Abstract
Preventing peritoneal implantation of ovarian carcinoma cells could prolong patient remission and survival. CA125 is expressed on most ovarian cancer cells and was reported to be a ligand of mesothelin, a peritoneal protein. We developed a cell adhesion assay with CA125-expresser ovarian cancer cells and human mesothelin-transfected cells and we confirmed that CA125 and mesothelin mediate cell attachment. We also showed that this assay supplies a high-throughput screening system for reagents able to block CA125/mesothelin-dependent cell attachment with a sensitive quantitative readout. We finally demonstrated that a mesothelin chimeric protein and anti-CA125 antibodies block CA125/mesothelin-dependent cell attachment.
Keywords: CA125, Mesothelin, High-throughput screening, Cell adhesion model system, Ovarian cancer
1. Introduction
Attachment of ovarian cancer cells to the mesothelial lining of the peritoneum is necessary for the metastatic spread typical of recurrent ovarian cancer. Mesothelial cells normally express mesothelin on their surface while most ovarian cancer cells express CA125 [1]. Rump and colleagues demonstrated that CA125 binds to mesothelin in a specific manner [2]. Thus, mesothelin/CA125 interaction may facilitate peritoneal metastasis by initiating cancer cell attachment to the mesothelial epithelium.
Mesothelin is a 40 kDa membrane-bound protein [3] that results from the cleavage of a 69 kDa preproprotein encoded by the MSLN gene. The alternative splicing of the MSLN gene results in at least two transcript variants. MSLN1 represents the predominant transcript or variant (1) (accession NM_005823) and encodes isoform 1 that we used to build up our cell adhesion assay. The transcript variant (2) MSLN2 (accession NM_013404) uses an alternate splice site in the coding region, resulting in a longer transcript that includes a 21 bp insertion in position 1229 of variant 1. The cleavage of MSLN-encoded preproprotein at the cationic motif TILRPRFRREVE releases the megakaryocyte potentiating factor (MPF), a 31 kDa soluble protein [4,5] while mesothelin remains membrane-bound. However, mesothelin also exists as a soluble form and has been detected in sera of ovarian carcinoma and mesothelioma patients [6–8], possibly after cleavage of its hydrophobic glycosylphosphatidylinositol (GPI) anchor [8] or as a variant lacking GPI anchor motif due to a reading frame shift [6]. Mouse mesothelin is 55% homologous to its human counterpart. The protease target sequence TVIHPRFRRDAE is conserved, although the mouse sequence may not be optimal for proteolytic cleavage (mouse R×RR compared to human R×R×RR). Mesothelin knockout mice have no obvious phenotype [9] and the biological function of mesothelin remains to be elucidated, as does that of CA125.
CA125 is a mucin-like protein of high molecular mass, estimated from 200 to 20,000 kDa, although smaller subunits have been reported [10,11]. In spite of its size, CA125 carries only two major antigenic domains, leading to only two groups of anti-CA125 antibodies: the OC125-like (group A) and the M11-like (group B) [12]. The rarity of CA125 antigenic domains may be due to its unusual structure, which consists of more than 60 repeat units of 156 amino acids (AA) [13,14] and to its immunosuppressive effects [15] that may prevent immunized mice from developing a diversified population of anti-CA125 antibodies. CA125 cell surface expression is upregulated when cells undergo metaplastic differentiation into a Müllerian-type epithelium [16] but is also detected on normal epithelia of the female genital tract [17,18] and on fetal coelomic epithelium and derivatives. Like mesothelin, CA125 is found in human sera and ascites. The release of CA125 soluble proteolytic fragments into the extracellular space [19] appears to be triggered by serine/threonine and/or tyrosine-dependant phosphorylation within the cytoplasmic domain [20] and is affected by cell cycle, proliferation, various growth factors and cytokines [10,21] and the conversion from benign to malignant cells [22]. CA125 is the most extensively studied biomarker for possible use in the early detection of ovarian carcinoma [23–28]. However, although CA125 is conserved in some mammals [29,30] its study is hampered by its lack of conservation in rodents. The absence of CA125 in rodents may be due to ovarian biological differences and thus it is difficult to extrapolate from mouse to human.
We have developed an all-human cell adhesion assay with an ovarian cancer cell line that expresses CA125 (OVCAR-3) [31] and immortalized embryonic kidney (HEK 293F) cell lines [32] transfected with MPF, mesothelin and MSLN1 coding sequences. This assay supplies a high-throughput screening for reagents that block CA125-mesothelin mediated cell adhesion with a sensitive quantitative readout. We screened a number of reagents and we demonstrated that CA125/mesothelin-dependent cell attachment is partially blocked by mesothelin fused to an Ig protein [33] and cross-linked with an anti-human Ig mouse monoclonal antibody (mAb), and completely blocked by several anti-CA125 mAbs of group B.
