Abstract
Basal ganglia striosomes, or patches, are rich in mu opioid receptors (MOR) and form a 3-dimiensional labyrinth of cells that extend throughout the mid and anterior striatum in mice. Though previous studies have suggested that striosomes could affect drug-induced motor output in rodents, the functional role of these compartmentalized MOR-rich striosomes is not well understood. To investigate any relationship between the striosomes and motor behavior we used the toxin dermorphin-saporin (DS) to selectively ablate MOR-rich striosomal cells. FVB mice were bilaterally infused with DS in the mid striatum alone or in the mid and anterior striatum, and were tested on three motor tasks and in an open field. Two volume measurement procedures and stereological cell counts were used to confirm the induced pathology. Mice that received DS injections showed significantly smaller volumes (−26 to −44%) and fewer cells (−30 to −49%) in the striosome compartment compared to mice that received control injections of saline or saporin. Striosome pathology was greatest in the dorsolateral striatum. The extrastriosomal matrix was not significantly affected, resulting in an imbalance in the ratio of striosome-to-matrix cells. Behaviorally, toxin injections caused deficits on an accelerating rotarod task and the deficit was worse in mice that received mid and anterior injections than those that received mid striatal injections alone. However, DS-injected mice did not differ from control mice on other motor tasks. We conclude that the MOR-rich cells of the striosomes are necessary for optimal rotarod performance, including learning and/or improvement on the task.
INTRODUCTION
The striatum is a critical nucleus for the basal ganglia’s modulation of motor control and its pathology accounts for many movement disorders. It is neurochemically compartmentalized into anatomically distinct clusters and strips of cells called striosomes that are embedded in an extrastriosomal matrix (Pert et al. 1976, Graybiel and Ragsdale, 1978, Herkenham and Pert, 1981, Gerfen et al. 1985, Tippett et al 2007). Several studies suggest that striosomes and matrix are functional units that differentially regulate sensorimotor processing and reward (Brown et al., 1996, Trytek et al., 1996,White and Hiroi, 1998, Canales and Graybiel, 2000, Brown et al., 2002, Tappe and Kuner, 2006). In rodents and cats, striosomes are rich in mu opioid receptors (MOR) and exist as a three-dimensional labyrinth (Desban et al., 1993, Groves et al., 1998). In the movement disorder Huntington’s disease (HD), the striatum shows marked atrophy and there is evidence that the striosome and matrix compartments are separately affected at early stages of the disease; furthermore, there is evidence from human studies that the integrity of these neurochemical compartments could contribute to lack of motor coordination and emotional deficits (Ferrante et al., 1987, Seto-Ohshima et al., 1988, Hedreen and Folstein, 1995, Tippett et al., 2007).
In some genetic mouse models of HD, degenerative signs in the striatum are greater in the striosomes than matrix (Menalled et al, 2002, Lawhorn et al., 2008). In the YAC128 mouse model, the striosome cells show a greater degree of loss in mutant mice than the surrounding matrix when compared to wild-type control mice (Lawhorn et al., 2008). Also, degeneration of cells in the dorsolateral striatum is more prominent than the surrounding striatal regions (Van Raamsdonk et al., 2007b, Lawhorn et al., 2008). A knock-in mouse model of HD showed more inclusions in the striosomes than matrix (Menalled et al., 2002). In addition, genetic models show motor skill and motor learning deficits on several tasks, especially the rotarod (Hodgson, 1999, Bates, 2002, Menalled and Chesselet, 2002, Rubinsztein, 2002, Slow et al., 2003, Van Raamsdonk et al., 2007a, Van Raamsdonk et al., 2007b, Lawhorn et al., 2008). We found that mice with striosome pathology also showed rotarod and balance beam deficits (Lawhorn et al., 2008).
To further investigate a role for the neurochemically distinct striosomes in motor control, we asked the question if the striosomes are critical to performance on balance and coordination tasks. Specifically, we wanted to know whether their perturbation could upset behavioral performance on complex motor tasks in wild-type mice. To address this question, we used a specific neurotoxin to lesion MOR-containing cells of the striosomes and examined motor behaviors before and after the lesion in 13-month-old FVB mice. We used 13-month-old mice because that is the age that HD mutants with partial lesions of the striosomes showed motor deficits (Lawhorn et al. 2008).
Dermorphin-saporin (DS) is an effective MOR-specific neurotoxin to cells in the spinal cord and the midbrain and has been associated with changes in pain response in rodents (Porreca et al., 2001, Burgess et al., 2002). This toxin was also used to lesion cells in the amygdala and was associated with abolishing fear extinction (Likhtik et al., 2008). Saporin is a protein that when conjugated to the MOR agonist dermorphin, binds to the cell surface of MOR-expressing neurons, inactivates ribosomes and causes protein inhibition and cell death. Cells that do not have the MOR cell surface marker are unaffected. Tokuno et al (2002) used the toxin in rats to determine efferent projections from the striosomes. The present study examined the FVB mouse strain, which is used in the YAC transgenic models for HD (Hodgson et al., 1999, Slow et al., 2003). We wanted to determine if the motor deficits reported in YAC HD mutants (Van Raamsdonk et al., 2005, Lawhorn et al., 2008) could be mimicked by a specific lesion to the striosome compartment in normal healthy mice at a similar age. We tested mice with DS lesions and with control lesions on an accelerating rotarod, balance beam and wire maneuver task to challenge their ability to complete a complex motor task. We also tested the mice for anxiety in Light-Dark environment, and for mild cognitive deficits in a Y-maze.
We chose to inject the toxin into the dorsolateral sensorimotor zone of the striatum because Costa et al. (2004) showed that accelerating rotarod behavior activates the dorsolateral striatum and there is cell loss in this area associated with motor deficits in the YAC128 mouse model of HD (Van Raamsdonk et al., 2007b, Lawhorn et al., 2008).
We used both stereological and direct measurements of striatal volume because we wanted to confirm our results using two methods. Also, stereology has been described as an unbiased method with greater consistency and reliability compared to direct measurements (West et al., 1991, Gundersen and Jensen, 1987). We had an opportunity to test the congruity of the two measurement procedures.
METHODS
Mice
We used FVB strain mice that were WT littermates of the YAC128 transgenic mouse model (Slow et al., 2003). Mice in this study were bred from breeders purchased from the Induced Mutant Resource Jackson Laboratory (Bar Harbor ME) stock name: Tg53Hay.
Behavioral Testing
Testing was done on male mice aged 13 months (n=10 per group) at the same time and following the same sequence each day. We followed a protocol of behavioral testing identical to Lawhorn et al (2008). Behavioral assays were carried out within the first 2 hours of the dark phase of the light-dark cycle. All mice were kept on a reverse light cycle (lights off at 07:00 hr and on at 19:00 hr). The Institutional Animal Care and Use Committee at the Albert Einstein College of Medicine approved all animal husbandry and experiments.
Briefly, we used the SHIRPA phenotype assessment and a grip strength meter as a quantitative tool to determine the general health state of each mouse (Rogers, 1997). The grip strength meter (San Diego Instruments) enabled us to evaluate forelimb and hindlimb strength. Mice included in this study had SHIRPA scores and grip strength measurements that were within two standard deviations of the group mean. None of the available mice needed to be excluded.
