varying affinity for different substrates. 3α-diol binds to both ERβ and GBRs (Gee et al. 1988), 3β-diol binds just to ERβ (Edinger and Frye 2007a), while androsterone will bind to GBRs (Fernández-Guasti and Martínez-Mota 2005). Radioimmunoassay (RIA) has confirmed that silastic implants provide a continuous amount of hormone to the animal, to produce circulating and brain levels that are comparable to physiological concentrations in intact male rats (Edinger and Frye 2006). Furthermore, RIA has also confirmed that animals administered oil/cholesterol vehicle do not have increased levels of hormones compared to GDX animals (Edinger and Frye 2006).
Procedure
This was a mixed, between- and within-subjects experimental design. Rats were assigned randomly to a hormone and/or vehicle condition. After receiving the implants, rats were tested repeatedly so that their performance could be assessed in each behavioral task (described below) once. Behavioral data were collected by trained observers and simultaneously video-recorded with a video-tracking system (Any-maze-Stoelting, Wood Dale, IL).
Behavioral testing
Elevated plus maze
The EPM was situated in a brightly lit room and consisted of four arms (two open without walls and two enclosed by 30 cm high walls) 49 cm long and 10 cm wide, elevated 50 cm off the ground. Rats were placed at the junction of the open and closed arms and the number of entries and time spent on the open and closed arms were recorded (as per Frye et al. 2000). Total arm entries made in the plus maze are an index of general motor behavior and an increase in time spent on the open arms indicates anti-anxiety behavior.
Light–dark transition task
The light/dark (LD) task, like EPM, and open field (Morgan and Pfaff 2002) takes advantage of the animals natural aversive reaction to bright white areas, and also is sensitive to steroid administration to produce consistent results in rats (Pan and Chen 2007; Schramm-Sapyta et al. 2007; Edinger and Frye. 2007b). Rats were placed on the side of a two-chambered box (30 × 40 × 40 cm) with white walls and floor and illuminated by a 40-watt light from above; the other side of the box was painted black and had a lid so it was not illuminated. The time spent on the light side of this chamber during 5 min compared to the dark side was recorded (Walf and Frye 2005). Increased time in the light side is indicative of anti-anxiety behavior.
Morris water maze
The MWM is used as a measure of spatial cognition. This experiment employed chronic regimens of several androgens, which effectively allows the hormones to be utilized by the animal during the critical memory acquisition/consolidation period that occurs in the 2 h following training (Packard 1998). We report the latency to find the hidden platform during the testing phase of the experiment as an index of spatial learning (Frye and Reed 1998; Morris 1984; Vongher and Frye 1999; Vorhees et al. 2009).
On day 1, the animals were trained in the cognitive spatial task. We filled a large circular water tank (175 cm diameter, 71 cm deep) with water (20–25°C), and then visually divided it into four quadrants. A clear Plexiglas platform with a top that measures 5.3 cm × 5.3 cm was placed in one of the quadrants 30 cm from the side of the pool. The water level was filled so that it was 2.5 cm above the top of the hidden platform. White toxic-free tempera paint was added to the water to make it appear opaque, and obscure the platform. The rat was then placed in the pool in one of the four quadrants. The rat was given 1 min to find the hidden platform. This was done four times until the rat has been placed in each of the four quadrants once.
On day 2, the animals were spatially tested. The pool was filled the same way, only with the platform removed from the pool, then tempera paint was added, and the rat was placed into one of the quadrants. We counterbalanced the quadrants, so that the rats were not always placed in the same one. Additionally, the animal was never placed in the quadrant where the platform was located. The amount of time it took the animal to find where the hidden platform was located was considered an index of its cognitive abilities.
Statistical analyses
One-way analyses of variance (ANOVAs), with Fisher’s post hoc tests, as appropriate, were used to evaluate effects of androgen condition (vehicle, 3α-diol, 3β-diol, or androsterone) on behavioral measures. The α level for statistical significance was a P-value of ≤0.05, a trend was considered P ≤ 0.10.