2. Material and methods
2.1. Constructs (Fig. 1)
Fig. 1.
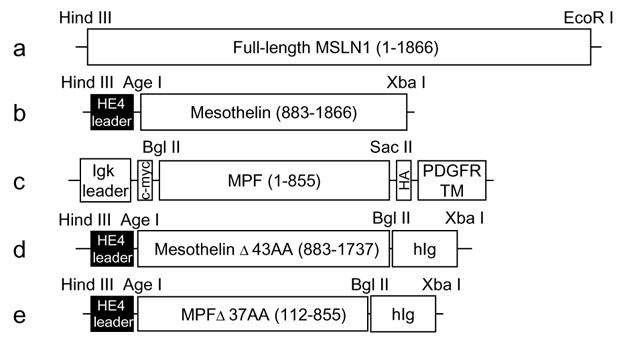
Constructs. (a)–(c) Constructs for cell surface expression: (a) full-length MSLN1 cDNA (1866 bp) was ligated to pcDNA3.1/−zeo(+) vector in between Hind III and EcoR I sites; (b) the N-terminal domain of MSLN1 cDNA that encodes mesothelin (984 bp) was ligated to a modified pcDNA3.1/hygro(+) vector in between Age I and Xba I sites and in frame with an HE4 leader sequence; (c) the C-terminal domain of MSLN1 cDNA that encodes MPF (855 bp) was ligated to pDisplay vector between Bgl II and Sac II sites and in frame with Igk leader sequence, c-myc tag, HA tag and PDGFR transmembrane domain. (d)–(e) Constructs for secreted chimeric proteins: truncated forms of (d) mesothelin (855 bp) and (e) MPF (744 bp) were ligated to a modified pcDNA3.1/hygro(+) vector between Age I and Bgl II sites and in frame with HE4 leader and human Ig sequence. The beginning and end positions of the inserts relative to the sequence of MSLN1 clone IMAGE no. 3957372 are shown in parenthesis. The number of truncated amino acids (AA) follows the Δ symbol.
cDNAs encoding full-length human mesothelin (MSLN1), MPF (MSLN1 amino-terminal domain), mesothelin (MSLN1 carboxy-terminal domain) were amplified by PCR from the clone MGC:10273 IMAGE:3957372 (ATCC, Manassas, VA). The full-length MSLN1 was amplified with the primers MSLN1 forward (5′-aagcttttcgaagccgccatggccttgccaacggctcgacccc-3′) and MSLN1 reverse (5′-tctagattatcaggccagtgtggaggctaggagcagtgc-3′) and ligated to pCR®-TOPO® vector (Invitrogen Corporation, Carlsbad, CA). After verification by sequencing, MSLN1 was excised with Hind III and EcoR I and ligated into Hind III/EcoR I-cut pcDNA3.1/zeo(+) (Invitrogen) (Fig. 1(a)). To express mesothelin at the cell surface, mesothelin cDNA was amplified with the primers Meso forward (5′-gccaccggtgcagaagtggagaagacagcctgtccttc-3′) and Meso reverse (5′-gcctctagattatcaggccagggtggaggctaggagcagtgccaggacggtgag-3′) to create a sequence with flanking Age I and Xba I sites and ligated to the Age I/Xba I-cut vector pcDNA3.1/hygro (+) (Invitrogen) modified by the insertion of a Kozak sequence [34] followed by the first 30 AA of HE4 leader [35] and an Age I site (Fig. 1(b)). To express MPF at the cell surface, MPF cDNA was amplified with the primers MPF forward (5′-ctagagatctatggccttgccaacggctcga-3′) and MPF reverse (5′-catgccgccggaggatggtccgttcaggctg-3′) to create a sequence flanked by Bgl II and Sac II sites and ligated to Bgl II/Sac II-cut pDisplay vector (Invitrogen). pDisplay-MPF encodes a protein fused to the PDGFR transmembrane domain and tagged with c-myc and HA (Fig. 1(c)).