Accelerating Rotarod
To measure a complex motor task that involves striatal circuitry (Costa et al., 2004) we used an accelerating rotarod (Columbus Instruments, Economex). The rod accelerated from 3–40 RPM in 5 minutes. Mice were tested for three days and then given bilateral injections of DS, saporin or saline. One set of mice received bilateral injections to the mid striatum. Another group received bilateral injections to both the mid and anterior striatum. Mice that received a mid-striatal injection were tested on the rotarod for 3 post-lesion days. Mice that received both a mid and anterior striatal injection were tested on the rotarod for 5 post-lesion days, to determine if they would reach a plateau. The intertrial interval was five minutes. The cut-off time for walking on the rod was 5 minutes. We used the best score of each mouse from the five trials on a given day for analysis (Wise, 1984, Wise and Raptis, 1986).
Balance Beam
As another measure of balance and coordination that is affected in HD mouse models (Lawhorn et al., 2008; Tang et al., 2007), we quantified the number of hindpaw slips that occurred when mice ambulated across a round beam that was 2.5 cm in diameter and 1 meter from the ground. Each mouse was given 5 trials. The mean number of hindpaw slips off the beam was recorded and analyzed.
Wire Maneuver
To investigate another complex motor behavior, we tested the capacity of the mice to grip and move along a wire suspended over a cage of bedding. This wire maneuver task was modified from the SHIRPA test. Mice were held by the tail and were vertically lowered to a suspended wire that was 1.5 mm in diameter. Upon grabbing the wire, the observer slowly brought the mouse to a horizontal position and then released it. Each mouse was placed on the wire for 5 trials with a 5-minute intertrial interval. Neurologically normal mice actively used a predictable combination of hindpaws, forepaws and tail to maneuver from one end of the wire to the other and to prevent themselves from falling. Each mouse was given five trials, and the longest time to maintain a grip on the wire or best score for each mouse was used in the analysis. There was no cut off period.
Open Field
We videotaped ambulatory behavior in an open field for eight-minutes. The open field was cleaned with ethanol between subjects and sessions were recorded in dim light. Ambulatory behavior such as distance traveled and velocity was obtained for each mouse and analyzed using Viewer Biobserve software (Bonn, Germany). Anxiety related behaviors such as Thigmotaxis (time spent in the periphery versus the center of the open field) and grooming and stereotypic behaviors were also recorded (Simon et al., 1994, Choleris et al., 2001, Zhu et al., 2007).
Light-dark anxiety test
We measured anxiety-like behavior in mice that were allowed to choose between spending time in a dark enclosed box or a brightly lit open field (Crawley, 2000). A standard plastic rat cage was divided so that 1/3 of the box was enclosed in darkness using opaque black plastic, while the remainder of the cage was transparent, without a lid and brightly lit. The mouse was placed in the dark side of the cage and the amount of time spent in the dark versus the light regions was recorded over a 10 minute period. The cage was cleaned with a vinegar and water solution prioro testsing each mouse.
Spontaneous Alternation
We measured spontaneous alternation in a Y-shaped maze. This behavior refers to a mouse’s innate tendency to want to explore novel environments and reduced tendency to return to the same location over successive trials (Lalonde 2002). Mice traveled down one of three arms in the maze in continuous patterns over 8 minutes. The percentage of time that the mice alternated was scored.
Lesions: Mid-Striatal and Mid+Anterior Striatal
After behavioral testing, all mice underwent general anesthesia with ketamine (100 mg/kg, ip.) and xylazine (7 mg/kg ip.).
Mid Striatal
In our first experiment, thirty mice were divided so that 10 mice received bilateral injections of dermorphin-saporin (DS1), 10 received bilateral injections of saporin (SAP; Advanced Targeting Systems, San Diego, CA, USA) and 10 received bilateral injections of saline (SAL1) into the striatum (AP + 1.0 anterior to Bregma, ML +/− 2.5 lateral to Bregma, V −3.5 mm below skull). A total volume of 0.5 µl of a 17 ng/µl dermorphin-saporin solution in physiological saline was injected through two 5-µl Hamilton microsyringes. The same amount of unconjugated saporin and saline was injected in the control mice. The syringes were attached so that bilateral injections were delivered simultaneously at a rate of 0.1 µl/30 seconds. Mice were given 1mg/kg of butorphanol at the end of surgery.
Mid + Anterior Striatal
In the second experiment, to make a larger lesion that would include the anterior striatum, an additional set of mice received bilateral injections at two AP locations. Ten mice received dermorphin-saporin lesions at the same striatal region as those in the first experiment and they also received injections at AP + 1.5 mm anterior to Bregma, +/− 2.3 mm lateral to Bregma, and −3.5 mm below skull (DS2). An additional 10 mice received saline control injections at the same coordinates (S2).
After an 8-day recovery period, the surviving mice were tested on the same battery of tests as before their surgery, and then deeply anesthetized with urethane (30 mg/kg) for perfusion and brain removal.
Immunohistochemistry
Mice were anesthetized and perfused intracardially with saline and 10% buffered formalin. Their brains were stored in 20% sucrose-formalin at 4°C. We sectioned 24 coronal serial slices per animal at 30 um thickness, beginning at approximately 0.3 mm anterior to bregma just anterior to where striosomes begin in the FVB mouse.
We used an immunohistochemistry protocol described in Lawhorn et. al. (2008) to stain for neurons (NeuN), and striosomes (MOR1), in the same sections. Briefly, free-floating sections were incubated in 50% formamide and 2X SSC for 2 hours, washed in .01M PBS, incubated in 2N HCl for 30 min, and washed in 0.1 M boric acid, pH 8.5, for 10 min. Standard ABC techniques were used to incubate sections in NeuN (1:800) primary antibody (Chemicon, Temecula, CA) in 0.01 M PBS for 24 hours followed by a horse secondary antibody (Vector, Burlingame, CA) (1:200) for 75 minutes. NeuN-labeled cells were briefly stained using DAB (Vectastain ABC Kit, Vector, Burlingame, CA) and were visible as darkly stained cell bodies. To visualize the mu opioid receptors (MOR) of the striosome compartment, we incubated the NeuN labeled sections in a MOR primary antibody MOR1 (Immunostar, Hudson, WI) (1:5000) for 48 h at room temperature followed by rabbit biotinylated secondary antibody (Vector, Burlingame, CA) (1:500) for 75 min at room temperature. Sections were then briefly incubated in DAB with the addition of nickel ammonium sulphate (Vectastain ABC Kit, Vector, Burlingame, CA). Inspection using a light microscope revealed brown neuronal cell bodies for the NeuN positive cells engulfed by either purplish-black striosomes labeled with MOR1 and a low background extrastriosomal matrix.
Before analyzing the data for these experiments we inspected histological sections to confirm that mice received an injection in similar striatal regions.
3D Reconstructions, Volume Measurement and Cell Counts
We processed serial sections from AP: +0.4 to +1.00 mm (Hof, 2000) the striatal regions that contain most of the striosomes in mice (Krebs et al., 1991). We used 3D reconstructions to measure volume, detect pattern changes and measure the number of striosomes. In a procedure described in Lawhorn et al (2008), twenty serial coronal sections from each animal were digitized into 256 gray levels. We aligned the processed serial images of the striosomes, striatum and cortex using Imaris Bitplane software (Zurich, Switzerland) and then compiled aligned images to build a 3D reconstruction. This 3D reconstruction procedure allowed for automated measurements of the entire volume of the striosomes, the matrix, the striatum and the overlying cortex using each striatal section. We also used a stereological procedure called the Cavalieri point counting technique as a second method to obtain the volume of the striatum, striosome and matrix that uses the principles of systematic, random sampling (Gundersen and Jensen, 198)
We used a stereological software program (Stereoinvestigator: Microbrightfield, Williston, VT, USA) to count the cells. The perimeter of the striatum and the striosomes were traced in alternate sections using a 4× objective. We used the optical fractionator feature to conduct counts on striosomes, matrix and the striatum separately. We used 50 × 50 µm counting frames spaced evenly throughout the striatum (striatal grid size was 450×450 µm) and counts were obtained using a 40× objective.