Results
Light/dark transition
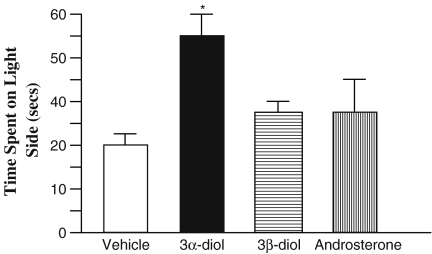
There was a significant main effect for chronic exposure to 3α-diol in time spent in the light (Fig. 1). Rats that received implants of 3α-diol spent significantly more time on the white side than those given vehicle (F3, 95 = 4.02, P < .01), and those given 3β-diol. Compared to vehicle and 3β-diol, rats given 3α-diol spent significantly more time on the white side.
Fig. 1.
Average time (+SEM) spent on the light side for the treatment groups. 3α-diol treatment (black) resulted in significantly greater time spent on the light side than did 3β-diol (horizontal lines) or vehicle (white)
Elevated plus maze
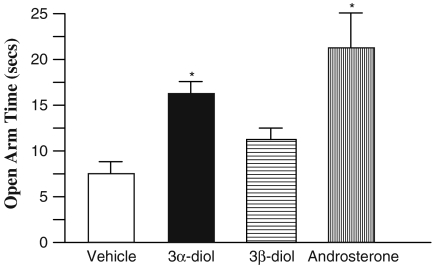
There was a significant main effect for chronic androsterone and 3α-diol treatment in time spent on the open arm (Fig. 2). Rats given androsterone or 3α-diol spent significantly more time on the open arm than did those given 3β-diol or vehicle (F3, 95 = 3.44, P < 0.05). There was no significant difference in closed arm entries (F3,95 = .129, P > 0.05) between any hormonal groups.
Fig. 2.
Average time (+SEM) spent on the open arms of the elevated plus maze (EPM) for the treatment groups. Androsterone treatment (vertical lines) resulted in significantly greater time spent on the open arms than 3β-diol (horizontal lines) and vehicle (white), while 3α-diol treatment (black) is significantly greater than vehicle
Morris water maze
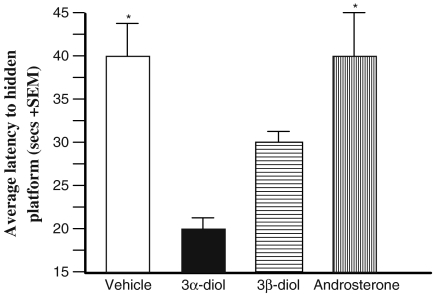
Rats with implants of 3α-diol and 3β-diol found the location of where the hidden platform was previously located during the training phase more readily than did control rats. Rats with implants of 3α-diol or 3β-diol had significantly shorter average latencies to find the hidden platform (Fig. 3) (F3,31 = 6.44, P < 0.01) than did rats with androsterone or cholesterol.
Fig. 3.
Average time (+SEM) that the rat took to find where the hidden platform was placed during the training period for implant treatment groups. Those given androsterone (vertical lines) or vehicle (white) treatments took significantly longer to locate where the hidden platform should have been located compared to the diol treatments
Discussion
Our postulation that actions at ERβ are important for mediating androgenic effects on anxiety-like and cognitive behavior was largely supported. Administration of 3α-diol, which has a high affinity for ERβ, was effective at enhancing anti-anxiety and cognitive behavior across all tasks. Administration of 3β-diol, which also has a high affinity for ERβ, was equally effective as 3α-diol in enhancing cognition in the MWM, but had no effects on anxiety measures. Slightly contrary to our hypothesis, androsterone, which binds to GBRs, but not ERβ, decreased anxiety-like behavior only in the EPM task, but in support of our hypothesis had no effects on cognition. Together, these findings suggest that actions at ERβ might be important for androgens’ anti-anxiety and cognitive-enhancing effects.
Our findings confirm past research indicating that androgens have anti-anxiety and cognitive-enhancing effects. Male GDX rats may experience increased anxiety, an effect that can be reversed with administration of T (Frye and Seliga 2001; Fernández-Guasti and Martínez-Mota 2003), and/or of T metabolites (Bitran et al. 1993; Edinger and Frye 2005). The present findings are consistent with past research illustrating the importance of T in anti-anxiety and cognitive improvement of rodents. Administration of DHT or 3α-diol has been found to be just as effective, if not more so, than sole administration of T, at reversing the negative effects of GDX on anxiety-like (Edinger and Frye 2004a, 2005) and cognitive processes (Ceccarelli et al. 2001; Edinger and Frye 2004). Our results confirm previous findings illustrating that administration of 3α-diol consistently enhances both anti-anxiety and cognitive behavior (Edinger et al. 2004; Frye et al. 2008). The present findings are also consistent with previous research indicating that ERβ may be important for androgens’ beneficial anxiolytic-like and cognitive effects (Edinger and Frye 2007a). Administration of antisense oligonucleotides for ERβ, but not ERα, to 3α-diol-replaced GDX rats resulted in a reversal of the beneficial effects of 3α-diol (Edinger and Frye 2007a). Similarly, in the present study, administration of 3α-diol, which can have actions at ERβ, was effective at reducing deficits in anxiety-like and cognitive behaviors caused by GDX. Furthermore, the positive effects of ERβ-binding androgens are exhibited in wildtype, but not mice deficient in ERβ (Frye et al. 2008). These findings, in conjunction with past research, indicate that ERβ is a likely target for the positive effects of T on anxiety-like and cognitive processes.