To produce soluble chimeric proteins, mesothelin and MPF cDNAs were truncated for, respectively, their 3′ and 5′ end terminal sequences because they are predicted by TMpred [36] to encode hydrophobic stretches that could interfere with protein secretion (Fig. 1(d) and (e)). A mesothelin fragment was PCR amplified with Meso forward and Meso-Ig reverse (5′-gccagatctgccctgtagccccagccccagcgtgtccaggtcgtcctgccg-3′) to create a sequence with flanking Age I and Bgl II sites (Fig. 1(d)). A MPF fragment was amplified with the primers MPF-Ig forward (5′-gccaccggtgctggagagacagggcaggctg cgcccctg-3′) and MPF-Ig reverse (5′-gccagatctggcgaggatggtccgttcaggctgccgccaggatgg-3′) to create a sequence flanked by Age 1 and Bgl II sites (Fig. 1(e)). Mesothelin and MPF fragments were cloned into the modified Age I/Bgl II-cut pcDNA3.1/hygro(+) vector. A 696 bp fragment encoding a truncated human IgG1 was PCR amplified from human B lymphocytes with the primers huIgG1 forward (5′-gccagatctggagcccaaatcttgtgacaaaactcacacatgcccaccgtgccca-3′) and huIgG1 reverse (5′-gcctctagattatcatttacccggagacagggagaggctcttctgcgtgtag-3′) to create a sequence with flanking Bgl II and Xba I sites and ligated to Bgl II/Xba I-cut modified pcDNA3 vector in frame with mesothelin or MPF (Fig. 1(d) and (e)).
2.2. Cell lines, transfections, cell culture and cell lysates
The constructs encoding cell-surface proteins and secreted Meso-Ig were stably transfected into HEK 293F cell lines (ATCC, Manassas, VA) with Lipofectamin 2000 (Invitrogen) according to the manufacturer’s instructions and selected with hygromycin B (Invitrogen) or Geneticin® (Sigma–Aldrich, Corp. St Louis, MO). The constructs encoding secreted MPF-Ig was transiently transfected into HEK 293F cell lines with lipofectamin 2000. Cells were incubated at 37 °C with 5% CO2 in a humidified atmosphere. HEK 293F cells and all transformants were grown in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen) supplemented with 10% FBS(ATCC), 100 units penicillin–streptomycin (Invitrogen) and 0.2 mM-glutamine (Invitrogen).
The ovarian carcinoma cell line OVCAR-3 (ATCC) was grown in RPMI-1640 (Invitrogen) supplemented with 20% FBS 100 units penicillin–streptomycin and 0.2 mM-glutamine.
2.3. Protein secretion and purification
HEK 293F cells secreting chimeric proteins were incubated in serum-free medium for 48 h before harvesting the medium. The Ig-fusion proteins were purified from the media using Ultralink Protein A (Pierce, Rockford, IL) according to standard procedures, dialyzed against phosphate buffered saline (PBS) and stored at −80 °C.
2.4. Flow cytometry analysis and sorting
Cells transfected with MSLN1 or mesothelin constructs were analyzed by flow cytometry on Becton Dickinson FACScan Cytometer for their binding to 4H3, an anti-mesothelin mouse monoclonal antibody (4H3 mAb) that does not recognize MPF [6]. 4H3 was detected with Alexa Fluor® 488 F(ab′)2 fragment of goat anti-mouse IgG (H+L) (488 anti-mIg) (Invitrogen). In the absence of MPF-specific antibody, MPF-transfected cells were analyzed for their cell surface expression of c-myc tagged protein with an anti c-myc mAb (Santa Cruz biotechnology, Santa Cruz, CA) and detected with 488 anti-mIg. The highest expressers were flow sorted with the Becton Dickinson FACS Vantage SE Cell Sorter and selectively grown.
2.5. Western blots
Purified chimeric proteins were mixed with 2× SDS loading buffer supplemented with 5% mercaptoethanol (Sigma–Aldrich), denatured by heating and separated by electrophoresis on a 4–12% NuPAGE Bis Tris gel (Invitrogen) using SDS running buffer (Invitrogen). The gels were transferred to a PVDF membrane (Invitrogen) in NuPAGE Transfer Buffer (Invitrogen) using an X-cell II blot module (Invitrogen). PVDF membranes were blocked with blocking buffer (Superblock, Pierce) supplemented with 0.05% Tween 20 (Fisher Biotech, Fair Lawn, NJ). The Ig-fusion proteins were detected with an HRP-conjugated F(ab′)2 fragment goat anti-human IgG (H+L) (HRP-anti-hIg) (Jackson Immuno Research Laboratory, Inc., West Grove, PA). Detection was performed with SuperSignal West Pico Chemiluminescent Substrate (Pierce) according to standard procedures.