Furthermore, we divided each striatum into quadrants to obtain regional measurements of volume and cell number.
Statistical Analyses
Data were analyzed using a two-way ANOVA for each behavioral and anatomical comparison. We used two-way ANOVAs (95 % confidence level) followed by a Fisher's LSD post hoc test (SPSS software) to analyze data. Treatment type and test day were used as factors in ANOVAs. Because wire maneuver data violated the principle of normality when displayed in a histogram (data were positively skewed under a curve), we used a Kruskall-Wallis H test followed by a Mann-Whitney U test to analyze fall latency (SPSS). A two-proportions t-test was used to examine ratios and a chi square analysis was used to determine regional differences in the striatum (Minitab). The Grubbs test (Prism Graphpad) was used to exclude outliers from our sample. A linear regression analysis was used to compare the relationship between behavioral and anatomical parameters (SPSS). All statistics were performed on absolute values. The data that is presented in the figures as percentages was done so that striosome and matrix values can be compared using the same axes. Data are reported as mean ± SEM.
RESULTS
Mid-striatal lesions resulted in striosome volume and cell loss
3D reconstructions of serial brain sections to measure volume
We found that the volume of MOR1 labeled striosomes in the DS1 animals was 28 ± 7 % smaller than in SAP animals, and 29 ± 4 % less than SAL1 Controls (p=.04; Fig 1, Fig 2A; Table 1). This amount of loss is similar to that seen in YAC128 HD mutant mice (Lawhorn et al., 2008). We also observed a change in the number of striosomes (DS1: 125 ± 11, SAL1: 198 ± 19, SAP: 191 ±18; p=.04) and there appeared to be fewer instances of strips that form a lattice (Brown et al 2002, Lawhorn et al., 2008). We saw no differences among the groups in the volumes of the matrix (p=.63; Fig 2B; Table 1), striatum (p=.27; Table 1) or cortex (p=.21 ; Table 1).
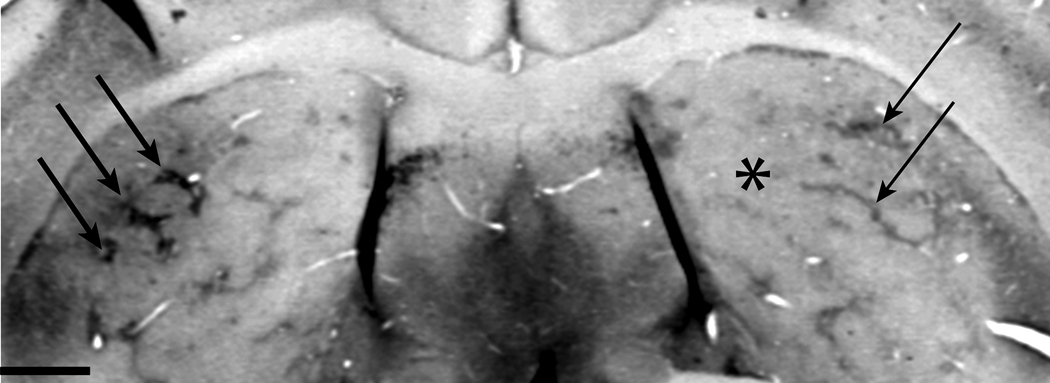
Figure 1.
Coronal section through the mouse striatum shows a unilateral injection of dermorphin-saporin to the right striatum (asterisk) and striosome label using a mu opioid receptor antibody. Striosomes are dark patches (arrows). The left striatum shows dark and full patches (thick arrows). In the right striatum striosomes are fewer and finer (thin arrows).
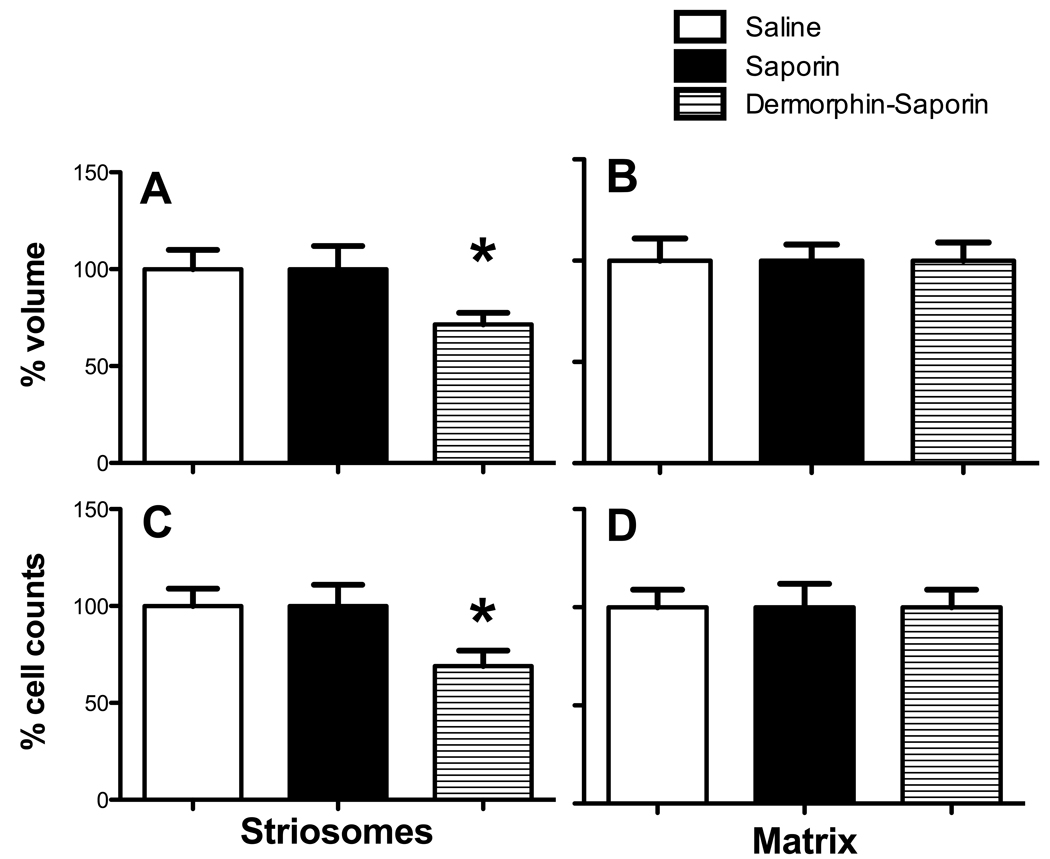
Figure 2.
Striosome and matrix volume and cell counts in Mid-Striatal lesion mice.
(A) In mice injected with DS, striosome volume was 28% less than WT mice. (B) There was no difference between treatment groups in the matrix volume. (C) Striosome cell counts were 31% fewer in Dermorphin-saporin mice than saline or saporin controls. (D) Matrix cell counts did not differ among groups. (n=8–10 per group; ANOVAs performed on raw data; *p<.05).
Table 1.