It must be taken into consideration that some effects observed may also be related to androgens’ actions at GBRs. Activation of these GBRs may produce sedative-like effects and anxiolysis (Da Settimo et al. 2007). In addition to binding to ERβ, 3α-diol also binds to GBRs to produce anxiolytic-like effects (Gee 1988). Furthermore, androsterone binds only to GBRs (Fernández-Guasti and Martínez-Mota 2005), and may produce anti-anxiety effects similar to 3α-diol in the EPM. However, activation of GBRs is often associated with an amnestic-like decline in cognitive abilities (Maubach 2003); since 3α-diol both decreased anxiety-like behavior and increased cognition, it is unlikely that its anti-anxiety effects were through activation of GBRs. Additionally, other studies have shown that attenuation of ERβ with antisense oligonucleotides eliminates both the cognitive and anxiolytic-like benefits associated with 3α-diol (Edinger and Frye 2007a). Furthermore, experiments have shown that, in conjunction with the addition of several antagonists for ERs, ARs, and GBRs, androsterone does have mild, task-specific, anti-anxiety effects, which are attenuated with the administration of flumazenil, which antagonizes GBRs (Frye et al. 2008; Fernández-Guasti and Martínez-Mota 2005). Furthermore, other studies have indicated that antagonizing GBRs in the presence of testosterone proprionate, which can be metabolized to androsterone, does not effect anxiolytic-like behavior (Fernández-Guasti and Martínez-Mota 2005). Given that previous experiments have indicated task-specific effects of androsterone for the EPM, this may indicate a particular sensitivity of that task to GBR ligation. However, the failure of androsterone to have any anxiolytic-like effects outside of the EPM in this study and others (Frye et al. 2008), indicates that reduction of anxiety-like behavior and increased cognitive ability may be more broadly and effectively mediated by androgenic activation of ERβ.
The current findings are clinically relevant for the aging male, in particular given the increased use of androgen-replacement therapies. The detrimental effects of ‘andropause’ has lead to increased interest in advancements in T-replacement therapies (Parsons et al. 2005), which unfortunately may carry an increased risk of prostate cancer (Guerini et al. 2005). Male hormone replacement therapy is becoming a more common treatment for men as they age and their T levels begin to decline, often resulting in cognitive and anxiety-like deficits, as well as sexual dysfunction (Heaton 2003). T levels can be correlated positively with the risk of prostate cancer metastasis (Raynaud 2006). In some, but not all studies, a correlation has been found between T replacement therapy, and an increased risk of prostate cancer in men (Marks et al. 2006). Development of prostate cancer often results from abnormal activation of ARs, which can be precipitated by circulating androgen levels (Guerini et al. 2005), in particular DHT because of its greater affinity for ARs than T, especially in the prostate (Grino et al. 1990; Kaufman and Pinsky 1983; Wilbert et al. 1983). As such, therapeutics often prescribed for some types of prostate cancer include finasteride, to prevent binding of DHT to those abnormal ARs (Rittmaster 2008). However, by blocking all T metabolites, these cancer therapies may be precipitating overall affective problems. The common duration of androgen-deprivation therapy (ADT) can result in unwanted changes in mood and cognition, and although these changes can revert back to baseline upon cessation of ADT (Cherrier et al. 2009), our findings suggest that an ADT that includes concomitant administration of 3α-diol/3β-diol may help to reduce some of these affective and cognitive detriments without enhancing the patient’s risk of prostate cancer. Our findings demonstrating 3α-diol’s ability to improve both affect and cognition holds great deal of promise for improving hormone replacement for aging men. Not only does 3α-diol not bind to AR, which limits its ability to increase prostate cancer risk, but activation of ERβ has powerful anti-metastatic properties, making it useful in restoring men’s vitality while preventing cancer progression (Guerini et al. 2005). By identifying precisely how T exerts its anxiolytic-like and cognitive-enhancing effects, and to which substrates its metabolites are binding, not only will men have access to better and safer hormone replacement options, but cancer treatments can be better tailored to provide increased survival rates with fewer detrimental side effects.