2.6. Heterotypic cell adhesion assay
Twenty-four to thirty-six hours prior to the assay, the adherent OVCAR-3 cells were distributed at 5×105 cells/ml into tissue culture sterile 96-well flat transparent bottom wells and black wall plates (Corning Costa3603, Corning, NY) and incubated at 37 °C to a confluence of 70–90%. On the day of the assay, wild-type HEK 293F cells or cells transfected with MPF, Mesothelin or MSLN1 constructs were fluorescently labeled with Vybrant® Cell Adhesion assay Kit (Invitrogen-GIBCO, Carlsbad CA) according to the manufacturer’s instructions. OVCAR-3 cells immobilized in the wells were washed once with 0.2 ml of warmed DMEM medium and labeled HEK 293 cells were distributed in triplicate at a final concentration of 2.5 or 5×106 cells/ml and incubated with the OVCAR-3 cells at 37 °C for 1 h. Fluorescence was measured before and after washes with 0.2 ml of prewarmed PBS with FL×800™ Multi-Detection Microplate Reader (Bio-Tek Instruments, Inc. Winooski, VT). For normalization, the after wash-fluorescent signals were divided by the fluorescent signal read before washing. The normalized values were averaged. Standard deviations were less than 4%.
2.7. Blocking agents for cell adhesion assay
HEK 293F cells were preincubated with 10 μg/ml of anti-mesothelin antibody in 0.1 ml DMEM medium for 30 min at 37 °C. Alternatively, OVCAR-3 cells were preincubated with 10 μg/ml of anti-CA125 antibody in 0.1 ml DMEM medium for 30 min at 37 °C or with 10 μg/ml of chimeric proteins alone or cross-linked. To cross-link the proteins, an equal amount of anti-human Ig mAb (Jackson ImmunoResearch Laboratories, Inc.) was added [37] and incubated at a final concentration of 10 μg/ml in DMEM medium for 30 min before the incubation with OVCAR-3 cells. Anti-mesothelin mAb 4H3 was acquired from the Hellström laboratory [6]. Anti-CA125 mAb were acquired from Fujirebio Diagnostics, Inc (FDI, Malvern, PA) (OC-125 and M11), Fitzgerald Industries International, Inc. (FIII, Concord, MA) (M8072320, M8072321, M002201 and M002203) and Research Diagnostics, Inc. (RDI, Flanders, NJ) (X306 and X52). M8072320, M002201 and X306 epitopes are OC-125-like (group A) while M8072321, M002203 and X52 are M11-like (group B) [17,38].
3. Results
3.1. Validation of HEK 293F transfected cells
Cells transfected with MSLN1 or mesothelin constructs were incubated with 4H3 and analyzed by flow cytometry (Fig. 2(a) and (b)). In the absence of MPF-specific antibody, HEK 293 cells transfected with MPF construct were incubated with anti-c-myc mAb to detect the transfected tagged-protein (Fig. 2(c)). The purified supernatants of HEK 293 cells transfected with Meso-Ig or MPF-Ig constructs were analyzed by western blot (Fig. 2(d) and (e)).
Fig. 2.
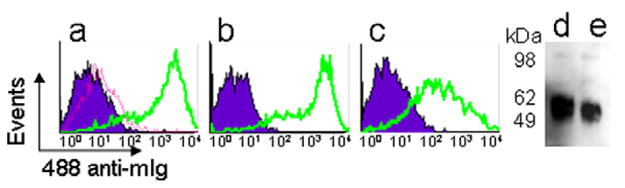
Validation of transfected HEK293F cells. (a)–(c) Flow cytometry analysis: HEK293F cells transfected with (a) MSLN1 or (b) mesothelin constructs were incubated with 10 μg/ml of 4H3 mAb (solid lines); (c) HEK293F cells transfected with MPF construct were incubated with 10 μg/ml of anti-c-myc tag mAb (solid lines). As negative controls, wild type (dotted line) or transfected cells (shade areas) were incubated with the secondary antibody only. (d)–(e) Western blot: (d) Meso-Ig and (e) MPF-Ig chimeric proteins were detected with an HRP-anti-hIg mAb.