Serial section reconstruction volume measurements show striosome reduction in dermorphin-saporin lesion mice (mean ± SEM µm3/1 ×107)
| Injection Region |
Treatment Groups |
Striosome | Matrix | Striatum | Cortex |
|---|---|---|---|---|---|
| Mid Striatum | Saline 1 | 327 ± 18 | 1256 ± 72 | 1491 ± 78 | 3119 ± 111 |
| Saporin | 329 ± 42 | 1112 ± 96 | 1487 ± 94 | 3210 ± 89 | |
| Dermorphin- saporin 1 |
224 ± 16** | 1204 ± 56 | 1466 ± 107 | 3412 ± 68 | |
|
Mid + Anterior Striatum |
Saline 2 | 309 ± 63 | 1311 ± 82 | 1507 ± 136 | 3218 ± 94 |
| Dermorphin- saporin 2 |
174 ± 15** | 1217 ± 112 | 1487 ± 89 | 3482 ± 196 |
Data represent the volume of 20 serial sections.
p<.01 (n=8–10 per group)
Volume estimates using the Cavalieri point-counting method
The volume of the striosomes was 21 ± 8 % smaller in DS1 mice than in SAP controls and 26 ± 9 % than in SAL1 controls (p=.02; Table 2), confirming the findings from the 3D reconstructions of 2D images. Also, Cavalieri method volume estimates for the matrix (p=.27; Table 2) the striatum (p=.33; Table 2) and the cortex did not differ among groups.
Table 2.
Cavalieri estimation volume measurements show striosome reductions that are not different from serial section measurements (mean ± SEM µm3/1 ×107)
| Injection Region |
Treatment Groups |
Striosome | Matrix | Striatum | Cortex |
|---|---|---|---|---|---|
| Mid Striatum | Saline 1 | 306 ±15 | 1116 ± 91 | 1426 ± 58 | 3121 ± 62 |
| Saporin | 324 ± 13 | 1109 ± 22 | 1471 ± 91 | 3307 ± 88 | |
| Dermorphin- Saporin 1 |
217 ± 29* | 1126 ± 31 | 1465 ± 81 | 3401 ± 62 | |
|
Mid + Anterior Stiatum |
Saline 2 | 311 ± 27 | 1108 ± 69 | 1391 ± 94 | 3431 ± 35 |
| Dermorphin- Saporin 2 |
181 ± 21 ** | 1161 ± 14 | 1387 ± 92 | 3701 ± 92 |
p<.01
p<.05 (n=5 per group)
Cell counts
DS1-lesion mice showed a 29 ± 7 % decrease in cell number in the striosome compartment compared to SAP and a 32 ± 6% decrease in cells number compared to SAL1 (p=.01; Fig 2C; Table 3). We did not observe a significant difference among groups for cell number in the matrix (p=.33; Fig 2D; Table 3). For the whole striatum, DS-lesion mice showed a 4 ± 1% decrease in the number of cells compared to saline and 2 ± 2 % compared to saporin controls (p=.21; Table 3).
Table 3.
Cell counts show striosome reduction in dermorphin-saporin lesion mice (mean ± SEM 1 × 103).
| Injection Region |
Treatment Group |
Striosome | Matrix | Striatum |
|---|---|---|---|---|
| Mid Striatum | Saline 1 | 121 ± 72 | 1721 ± 69 | 1837 ± 48 |
| Saporin | 117 ± 36 | 1674 ± 78 | 1762 ± 89 | |
| Dermorphin- Saporin 1 |
80 ± 17* | 1589 ± 82 | 1843 ± 88 | |
|
Mid + Anterior Striatum |
Saline 2 | 119 ± 28 | 1636 ± 89 | 1787 ± 71 |
| Dermorphin- Saporin 2 |
60 ± 12 ** | 1682 ± 56 | 1752 ± 69 |
p<.05
p<.01 (n=5–10 per group)
Striosome-Matrix Ratios
We used a 2-proportions t-test to examine the ratio of striosome to matrix cells among DS1, SAP and SAL1 groups. As expected, the ratio of striosome to matrix cells was significantly different among treatment groups. Per every 100 cells of matrix, we counted 5 striosomal cells in DS1 mice compared to 7 striosomal cells in SAP (p=.02), and SAL1 controls (p =.01).
Regional specificity
Using a chi square analysis, we observed significant volume reductions in all areas of DS1 mice, but the smallest striosome volume among the quadrants was in the dorsolateral region (p<.03; Table 4). The volume of the extrastriosomal matrix did not differ among regions (p=.36; Table 4). In addition, our stereological analysis of cell number revealed degeneration effects in all quadrants, but the fewest cells in striosomes were in the dorsolateral quadrant for the DS1 group (p<.01; Table 4). There was no significant difference in cell counts among regions in the extrastriosomal matrix (p=.32; Table 4).
Table 4.
Cavalieri volume1 and cell counts2 show regional reduction (MOR1 label) in dermorphin-saporin lesioned mice (mean ± SEM)
| Injection Region |
Treatment Groups |
DL | DM | VL | VM | |
|---|---|---|---|---|---|---|
|
Mid Striatum |
Striosomes Volume |
Saline 1 | 61 ± 19 | 62 ± 11 | 59 ± 15 | 62 ± 33 |
| Saporin | 63 ± 19 | 61 ± 21 | 63 ± 23 | 62 ± 19 | ||
| Dermorphin- Saporin 1 |
44 ± 7* | 46 ± 9* | 42 ± 11* | 45 ± 17* | ||
| cell counts | Saline 1 | 28 ± 4 | 24 ± 1 | 18 ± 3 | 28 ± 1 | |
| Saporin | 29 ± 3 | 27 ± 5 | 19 ± 2 | 25 ± 2 | ||
| Dermorphin- Saporin 1 |
11 ± 3**† | 17 ± 3* | 16 ± 3* | 19 ± 5* | ||
|
Matrix Volume |
Saline 1 | 254 ± 81 | 288 ± 31 | 260 ± 36 | 271 ± 21 | |
| Saporin | 296 ± 83 | 271 ± 45 | 288 ± 47 | 269 ± 38 | ||
| Dermorphin- Saporin 1 |
278 ± 48 | 276 ± 43 | 269 ± 67 | 260 ± 76 | ||
| cell counts | Saline 1 | 392 ± 29 | 371 ± 78 | 388 ± 96 | 361 ± 82 | |
| Saporin | 359 ± 37 | 396 ± 57 | 364 ± 79 | 371 ± 71 | ||
| Dermorphin- Saporin 1 |
||||||
| 376 ± 69 | 382 ± 91 | 379 ± 67 | 391 ± 78 | |||
|
Mid + Anterior Striatum |
Striosomes Volume |
Saline 2 | 63 ± 26 | 65 ± 19 | 68 ± 4 | 69 ± 21 |
| Dermorphin- Saporin 2 |
27 ± 19** | 39 ± 11* | 40 ± 9* | 36 ± 18* | ||
| cell counts | Saline 2 | 29 ± 2 | 24 ± 1 | 23 ± 1 | 24 ± 1 | |
| Dermorphin- Saporin 2 |
9 ± 5**† | 13 ± 3* | 14 ± 1* | 16 ± 2* | ||
|
Matrix Volume |
Saline 2 | 261 ± 17 | 280 ± 22 | 277 ± 33 | 281 ± 19 | |
| Dermorphin- Saporin 2 |
252 ± 23 | 271 ± 16 | 269 ±22 | 260 ± 9 | ||
| cell counts | Saline 2 | 386 ± 88 | 363 ± 11 | 366 ± 17 | 380 ± 20 | |
| Dermorphin- Saporin 2 |
391 ± 41 | 356 ± 67 | 380 ± 24 | 391 ± 37 | ||
volume values expressed are 1 × 107 µm32 cell count values are expressed as 1 × 103
p <.05
p<.01 show differences between groups
chi square analysis shows differences within dermorphin-saporin group; DM = dorsomedial, DL= dorsolateral, VM- ventromedial, VL = ventrolateral
Thus, DS injections into the mid striatum of FVB mice induced a partial striosome-lesion that affected the volume and number of cells in the striosomes relatively specifically. The DS toxin did not have a significant effect on the extrastriosomal matrix, striatum or cortex. The lesion affected all striatal areas, but had its greatest effect in the dorsolateral quadrant, indicated by a greater degree of volume and cell loss to that region. In addition, the greater percentage of cells lost in the striosomes resulted in a compartmental imbalance in the ratio of cells between the compartments. Of interest, the measurements did not detect a difference in the volume of the overall striatum, even though striosomes were significantly affected.