In conclusion, 3α-diol consistently produced improvements in anxiety-like behavior and cognition over vehicle control in LD transition, EPM, and MWM, while 3β-diol was equally effective in the MWM. Thus, actions at ERβ, through T’s metabolites 3α-diol and 3β-diol, may produce anti-anxiety and cognitive-enhancing effects.
Acknowledgments
This research was supported, in part, by grants from the Karo Bio Research Foundation, National Science Foundation (IBN03–16083), and National Institute of Mental Health (MH0676980). Additional assistance provided by Carolyn Koonce, Kassandra Edinger, and Alicia Walf is greatly appreciated.
Footnotes
This article has previously been published in issue 31/2, under 10.1007/s11357-009-9088-1
References
- Alexander GM, Swerdloff RS, Wang C, Davidson T, McDonald V, Steiner B, Hines M (1998) Androgen-behavior correlations in hypogonadal men and eugonadal men. II. Cognitive abilities. Horm Behav 33:85–94. doi:10.1006/hbeh.1998.1439 [DOI] [PubMed]
- Bitran D, Kellog CK, Hilvers RJ (1993) Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav 27:568–583. doi:10.1006/hbeh.1993.1041 [DOI] [PubMed]
- Brown TJ, Adler GH, Sharma M, Hochberg RB, MacLusky NJ (1994) Androgen treatment decreases estrogen receptor binding in the ventromedial nucleus of the rat brain: a quantitative in vitro autoradiographic analysis. Mol Cell Neurosci 5:549–555. doi:10.1006/mcne.1994.1067 [DOI] [PubMed]
- Ceccareli I, Scaramuzzino A, Aloisi AM (2001) Effects of gonadal hormones and persist pain on non-spatial working memory in male and female rats. Behav Brain Res 123:65–75. doi:10.1016/S0166-4328(01)00195-4 [DOI] [PubMed]
- Cherrier MM, Aubin S, Higano CS (2009) Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology (in press). doi: 10.1002/pon.1401 [DOI] [PMC free article] [PubMed]
- Da Settimo F, Taliani S, Trincavelli ML, Montali M, Martini C (2007) GABAA/Bz receptor subtypes as targets for selective drugs. Curr Med Chem 14:2680–2701. doi:10.2174/092986707782023190 [DOI] [PubMed]
- Delhez M, Hansenne M, Legros JJ (2003) Andropause and psychopathology: minor symptoms rather than pathological ones. Psychoneuroendocrinology 28:863–874. doi:10.1016/S0306-4530(02)00102-6 [DOI] [PubMed]
- Earls F (1987) Sex differences in psychiatric disorders: origins and developmental influences. Psych Dev 5:1–23 [PubMed]
- Edinger KL, Frye CA (2004) Testosterone’s analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Behav Neurol 118:1352–1364. doi:10.1037/0735-7044.118.6.1352 [DOI] [PubMed]
- Edinger KL, Frye CA (2005) Testosterone’s anti-anxiety and analgesic effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology 30:418–430. doi:10.1016/j.psyneuen.2004.11.001 [DOI] [PubMed]
- Edinger KL, Frye CA (2006) Intrahippocampal administration of an androgen receptor antagonist, flutamide, can increase anxiety-like behavior in intact and DHT-replaced male rats. Horm Behav 50:216–222. doi:10.1016/j.yhbeh.2006.03.003 [DOI] [PubMed]
- Edinger KL, Frye CA (2007a) Androgens effects to enhance learning and memory may be mediated in part by actions at estrogen receptor-beta in the hippocampus. Neurobiol Learn Mem 87:78–85. doi:10.1016/j.nlm.2006.07.001 [DOI] [PMC free article] [PubMed]
- Edinger KL, Frye CA (2007b) Sexual experience of male rats influences anxiety-like behavior and androgen levels. Physiol Behav 92(3):443–453. doi:10.1016/j.physbeh.2007.04.018 [DOI] [PubMed]
- Edinger KL, Lee B, Frye CA (2004) Mnemonic effects of testosterone and its 5α-reduced metabolites in the Conditioned Fear and inhibitory avoidance tasks. Pharmacol Biochem Behav 78:559–568. doi:10.1016/j.pbb.2004.04.024 [DOI] [PubMed]