3.2. Development of a CA125/mesothelin-dependant cell adhesion assay
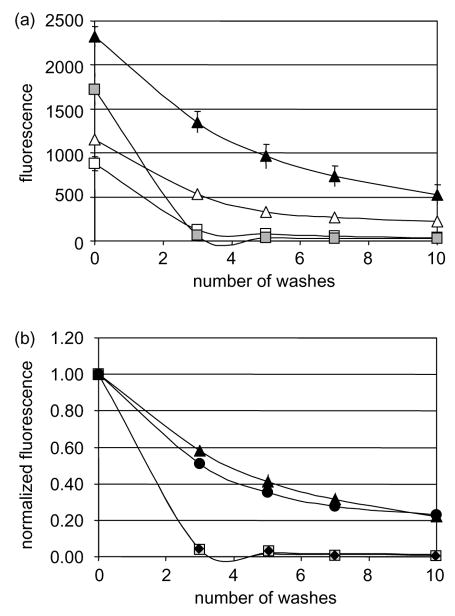
Fluorescent-labeled HEK 293F cells were incubated with an adherent monolayer of OVCAR-3 cells in 96-well plates. Gentle washing quickly decreased the fluorescent signal given by wild type HEK 293F cells, but mesothelin-transfected cells were resistant to washing (Fig. 3(a)). We observed that although the fluorescent signals were clearly proportional to the quantity of labeled cells, they were also variable from one staining to another (from 1000 to 3000 for 2×106 cells), hence the necessity to normalize our readings. Fig. 3(b) shows that after normalization of the fluorescent signals, mesothelin- and MSLN1-trans-fected HEK293F adhere equally well to OVCAR-3 cells and more strongly than wild type cells or MPF-transfected cells. This confirms that mesothelin mediates cell attachment to OVCAR-3 and demonstrates that CA125/mesothelin interaction can be assessed by an assay compatible with high-throughput screening.
Fig. 3.
CA125/mesothelin-dependant cell adhesion assay. Ninety six-well plates containing OVCAR-3 adherent cells and fluorescently-labeled HEK 293 cells, wild type at 2.5×106 cells/ml (white squares) or 5×106 cells/ml (gray squares); or transfected with mesothelin at 2.5×106 cells/ml (white triangles) or 5×106 cells/ml (black triangles); MPF at 5×106 cells/ml (black diamonds); MSLN1 at 5×106 cells/ml (black circles) were washed up to 10 times. (a) Remaining fluorescence was plotted after each wash or (b) normalized to the original fluorescence for each well. The assay was performed in triplicates and the values were averaged. All standard deviations were less than 4%.
3.3. Identification of agents able to block CA125/mesothelin-dependant cell adhesion
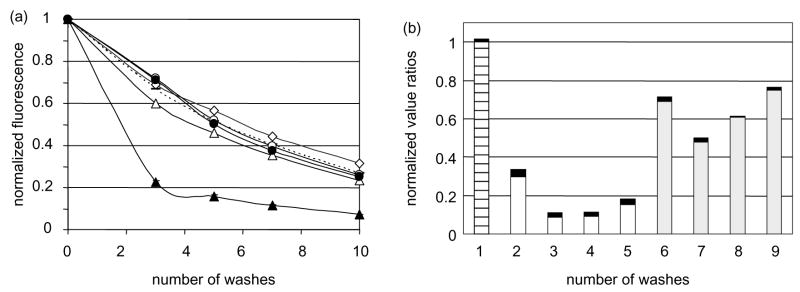
Identifying agents that block CA125/mesothelin interaction could help design treatments to prevent peritoneal spreading of ovarian carcinoma cells. Using the CA125/mesothelin cell adhesion assay, we tested several agents for their ability to interfere with cell attachment. First, we tested chimeric proteins. Fig. 4(a) shows that Meso-Ig cross-linked with an anti-Ig antibody interfered with CA125/mesothelin-dependant cell attachment while Meso-Ig alone did not; neither MPF-Ig nor cross-linked MPF-Ig affected the cell attachment. We also tested several antibodies directed against CA125 and mesothelin. Fig. 4(b) shows that all anti-CA125 mAb tested interacted with the cell adhesion and decreased the fluorescence signal by 30% or more. Three out of the four M11-like anti-CA125 mAb decreased the fluorescence signal by 85% or more, which suggested that anti-CA125 antibodies of group B are more efficient that antibodies of group A for blocking CA125/mesothelin-dependant cell adhesion. The addition of the anti-mesothelin mAb 4H3 during the incubation did not block the MSLN1-transfected 293F cell adhesion to OVCAR-3 cell line (data not shown).
Fig. 4.
Cross-linked Meso-Ig and anti-CA125 mAb block cell adhesion (a) The cell adhesion assay was conducted in presence of 10 μg/ml of MPF-Ig alone (white circles) or cross-linked with anti-Ig mAb (black circles), or Meso-Ig alone (white triangles) or cross linked (black triangles). As a positive control for cell attachment, the assay was performed in medium only (white diamonds) or in medium supplemented with anti-hIg (dotted line). The assays were performed in triplicates. All standard deviations were less than 4%. (b) The cell adhesion assay was conducted after preincubation of OVCAR3 cells with 10 μg/ml of anti-CA125 antibodies (2=M002203; 3=M11; 4=M8072321; 5=X52; 6=M002201; 7=OC-125; 8=M8072320; 9=X306). The normalized fluorescent signals after five washes were divided by the fluorescent signal of the adhesion assay performed in medium only (1). The gray bars correspond to the anti-CA125 mAb of group A and the white bars to the group B. The black boxes represent the standard deviations and the stripped bar is the positive control for cell attachment.