Mid + Anterior striatal lesions resulted in a large volume and cell loss to striosomes
3D reconstruction volume measurements
Compared to SAL2 control mice, DS2 mice with mid + anterior striatal lesions showed a 44 ± 6 % (Fig 3A; p=.007) smaller striosomal volume. This volume decrease was greater than was seen in mice with mid striatal lesions (p=.03). The mid + anterior striatal lesion mice did not differ from SAL2 controls in the volume of the matrix (p=.36; Fig 3B; Table 1) or total striatum, (p=.45; Table 1) or cortex (p=.27; Table 1). The volume of the matrix also did not differ between DS2 and SAL2 groups (p=.43). In addition, the number of striosomal objects decreased by 50% in DS2 mice compared to SAL2 controls (DS2: 87 ± 7 striosomal objects, SAL2: 188 ± 18 striosomal objects, p=.01). The number of striosomes was significantly different between DS1 and DS2 lesion mice (p =.05).
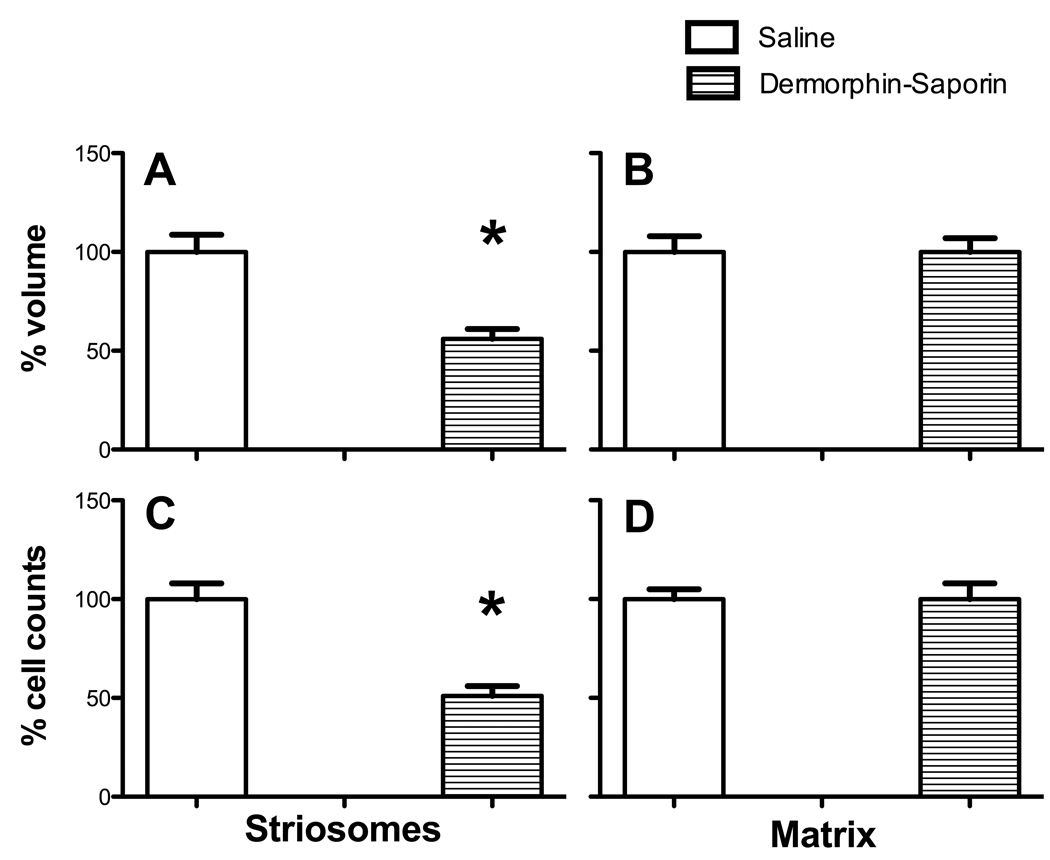
Figure 3.
Striosome and matrix volume and cell counts in Mid+Anterior Striatal lesion mice. (A) In DS mice, striosome volume was 46 % less than WT mice. (B) There was no difference between treatment groups in the matrix volume. (C) Striosome cell counts were 49 % fewer in Dermorphin-saporin mice than saline controls. (D) Cell counts in the matrix did not differ between groups. (n=10 per group; ANOVAs performed on raw data; *p<.05).
Cavalieri volume estimates
Cavalieri estimates confirmed the amount of loss found using all serial sections (Table 2). Compared to SAL2 controls, mice with DS2 injections showed a 46 ± 8 % volume loss in striosomes, and no significant difference in the matrix (p=.26) or the overall striatum (p=.22).
Cell Counts
DS2 animals showed a 49 ± 4% decrease in the number of cells in the striosome compartment compared to SAL2 controls (p=.009; Fig 3C; Table 3). This decrease in striosomal cells was greater than was observed in DS1 mice with mid striatal lesions (p=.04). In the whole striatum, cell counts in mid + anterior lesion mice showed an 8 ± 4% decrease compared to saline (p=.32). We did not observe a significant difference among mid + anterior striatal lesion groups in whole-striatum cell counts (p=.11) or in the matrix (p=.33; Fig 3F; Table 3).
Striosome-Matrix Ratios
When we examined the ratio of striosome-to-matrix cells between DS2 and SA2, groups, the ratio of striosome to matrix cells was significantly different; per every 100 cells of matrix, we counted 3 striosomal cells in DS2 mice compared to 7 striosomal cells in SAL2 controls, (p=.006).
Regional Specificity
A chi square analysis showed that all striatal regions were affected by the DS2 lesion, but the smallest striosome volume among the quadrants was in the dorsolateral region (p<.01; Table 4). The volume of the extrastriosomal matrix did not differ across regions (p=.42; Table 4). Our stereological cell counts also revealed the fewest number of cells in striosomes in the dorsolateral quadrant for DS2 compared to other regions, (p<.001; Table 4) and no significant difference in cell counts among quadrants in the extrastriosomal matrix (p=.11; Table 2).
Thus, dermorphin-saporin lesions made to both the anterior and mid striatum resulted in a greater percentage of volume loss, number of striosomes cells lost and number of striosomes lost than mice that received a single injection in the mid striatum. These D2 mice also demonstrated greater loss of striosomes than D1 mice, and the ratio of striosome to matrix cells also showed an imbalance.
Mice with striosome pathology performed poorly on the rotarod
Prior to lesion injections, all mice showed equal performance across three days of training (Fig 4A–4B; days 1–3). After DS1 mid striatal injections, there was a significant main effect of TREATMENT (Fisher’s LSD post hoc tests p<.05; Fig. 4A). The DS1 group was worse than SAL1 and SAP, but SAL1 and SAP were not different from each other (p=.36). There was also a significant main effect of DAY, suggesting all the animals were getting better over time (p<.05). However, there was no DAY by TREATMENT interaction (Fig 4A).