- Fernández-Guasti A, Martínez-Mota L (2003) Orchidectomy sensitizes male rats to the action of diazepam on burying behavior latency: role of testosterone. Pharmacol Biochem Behav 75:473–479. doi:10.1016/S0091-3057(03)00142-4 [DOI] [PubMed]
- Fernández-Guasti A, Martínez-Mota L (2005) Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology 30:762–770. doi:10.1016/j.psyneuen.2005.03.006 [DOI] [PubMed]
- Frye CA, Reed TA (1998) Androgenic neurosteroids: anti-seizure effects in an animal model of epilepsy. Psychoneuroendocrinology 23(4):385–399. doi:10.1016/S0306-4530(98)00009-2 [DOI] [PubMed]
- Frye CA, Seliga AM (2001) Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci 1:371–381. doi:10.3758/CABN.1.4.371 [DOI] [PubMed]
- Frye CA, Petralia SM, Rhodes ME (2000) Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav 67:587–597. doi:10.1016/S0091-3057(00)00392-0 [DOI] [PubMed]
- Frye CA, Edinger K, Sumida K (2008) Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharm 33:1049–1061 [DOI] [PMC free article] [PubMed]
- Gee KW (1988) Steroid Modulation of the GABA/benzodiazepine receptor-linked chloride ionophore. Mol Neurobiol 2:291–317. doi:10.1007/BF02935636 [DOI] [PubMed]
- Gouchie C, Kimura D (1991) The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinlogy 16:323–334. doi:10.1016/0306-4530(91)90018-O [DOI] [PubMed]
- Grino PB, Griffen JE, Wilson JD (1990) Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology 126:1165–1172 [DOI] [PubMed]
- Guerini V, Sau D, Scacciancoce E, Rusmini P, Ciana P, Maggi A, Martini P, Katzenellenbogen BS, Martini L, Motta M, Poletti A (2005) The androgen derivative 5α-Androstane-3α,17β-diol inhibits prostate cancer cell migration through activation of estrogen receptor beta subtype. Cancer Res 65:5445–5453. doi:10.1158/0008–5472.CAN-04–1941 [DOI] [PubMed]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L (2007) An alternate pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav 53:741–752 [DOI] [PMC free article] [PubMed]
- Heaton JP (2003) Hormone treatments and preventive strategies in the aging male: whom and when to treat? Rev Urol 1:S16–S21 [PMC free article] [PubMed]
- Heyns CF, Simonin MP, Grosgurin P, Schall R, Porchet HV (2003) Comparative efficancy of triptorelinpamoate and leuprolide acetate in men with advanced prostate cancer. Br J Urol Int 92:226–231 [DOI] [PubMed]
- Howell S, Shalet S (2001) Testosterone deficiency and replacement. Horm Res 56:86–92. doi:10.1159/000048142 [DOI] [PubMed]
- Janowsky JS (2006) The role of androgens in cognition and brain aging in men. Neurosci 138:1015–1020. doi:10.1016/j.neuroscience.2005.09.007 [DOI] [PubMed]
- Janowsky JS, Oviatt SK, Orwoll ES (1994) Testosterone influences spatial cognition in older men. Behav Neurosci 108:325–332. doi:10.1037/0735-7044.108.2.325 [DOI] [PubMed]
- Kaminetsky JC (2005) Benefits of a new testosterone gel formulation for hypogonadal men. Clin Cornerstone 7:8–12. doi:10.1016/S1098-3597(05)80091-2 [DOI] [PubMed]
- Kaufman M, Pinsky L (1983) The dissociation of testosterone- and 5α-dihydrotestosterone-receptor complexes formed within cultured human genital skin fibroblasts. J Steroid Biochem 18:121–125. doi:10.1016/0022-4731(83)90077-8 [DOI] [PubMed]
- Li JY, Zhu JC, Dou JT, Bai WJ, Deng SM, Li M, Huang W, Jin H (2002) Effects of androgen supplementation therapy on partial androgen deficiency in the aging male: a preliminary study. Aging Male 5:47–51. doi:10.1080/713604649 [DOI] [PubMed]
- Marks LS, Mazer NA, Mostaghel E, Hess DL, Dorey FJ, Epstein JI, Veltri RW, Makarov DV, Partin AW, Bostwick DG, Macairan ML, Nelson PS (2006) Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA 296:2351–2361. doi:10.1001/jama.296.19.2351 [DOI] [PubMed]