4. Discussion
Spreading and implantation of ovarian carcinoma cells to adjacent organs through peritoneal fluid is characteristic of ovarian carcinoma and contributes to the grim prognosis of the disease. Blocking the CA125/mesothelin interaction is a potential therapeutic target, but the absence of a CA125 homolog in rodents makes it difficult to test therapeutic approaches in vivo.
Here, we present a cell adhesion assay using human cell lines that can measure 100s of conditions in a single experiment and thus supplies a high-throughput screening system with a sensitive quantitative readout for reagents that block CA125-mesothelin mediated cell adhesion. Using this assay, we confirmed that CA125 and human mesothelin mediate cell attachment and we showed that cells transfected with MSLN1, full-length or carboxy-terminal domain (mesothelin), bind equally well to CA125-expresser cells. This was not surprising because mesothelin results from the cleavage of a preproprotein encoded by MSLN1. It would be of interest to study the pathways that trigger the preproprotein cleavage since interfering with it might prevent CA125/mesothelin-dependant cell adhesion.
We showed that the CA125/mesothelin-dependent cell adhesion could be partially blocked by a cross-linked chimeric mesothelin protein. The need to cross-link mesothelin to disrupt cell attachment hints at the importance of the cooperativity of blocking compounds. Soluble mesothelin is detected in patient sera and ascites and a recent publication demonstrates the existence of a humoral immune response to mesothelin in mesothelioma and ovarian cancer patients [39]. The presence of soluble mesothelin in patients does not seem to be linked to a better prognosis or survival (observation based on 33 ovarian cancer cases followed for more than 2 years—Urban N, unpublished data) but to our knowledge the presence of both soluble mesothelin and anti-mesothelin-autoantibodies has not been correlated to patient outcome. Addressing whether mesothelin/anti-mesothelin mAb complexes could interfere with ovarian cancer cell attachment to the peritoneum is clinically relevant. A positive correlation between favorable prognosis and presence of both circulating mesothelin and anti-mesothelin auto-antibodies may be an incentive for the development of anti-mesothelin antibodies as well as vaccination strategies against mesothelin aimed to prevent metastastatic disease.
Although 4H3, an anti-mesothelin antibody, could not block CA125/mesothelin-dependent cell adhesion, we believe that other antibodies directed against different mesothelin epitopes might demonstrate some activity in this model system. In contrast, CA125/me-sothelin-dependent cell attachment could be completely blocked with three anti-CA125 mAb of group B and partially blocked with five other anti-CA125 mAbs. Although the two anti-CA125 antibody groups do not overlap, the epitopes of both groups lie within the 156 AA repeat units of CA125. This suggests that CA125 binds to mesothelin through a site located in the 156 AA repeat units and that the mesothelin binding site shares more homologies with M11 epitope than with OC-125 epitope.
Metastasis remains a major challenge in the clinical management of ovarian cancer [40–42]. Various approaches to maintenance therapy are being evaluated but they rely on continued treatment with highly toxic chemotherapeutic agents. Biologics that are better tolerated by the patients, like Herceptin, an anti-Her2/Neu used for breast cancer therapy [43,44] are urgently needed as a way to maintain remission. We developed a robust and high throughput cell adhesion assay that makes it possible to test small molecules and new generations of biologics for their ability to disrupt the cell attachment that underlies ovarian cancer metastasis. Our results suggest that developing therapeutic agents such as humanized or recombinant antibodies against CA125 or mesothelin may help to prevent the implantation of ovarian carcinoma cells in the peritoneum.
Acknowledgments
We thank Paul Lampe for thoughtful advice and Kathy O’Briant for technical help. The work presented was supported by grant GM 17709 from the National Institutes of Health, the Canary Foundation and the Pacific Ovarian Cancer Research Consortium/SPORE in Ovarian Cancer Developmental Research Program (P50 CA83636).