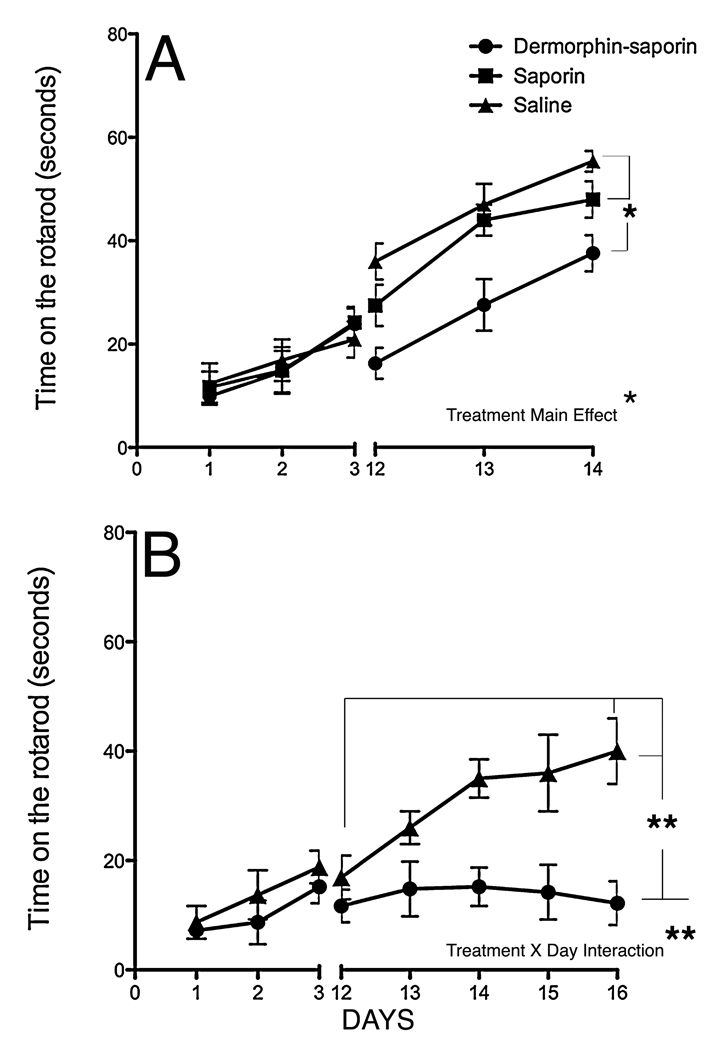
Figure 4.
Accelerating rotarod performance. (A) Mice with a Mid-striatal DS lesion showed poorer mean performance on the rotarod on days 12–14, compared to saporin and saline controls (ANOVA, main effect of treatment; *p<.05) but similar improvement over time. (B) Mice with Mid+Anterior striatal DS lesions showed poorer performance on the rotarod and no improvement over time on days 12–17, compared to saline controls (ANOVA, Day by Treatment interaction; **p<.01; n=10 per group)
DS2 lesion mice showed poorer performance on the rotarod than DS1 mice, and no improvement over time compared to SAL2 controls. In the DS2 group, a 2-way ANOVA revealed that there was a significant main effect of TREATMENT (DS2 mice were worse performers than SAL2; p<.05) and there was a significant DAY by TREATMENT interaction; p<.05 (Fig 4B). The D2 animals did not improve over the 5 days of post-lesion testing. When we compared the best performance of DS1 and DS2 lesion mice, DS2 mice were significantly worse (p=.01)
We also tested mice in the open field, wire maneuver and balance beam and saw no difference among the groups on any task before and after the lesion (Table 5). The mice from both the lesion and control groups showed no evidence of improvement on these tasks over time. Because striosomes have also been indicated in emotional deficits (Tippett et al. 2007), we measured thigmotaxis in the open field and also used the light dark-test anxiety test, as a means to determine if striosome lesions induced anxiety-like behaviors in the mice. We also saw no deficits in anxiety-like tasks after striatal lesions (Table 5). Finally, to determine if there were gross cognitive deficits we used a simple spontaneous alternation task using a Y-maze and observed no deficits between groups (Table 5).
Table 5.
Performance on behavioral tasks with no effects (ANOVA).
| Injection Region |
Behavioral test | Saline | Saporin | Dermorphin- Saporin |
|---|---|---|---|---|
| Mid Striatum | Balance beam (slips) | 6 ± 1 | 5 ± .6 | 5 ± .4 |
| Wire Maneuver (seconds) |
48 ± 3 | 46 ± 4 | 47 ± 3 | |
| Open Field (distance traveled in cm; velocity in cm/sec; time spent in center in sec) |
2903 ± 54 5 ± .2 48 ± 1.5 |
2841 ± 46 5 ± .2 49 ± 1.4 |
2881 ± 40 5 ± .1 50 ± 1.5 |
|
| Light Dark test (time spent in light in sec) |
172.6 ± 31.5 | 149.9 ± 22.8 | 163.8 ± 53.9 | |
| Y-maze (% alternation) |
53 ± 3 | 55 ± 5 | 57 ± 4 | |
|
Mid + Anterior Striatum |
7± 2 | 6 ± 1 | ||
| Balance beam (slips) | ||||
| Wire Maneuver (seconds) |
54 ± 8 | 55 ± 6 | ||
| Open Field (distance traveled in cm; velocity in cm/sec; time spent in center sec) |
2885 ± 46 6 ± 1 52 ± 3 |
2900 ± 52 5 ±.1 49 ±2 |
||
| Light Dark test (time spent in light in sec) |
160 ± 25.4 | 210 ± 48 | ||
| Y-maze (% alternation) |
50 ± 6 | 51 ± 6 |
(n=10 per group)
Data are the difference scores of before and after injection (mean ± SEM).
Thus, striosome cell-specific lesions were followed by rotarod performance and daily improvement deficits, but no deficits in other behavioral tasks. Furthermore, increasing the size of the lesion increased the striosome degeneration and the imbalance of the ratio of matrix to striosome cells. This pathology resulted in a greater deficit on the accelerating rotarod task than smaller lesions, and the lack of improvement over 5 days suggests a motor learning deficit.
DISCUSSION
This is the first report of the use of the specific neurotoxin DS to ablate the MOR expressing cells that represent the striosomes in the striatum to evaluate motor behavior. It is also the first use of the toxin in mice. Both striosome volume (−26%) and cell counts (−31%) were affected in mice that received bilateral mid striatal lesions. In mice that received lesions to both the mid and anterior striatum, striosome volume (−44 %) and cells counts (−49%) were more affected than in the single-lesion group. The DS injections did not significantly change the number of cells in the matrix or overall striatum compared to controls. Thus, an imbalance in the matrix-to-striosome compartmental activation was created that could account for some motor deficits, as well as the simple loss of striosome cell output controlling dopamine cell activity in the substantia nigra and striatum.
These data also demonstrate that the greater the striatal MOR-containing cell lesion, the worse the performance on an accelerating rotarod task. The data suggest that the striosomes are critical to performance and perhaps learning on the accelerating rotarod task. Striosome pathology was associated with the same learning deficits observed in YAC mouse models for HD (Lawhorn et al., 2008). The two methods we used to measure volume provide strong evidence for the volume loss, and are an example of the success of estimation procedures (West, 1991, Gundersen and Jensen 1987). We conclude that (1) DS provides a unique tool to investigate the functional significance of the striosomes, (2) striosomes in normal mice play a role in a motor skill learning task that involves adjusting to acceleration (3) the loss of striosome MOR-containing cells may account for rotarod deficits observed in the TAC128 model of HD. Learning the rotarod task may be affected because feedback is poor, because central motor responses are compromised, or because a critical link for adjusting movement is disrupted. The normal responses on other motor tasks suggest that the animals are not motorically impaired enough centrally or peripherally to account for the deficits.
Striosome lesions and rotarod learning deficits?