- Maubach K (2003) GABA(A) receptor subtype selective cognition enhancers. Curr Drug Target CNS Neurol Disord 2(4):233–239. doi:10.2174/1568007033482779 [DOI] [PubMed]
- Morgan MA, Pfaff DW (2002) Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behav Brain Res 132(1):85–93. doi:10.1016/S0166-4328(01)00398-9 [DOI] [PubMed]
- Morris RGM (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60. doi:10.1016/0165-0270(84)90007-4 [DOI] [PubMed]
- Packard MG (1998) Posttraining estrogen and memory modulation. Horm Behav 34(2):126–139. doi:10.1006/hbeh.1998.1464 [DOI] [PubMed]
- Palomba S, Orio F, Falbo A, Oppedisano R, Tolino A, Zullo F (2008) Tibolone reverses the cognitive effects caused by leuprolide acetate administration, improving mood and quality of life in patients with symptomatic uterine leiomyomas. Fertil Steril 90:165–173 [DOI] [PubMed]
- Pan HZ, Chen HH (2007) Hyperalgesia, low-anxiety, and impairment of avoidance learning in neonatal caffeine-treated rats. Psychopharmacology (Berl) 191(1):119–125. doi:10.1007/s00213-006-0613-y [DOI] [PubMed]
- Parsons JK, Carter HB, Platz EA, Wright EJ, Landis P, Metter EJ (2005) Serum testosterone and the risk of prostate cancer: potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev 14:2257–2260. doi:10.1158/1055-9965.EPI-04-0715 [DOI] [PubMed]
- Raynaud JP (2006) Prostate cancer risk in testosterone-treated men. J Steroid Biochem Mol Biol 102:261–266. doi:10.1016/j.jsbmb.2006.09.032 [DOI] [PubMed]
- Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA (2002) Disruption of estrogen receptor b gene impairs spatial learning in mice. Proc Natl Acad Sci USA 99:3996–3901. doi:10.1073/pnas.012032699 [DOI] [PMC free article] [PubMed]
- Rittmaster RS (2008) 5alpha-reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab 22(2):389–402. doi:10.1016/j.beem.2008.01.016 [DOI] [PubMed]
- Roselli CE, Horton LE, Resko JA (1987) Time-course and steroid specificity of aromatase induction in rat hypothalamus-preoptic area. Biol Reprod 37:628–633. doi:10.1095/biolreprod37.3.628 [DOI] [PubMed]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM (2007) Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology (Berl) 191(4):867–877. doi:10.1007/s00213-006-0676-9 [DOI] [PubMed]
- Vongher JM, Frye CA (1999) Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav 64(4):777–785. doi:10.1016/S0091-3057(99)00140-9 [DOI] [PubMed]
- Vorhees CV, Johnson HL, Burns LN, Williams MT (2009) Developmental treatment with the dopamine D2/3 agonist quinpirole selectively impairs spatial learning in the Morris water maze. Neurotoxicol Teratol 31:1–10 [DOI] [PubMed]
- Walf AA, Frye CA (2005) ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacol 30:1598–1609. doi:10.1038/sj.npp.1300713 [DOI] [PubMed]
- Walf AA, Rhodes ME, Frye CA (2004) Antidepressants effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav 78:523–529. doi:10.1016/j.pbb.2004.03.023 [DOI] [PubMed]
- Walf AA, Koonce CJ, Frye CA (2008) Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci (5):974–981. doi:10.1037/a0012749 [DOI] [PMC free article] [PubMed]
- Walf AA, Koonce C, Manley K, Frye CA (2009) Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res 196:254–260 [DOI] [PMC free article] [PubMed]
- Wilbert DM, Griffen JE, Wilson JD (1983) Characterization of the cytosol androgen receptor of the human prostate. J Clin Endocrinol Metab. 56:113–20 [DOI] [PubMed]
- Zhang JQ, Cai WQ, DeZhou S, Su BY (2002) Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Research 935:73–80 [DOI] [PubMed]