References
- 1.Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68(5):1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279(10):9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 3.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93(1):136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi N, Yamamura Y, Konishi E, Ueda K, Kojima T, Hattori K, et al. Characterization, molecular cloning and expression of megakaryocyte potentiating factor. Stem Cells. 1996;14(Suppl 1):62–74. doi: 10.1002/stem.5530140708. [DOI] [PubMed] [Google Scholar]
- 5.Kojima T, Oheda M, Hattori K, Taniguchi Y, Tamura M, Ochi N, et al. Molecular cloning and expression of megakaryocyte potentiating factor cDNA. J Biol Chem. 1995;270(37):21984–21990. doi: 10.1074/jbc.270.37.21984. [DOI] [PubMed] [Google Scholar]
- 6.Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellstrom KE, et al. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci USA. 1999;96(20):11531–11536. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362(9396):1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 8.Hassan R, Remaley AT, Sampson ML, Zhang J, Cox DD, Pingpank J, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006;12(2):447–453. doi: 10.1158/1078-0432.CCR-05-1477. [DOI] [PubMed] [Google Scholar]
- 9.Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol. 2000;20(8):2902–2906. doi: 10.1128/mcb.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien TJ, Tanimoto H, Konishi I, Gee M. More than 15 years of CA 125: what is known about the antigen, its structure and its function. Int J Biol Markers. 1998;13(4):188–195. doi: 10.1177/172460089801300403. [DOI] [PubMed] [Google Scholar]
- 11.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276(29):27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 12.Nustad K, Bast RC, Jr, Brien TJ, Nilsson O, Seguin P, Suresh MR, et al. Specificity and affinity of 26 monoclonal antibodies against the CA 125 antigen: first report from the ISOBM TD-1 workshop. International Society for Oncodevelopmental Biology and Medicine. Tumour Biol. 1996;17(4):196–219. doi: 10.1159/000217982. [DOI] [PubMed] [Google Scholar]
- 13.Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer. 2002;98(5):737–740. doi: 10.1002/ijc.10250. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien TJ, Beard JB, Underwood LJ, Dennis RA, Santin AD, York L. The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumour Biol. 2001;22(6):348–366. doi: 10.1159/000050638. [DOI] [PubMed] [Google Scholar]
- 15.Patankar MS, Yu J, Morrison JC, Belisle JA, Lattanzio FA, Deng Y, et al. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol Oncol. 2005;99(3):704–713. doi: 10.1016/j.ygyno.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Feeley KM, Wells M. Precursor lesions of ovarian epithelial malignancy. Histopathology. 2001;38(2):87–95. doi: 10.1046/j.1365-2559.2001.01042.x. [DOI] [PubMed] [Google Scholar]
- 17.Kabawat SE, Bast RC, Jr, Bhan AK, Welch WR, Knapp RC, Colvin RB. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Pathol. 1983;2(3):275–285. doi: 10.1097/00004347-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Zeimet AG, Offner FA, Muller-Holzner E, Widschwendter M, Abendstein B, Fuith LC, et al. Peritoneum and tissues of the female reproductive tract as physiological sources of CA-125. Tumour Biol. 1998;19(4):275–282. doi: 10.1159/000030018. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd KO, Yin BW. Synthesis and secretion of the ovarian cancer antigen CA 125 by the human cancer cell line NIH:OVCAR-3. Tumour Biol. 2001;22(2):77–82. doi: 10.1159/000050600. [DOI] [PubMed] [Google Scholar]
- 20.Fendrick JL, Konishi I, Geary SM, Parmley TH, Quirk JG, Jr, O’Brien TJ. CA125 phosphorylation is associated with its secretion from the WISH human amnion cell line. Tumour Biol. 1997;18(5):278–289. doi: 10.1159/000218041. [DOI] [PubMed] [Google Scholar]
- 21.Marth C, Zeimet AG, Widschwendter M, Daxenbichler G. Regulation of CA 125 expression in cultured human carcinoma cells. Int J Biol Markers. 1998;13(4):207–209. [PubMed] [Google Scholar]