One of the principal methods to approach the study of brain function has been to experimentally destroy a structure and examine ensuing behavior. Several studies of the basal ganglia have successfully lesioned striatal regions to determine its role in motor behavior (Cromwell and Berridge, 1996, Aldridge et al., 1997, Brooks et al., 2007). Yet a major question that remains is: what is the functional role of the striatum’s striosome and matrix compartmentalization? Several groups have sought to determine their role in both healthy and disease states (Seto-Ohshima et al., 1988, Ferrante et al., 1987, White and Hiroi, 1998, Canales and Graybiel, 2000, Brown et al., 2002, Tappe and Kuner, 2006). Data from this study suggests striosomes could be involved in motor learning.
Mice that received a double DS injection to both the mid and anterior showed significantly worse performance than the single lesion mice and than controls, and did not show improvement over days. Several factors might have contributed to the differences in behavioral outcomes between these two groups. First, the extent of the lesion encompassed a greater striatal region in the double lesion group suggesting that a greater extent of damage would correspond to an increased deficit in that group. Second, there is also the possibility that the regional differences and patterns of connectivity between the mid and anterior striatum contribute to the differences in motor performance (Gerfen, 1988, Levesque et al., 2003). Lesions to dorsolateral anterior regions have been associated with disruption of complex sequencing tasks in rodents (Cromwell and Berridge, 1996) and therefore may be well-positioned to cause impaired performance on the rotarod task. Future studies in which the striosomes of just the anterior striatum are damaged are necessary.
Furthermore, striosomes make discrete projections to the substantia nigra compacta region, which provides the major dopaminergic input to the striatum. It is likely that striosome perturbation could disrupt striatal dopamine levels (Gerfen et al., 1985). Based on the anatomical projections of striosomes, one group interpreted these findings to suggest that dopamine dysregulation leads to the unintentional and inappropriate release of motor programs such as those seen in the constant involuntary movements of patients with HD (Hedreen and Folstein, 1995). In congruence with this theory, striosomes may be positioned to facilitate dopaminergic feedback to matrix and cortical circuits that facilitate reinforcement learning of the rotarod (Joel et al., 2002). Dopamine depletion in the striatum has been associated with motor skill learning deficits on the rotarod in rats (Ogura et al., 2005). Dopamine d1 receptor antagonists in the striatum have been shown to interfere with motor skill learning in rats (Willuhn and Steiner, 2008). A role in learning would also be consistent with the reward function proposed by White and Hiroi (1998) for the striosomes. A perturbation of striosomes such as was caused by DS injections might therefore disrupt an animal’s ability to learn the rotarod task at the level of mice with control lesions.
Interestingly, Costa et al (2004) used microelectrodes to demonstrate that different phases of motor skill learning (fast and slow) activate different ensembles of neurons in the striatum and motor cortex. We measured the post-lesion rotarod performance over 5 days (Fig 4B) and noticed that saline animals reach a plateau indicating a transition from fast to slow phase of learning, while mice with large (DS2) lesions show no fast learning over the post-lesion days. Our lesions may have affected regions that are involved in fast motor skill learning. Future studies that address the neuronal firing, learning phases and plasticity in mice with DS2-like lesions are needed.
Implications for Huntington’s disease mouse models
The rotarod is a universally administered apparatus in HD research. It is regarded as superior because the behavior is easy to quantify, requires minimal handling and training (Bain, 2002). Hence any understanding of the neural structures that contribute to the circuitry involved in this task makes a significant contribution to the field of mouse models for HD. Interestingly, though mice with DS lesions showed a large percentage of striosomal loss in this study, their behavioral deficits still did not equal those of age matched YAC128 mutants (Slow et al., 2003, Tang et al., 2007, Van Raamsdonk et al., 2007a, Lawhorn et al., 2008). Symptom differences between the lesion mice and the YAC mutants may be caused in part by differences in the regional pattern of cell loss between the groups, and/or the additional cortical systems and matrix cells affected in HD mice.
Limitations
While we made a select lesion to striosomes that largely spared cells from the matrix, we cannot rule out the possibility that the behavioral deficits that resulted could have also occurred because there were undetectable changes in matrix cells. Matrix and striosome cells are both comprised of medium spiny neurons and a small portion of mu opioid receptors exist in the matrix and those cells could have been affected. In addition, we cannot assume that because matrix cells appear structurally healthy, they continue to function exactly the same as they did before the lesion. It should also be noted that we affected only a portion of the striosomes and that an experiment that obliterates the entire population might produce different results. In addition, new siRNA techniques that target the gene-specific sequences of MORs and can “silence” striosomes may also prove useful for further determining and/or confirming their role in motor behavior and learning (Zhang et al, 2009). Also, we have to assume that a certain level of brain reconfiguration is possible with any technique that reduces the striosomes, which makes it difficult to infer an exact striosome function. Finally, this was a partial lesion. Like dopamine depletion, a larger loss may be needed to detect greater deficits.
CONCLUSION
In summary, our results demonstrate that the MOR expressing cells in the striatum that represent the striosomes are critically involved in the expression of normal accelerating rotarod performance. In addition, the relatively stable performance of lesioned mice on other tasks despite marked striosome degradation indicates the importance of extrastriosomal matrix neurons for maintaining motor function (Brown et al., 2002). By gaining an increased awareness of the complex relationship between striosome function and motor behaviors we can help to form new hypotheses for their role in motor skill learning and potential treatment of neurological disorders like Huntington’s disease.
Acknowledgments
Grant Support: NINDS - R01 21356; F31 NS055592-02; APA - Diversity Fellowship Program in Neuroscience
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aldridge JW, Thompson JF, Gilman S. Unilateral striatal lesions in the cat disrupt well-learned motor plans in a GO/NO-GO reaching task. Exp Brain Res. 1997;113:379–393. doi: 10.1007/pl00005592. [DOI] [PubMed] [Google Scholar]
- Bain L. Behavioral Assessment in Mouse Models of Huntington’s Disease. Cardiff: Hereditary Disease Foundation: Workshop Reports. 2002 [Google Scholar]
- Brown LL, Hand PJ, Divac I. Representation of a single vibrissa in the rat neostriatum: peaks of energy metabolism reveal a distributed functional module. Neuroscience. 1996;75(3):717–728. doi: 10.1016/0306-4522(96)00310-7. [DOI] [PubMed] [Google Scholar]
- Bates G, Harper P, Jones L. Huntington's Disease. Oxford: Oxford University Press; 2002. [Google Scholar]
- Brooks SP, Trueman RC, Dunnett SB. Striatal lesions in the mouse disrupt acquisition and retention, but not implicit learning, in the SILT procedural motor learning task. Brain Res. 2007;1185:179–188. doi: 10.1016/j.brainres.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Brown LL, Feldman SM, Smith DM, Cavanaugh JR, Ackermann RF, Graybiel AM. Differential metabolic activity in the striosome and matrix compartments of the rat striatum during natural behaviors. J Neurosci. 2002;22:305–314. doi: 10.1523/JNEUROSCI.22-01-00305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What's wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. New York: Wiley Liss; 2000. [Google Scholar]