- 22.Meyer T, Rustin GJ. Role of tumour markers in monitoring epithelial ovarian cancer. Br J Cancer. 2000;82(9):1535–1538. doi: 10.1054/bjoc.2000.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenemans P, Yedema CA, Bon GG, von Mensdorff-Pouilly S. CA 125 in gynecological pathology–a review. Eur J Obstet Gynecol Reprod Biol. 1993;49(1–2):115–124. doi: 10.1016/0028-2243(93)90135-y. [DOI] [PubMed] [Google Scholar]
- 24.Tamakoshi K, Kikkawa F, Hasegawa N, Ishikawa H, Mizuno K, Kawai M, et al. Clinical value of a new serum tumor marker, CA125II, in gynecologic disease: comparison with CA125. Gynecol Obstet Invest. 1995;39(2):125–129. doi: 10.1159/000292393. [DOI] [PubMed] [Google Scholar]
- 25.Dorum A, Kristensen GB, Abeler VM, Trope CG, Moller P. Early detection of familial ovarian cancer. Eur J Cancer. 1996;32A(10):1645–1651. doi: 10.1016/0959-8049(96)00137-2. [DOI] [PubMed] [Google Scholar]
- 26.Eagle K, Ledermann JA. Tumor markers in ovarian malignancies. Oncologist. 1997;2(5):324–329. [PubMed] [Google Scholar]
- 27.Fures R, Bukovic D, Hodek B, Klaric B, Herman R, Grubisic G. Preoperative tumor marker CA125 levels in relation to epithelial ovarian cancer stage. Coll Antropol. 1999;23(1):189–194. [PubMed] [Google Scholar]
- 28.Jacobs IJ, Menon U. Progress and challenges in screening for early detection of ovarian cancer. Mol Cell Proteomics. 2004;3(4):355–366. doi: 10.1074/mcp.R400006-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Nouwen EJ, Dauwe S, De Broe ME. Occurrence of the mucinous differentiation antigen CA125 in genital tract and conductive airway epithelia of diverse mammalian species (rabbit, dog, monkey) Differentiation. 1990;45(3):192–198. doi: 10.1111/j.1432-0436.1990.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 30.McDonnel AC, Van Kirk EA, Austin KJ, Hansen TR, Belden EL, Murdoch WJ. Expression of CA-125 by progestational bovine endometrium: prospective regulation and function. Reproduction. 2003;126(5):615–620. [PubMed] [Google Scholar]
- 31.Sweet F, Rosik LO, Sommers GM, Collins JL. Daunorubicin conjugated to a monoclonal anti-CA125 antibody selectively kills human ovarian cancer cells. Gynecol Oncol. 1989;34(3):305–311. doi: 10.1016/0090-8258(89)90163-7. [DOI] [PubMed] [Google Scholar]
- 32.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 33.Shan D, Press OW, Tsu TT, Hayden MS, Ledbetter JA. Characterization of scFv-Ig constructs generated from the anti-CD20 mAb 1F5 using linker peptides of varying lengths. J Immunol. 1999;162(11):6589–6595. [PubMed] [Google Scholar]
- 34.Kozak M, Shatkin AJ. Characterization of translational initiation regions from eukaryotic messenger RNAs. Methods Enzymol. 1979;60:360–375. doi: 10.1016/s0076-6879(79)60034-4. [DOI] [PubMed] [Google Scholar]
- 35.Kirchhoff C, Habben I, Ivell R, Krull N. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod. 1991;45(2):350–357. doi: 10.1095/biolreprod45.2.350. [DOI] [PubMed] [Google Scholar]
- 36.Hofmann K, Stoffel W. TMbase—a database of membrane spanning proteins segments. Biol Chem Hoppe-Seyler. 1993;374:166. [Google Scholar]
- 37.Kanner SB, Damle NK, Blake J, Aruffo A, Ledbetter JA. CD2/LFA-3 ligation induces phospholipase-C gamma 1 tyrosine phosphorylation and regulates CD3 signaling. J Immunol. 1992;148(7):2023–2029. [PubMed] [Google Scholar]
- 38.Nap M, Vitali A, Nustad K, Bast RC, Jr, O’Brien TJ, Nilsson O, et al. Immunohistochemical characterization of 22 monoclonal antibodies against the CA125 antigen: 2nd report from the ISOBM TD-1 workshop. Tumour Biol. 1996;17(6):325–331. [PubMed] [Google Scholar]
- 39.Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, Pastan I. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 40.Ozols RF. Update on the management of ovarian cancer. Cancer J. 2002;8(Suppl 1):S22–S30. [PubMed] [Google Scholar]
- 41.Almadrones LA. Treatment advances in ovarian cancer. Cancer Nurs. 2003;26(6 Suppl):16S–20S. doi: 10.1097/00002820-200312001-00005. [DOI] [PubMed] [Google Scholar]
- 42.Eltabbakh GH. Recent advances in the management of women with ovarian cancer. Minerva Ginecol. 2004;56(1):81–89. [PubMed] [Google Scholar]
- 43.Emens LA, Reilly RT, Jaffee EM. Breast cancer vaccines: maximizing cancer treatment by tapping into host immunity. Endocr Relat Cancer. 2005;12(1):1–17. doi: 10.1677/erc.1.00671. [DOI] [PubMed] [Google Scholar]
- 44.Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z. Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther. 2003;2(11):1113–1120. [PubMed] [Google Scholar]