- Cromwell HC, Berridge K. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J Neurosci. 1996;16:3444–3458. doi: 10.1523/JNEUROSCI.16-10-03444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desban M, Kemel ML, Glowinski J, Gauchy C. Spatial organization of patch and matrix compartments in the rat striatum. Neuroscience. 1993;57(3):661–671. doi: 10.1016/0306-4522(93)90013-6. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Kowall NW, Beal MF, Martin JB, Bird ED, Richardson EP., Jr Morphologic and histochemical characteristics of a spared subset of striatal neurons in Huntington's disease. J Neuropathol Exp Neurol. 1987;46:12–27. doi: 10.1097/00005072-198701000-00002. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Miller JJ. The neostriatal mosaic: compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc Natl Acad Sci USA. 1985;82:8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. Synaptic organization of the striatum. J Electron Microsc Tech. 1988;10:265–281. doi: 10.1002/jemt.1060100305. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Ragsdale CW., Jr Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad of Sci USA. 1978;75:5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Martone M, Young SJ, Armstrong DM. Three-dimensional pattern of enkephalin-like immunoreactivity in the caudate nucleus of the cat. J Neurosci. 1988;8(3):892–900. doi: 10.1523/JNEUROSCI.08-03-00892.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H, Jensen E. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hedreen JC, Folstein SE. Early loss of neostriatal striosome neurons in Huntington's disease. J Neuropathol Exp Neurol. 1995;54:105–120. doi: 10.1097/00005072-199501000-00013. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Pert CB. Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature. 1981;291:415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, Smith DJ, Bissada N, McCutcheon K, Nasir J, Jamot L, Li XJ, Stevens ME, Rosemond E, Roder JC, Phillips AG, Rubin EM, Hersch SM, Hayden MR. A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron. 1999;23:181–192. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative cytoarchitectonic atlas of the c57bl/6 and 129/sv mouse brain. Amsterdam: Elsevier; 2000. [Google Scholar]
- Joel D, Niv Y, Ruppin E. Actor-critic models of the basal ganglia: new anatomical and computational perspectives. Neural Netw. 2002;15:535–547. doi: 10.1016/s0893-6080(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Krebs MO, Trovero F, Desban M, Gauchy C, Glowinski J, Kemel ML. Distinct presynaptic regulation of dopamine release through NMDA receptors in striosome- and matrix-enriched areas of the rat striatum. J Neurosci. 1991;11:1256–1262. doi: 10.1523/JNEUROSCI.11-05-01256.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lawhorn C, Smith DM, Brown LL. Striosome-matrix pathology and motor deficits in the YAC128 mouse model of Huntington's disease. Neurobiol Dis. 2008;32:471–478. doi: 10.1016/j.nbd.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Lawhorn C, Smith DM, Leavit BR, Brown LL. Society for Neuroscience Annual meeting Abstracts. New Orleans, LA: 2003. Motor deficits and striosome volume in a YAC mouse model of Huntington's disease. [Google Scholar]
- Levesque M, Bedard A, Cossette M, Parent A. Novel aspects of the chemical anatomy of the striatum and its efferents projections. J Chem Neuroanat. 2003;26:271–281. doi: 10.1016/j.jchemneu.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menalled LB, Sison JD, Wu Y, Olivieri M, Li XJ, Li H, Zeitlin S, Chesselet MF. Early motor dysfunction and striosomal distribution of huntingtin microaggregates in Huntington's disease knock-in mice. J Neurosci. 2002;22:8266–8276. doi: 10.1523/JNEUROSCI.22-18-08266.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T, Ogata M, Akita H, Jitsuki S, Akiba L, Noda K, Hoka S, Saji M. Impaired acquisition of skilled behavior in rotarod task by moderate depletion of striatal dopamine in a pre-symptomatic stage model of Parkinson's disease. Neurosci Res. 51:299–308. doi: 10.1016/j.neures.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Pert CB, Kuhar MJ, Snyder SH. Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci USA. 1976;73:3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8:711–713. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC. Lessons from animal models of Huntington's disease. Trends Genet. 2002;18:202–209. doi: 10.1016/s0168-9525(01)02625-7. [DOI] [PubMed] [Google Scholar]
- Seto-Ohshima A, Emson PC, Lawson E, Mountjoy CQ, Carrasco LH. Loss of matrix calcium-binding protein-containing neurons in Huntington's disease. Lancet. 1988;4:1252–1255. doi: 10.1016/s0140-6736(88)92073-9. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y, Oh R, Bissada N, Hossain SM, Yang YZ, Li XJ, Simpson EM, Gutekunst CA, Leavitt BR, Hayden MR. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet. 2003;12:1555–1567. doi: 10.1093/hmg/ddg169. [DOI] [PubMed] [Google Scholar]
- Tang TS, Chen X, Liu J, Bezprozvanny I. Dopaminergic signaling and striatal neurodegeneration in Huntington's disease. J Neurosci. 2007;27:7899–7910. doi: 10.1523/JNEUROSCI.1396-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe A, Kuner R. Regulation of motor performance and striatal function by synaptic scaffolding proteins of the Homer1 family. Proc Natl Acad Sci USA. 2006;103:774–779. doi: 10.1073/pnas.0505900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippett LJ, Waldvogel HJ, Thomas SJ, Hogg VM, van Roon-Mom W, Synek BJ, Graybiel AM, Faull RL. Striosomes and mood dysfunction in Huntington's disease. Brain. 2007;130:206–221. doi: 10.1093/brain/awl243. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Chiken S, Kametani K, Moriizumi T. Efferent projections from the striatal patch compartment: anterograde degeneration after selective ablation of neurons expressing mu-opioid receptor in rats. Neurosci Lett. 2002;332:5–8. doi: 10.1016/s0304-3940(02)00837-6. [DOI] [PubMed] [Google Scholar]
- Trytek ES, White IM, Schroeder DM, Heidenreich BA, Rebec GV. Localization of motor and nonmotor related neurons within the matrix -striosome organization of rat striatum. Brain Res. 1996;707(2):221–227. doi: 10.1016/0006-8993(95)01261-3. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Metzler M, Slow E, Pearson J, Schwab C, Carroll J, Graham RK, Leavitt BR, Hayden MR. Phenotypic abnormalities in the YAC128 mouse model of Huntington disease are penetrant on multiple genetic backgrounds and modulated by strain. Neurobiol Dis. 2007a;26:189–200. doi: 10.1016/j.nbd.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Warby SC, Hayden MR. Selective degeneration in YAC mouse models of Huntington disease. Brain Res Bull. 2007b;72:124–131. doi: 10.1016/j.brainresbull.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Pearson J, Slow EJ, Hossain SM, Leavitt BR, Hayden MR. Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington's disease. J Neurosci. 2005;25:4169–4180. doi: 10.1523/JNEUROSCI.0590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Steiner H. Motor-skill learning in a novel running-wheel task is dependent on D1 dopamine receptors in the striatum. Neuroscience. 2008;153:249–258. doi: 10.1016/j.neuroscience.2008.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM, Hiroi N. Preferential localization of self-stimulation sites in striosomes/patches in the rat striatum. Proc Natl Acad Sci USA. 1998;95:6486–6491. doi: 10.1073/pnas.95.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R, Colle L. Pimozide attenuates free feeding: best scores analysis reveals a motivational deficit. Psychopharmacol. 1984;66:219–225. doi: 10.1007/BF00431448. [DOI] [PubMed] [Google Scholar]
- Wise RA, Raptis L. Effects of naloxone and pimozide on initiation and maintenance measures of free feeding. Brain Res. 1986;368:62–68. doi: 10.1016/0006-8993(86)91042-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Landthaler M, Schlussman SD, Yuferov V, Ho A, Tuschl T, Kreek MJ. Mu opioid receptor knockdown in the substantia nigra/ventral tegmental area by synthetic small interfering RNA blocks the rewarding and locomotor effects of heroin. Neuroscience. 2009;158:474–483. doi: 10.1016/j.neuroscience.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Lee M, Agatsuma S, Hiroi N. Pleiotropic impact of constitutive fosB inactivation on nicotine-induced behavioral alterations and stress-related traits in mice. Hum Mol Genet. 2007;16:820–836. doi: 10.1093/hmg/ddm027. [DOI] [PubMed] [Google Scholar]