Abstract
Objectives. To quantify the sibling risk of RA, SLE and AS. To analyse the concordant and discordant associations between RA, SLE and AS.
Methods. Follow-up study of all individuals and their siblings born in or after 1932 and hospitalized for RA, SLE or AS between 1973 and 2004 (32 yrs). Data were retrieved from a comprehensive dataconstructed by using several national Swedish data registers, including the Total Population Register, the Swedish Hospital Discharge Register and the Multigeneration Register. Standardized incidence ratios (SIRs) were used to estimate sibling risks.
Results. For males, the overall significant SIRs were 4.72, 4.35 and 4.14 for RA, SLE and AS, respectively, if a sibling was affected by any inflammatory disease. The corresponding significant SIRs for females were 4.12, 3.73 and 4.73. The concordant significant SIRs in siblings were 5.12, 17.02 and 17.14 for RA, SLE and AS, respectively. There were also discordant associations between RA and SLE, whereas AS was only associated with AS.
Conclusions. This study was able objectively to quantify the sibling risk of RA, SLE and AS, which represents useful knowledge for clinicians and geneticists. The analysis of concordant and discordant associations may be useful in future studies aimed at finding specific genes associated with these diseases.
Keywords: Ankylosing spondylitis, Rheumatoid arthritis, Siblings, Sweden, Systemic lupus erythematosus
Introduction
RA, SLE and AS (Bechterew's disease) are caused by misdirected inflammation in the joints, internal organs and/or spine. The prevalence of RA and AS in industrialized countries is 1–2% and 0.5–4%, respectively [1]. The prevalence rates of SLE are ∼4/100 000 men and 45/100 000 women [2, 3].
These inflammatory diseases are incurable and they are characterized by chronic pain, joint deformities, neuropsychiatric disorders [4–9] and a reduced quality of life [4, 10–12].
Although novel medical treatments for these chronic diseases (e.g. TNF-α blocking agents in RA and rituximab in SLE) have produced encouraging results, the required medication could be lifelong and associated with adverse side-effects. Much effort has therefore been invested in genetic studies in order to find better targets for treatment. Recent genetic studies have resulted in substantial progress with several strong candidate genes being detected [13], although there is still much room for improved knowledge.
Twin and family studies show that susceptibility to RA has a heritable basis with some identified candidate genes [14, 15]. However, HLA-DRB1 is the only genetic risk factor for RA that has been consistently observed across populations [16]. It has been estimated that 30% of the genetic contribution to RA can be attributed to HLA genes [17]. Other genes such as PTPN22, PAD14 and FCRL3 have been shown to be associated with RA susceptibility in some populations [18].
Previous studies of SLE have resulted in the identification of several susceptibility genes at several loci. However, the heterogeneity of these findings across population groups has complicated their interpretation [19, 20].
The susceptibility to AS is largely genetically determined and linked to HLA-B27 as the major gene. Previous studies have shown that the concordance in identical twins is 63% although <5% of HLA-B27-positive people in the general population develop the disease [21, 22].
Clinical risk estimates of RA, SLE and AS among first-degree relatives have mostly been based on relatively small sample sizes, case–control studies and/or have suffered from recall bias. A novel approach of the present large-scale study is that it was based on data from the unique national registers in Sweden that include remarkably complete population data linked to individual hospitalization data. This gave us the opportunity to include all hospitalizations in Sweden for these inflammatory diseases between 1973 and 2004. The use of hospitalization data and the inclusion of the entire Swedish population gave us enough statistical power to objectively quantify the sibling risk for these diseases, which was the primary aim of this study. In addition, the large-scale approach gave us the opportunity to analyse the concordant and discordant associations between RA, SLE and AS in siblings during a 32-yr period, which could be helpful in future studies aimed at finding specific genes associated with these diseases.
Methods
Data used in this study were retrieved from the MigMed database, located at the Center for Family and Community Medicine at the Karolinska Institute in Stockholm. MigMed is a single, comprehensive database that has been constructed using several national Swedish data registers, including, but not limited to, the Total Population Register and the Swedish Hospital Discharge Register [23, 24].
Since the database also contains information from the Multigeneration Register, it is possible to link index persons (persons born in or after 1932 and registered in Sweden at any time since 1947) with their biological siblings and parents, i.e. more than 3.2 million families. The latest version of the Multigeneration Register, which has been incorporated in the MigMed database, includes supplementary data from church records on index persons domiciled in Sweden between 1947 and 1961.
Information from the various registers in the database is linked at the individual level via the 10-digit national registration number assigned to each person in Sweden for his or her lifetime. Prior to inclusion in the MigMed database, national registration numbers were replaced by serial numbers to ensure the anonymity of all individuals.
Diagnostic codes were retrieved from the Swedish Hospital Discharge Register reported according to the 8th (1973–86), 9th (1987–96) and 10th (1997–2004) versions of the International Classification of Diseases (ICD 8, ICD 9 and ICD 10). The Swedish Hospital Discharge Register covers all hospitals in Sweden. The following ICD codes were included:
AS
ICD 8: 712.4; ICD 9: 720.A; ICD 10: M45, M081.
SLE
ICD 8: 734.1; ICD 9: 710.A; ICD 10: M32.
RA
ICD 8: 712.1, 712.3; ICD 9: 714 (except 714.E and 714.X); ICD 10: M05, M06, M080, M082.
Only first hospitalizations during the study period were included. The juvenile forms of the rheumatic disorders listed above were also included.
Individual variables
Gender
Males and females.
Age
At hospital diagnosis age was categorized in 5-yr groups and the groups were merged as necessary.
Geographic region
It was divided into large cities (cities with a population of >200 000, i.e. Stockholm, Gothenburg and Malmö), Southern Sweden and Northern Sweden. Southern and Northern Sweden were divided at the river Dalälven, which is regarded as the traditional ‘border’ between Southern and Northern Sweden. Geographic region was included as an individual variable to adjust for possible differences between geographic regions in Sweden with regard to hospital admissions for psychotic disorders.
Time period
It was included in order to adjust for possible differences in hospitalization rates over time.
Statistical analysis
Person-years were calculated for siblings with at least one affected sibling and for siblings with no affected sibling. The follow-up started on 1 January 1973 and proceeded until the hospital diagnosis, death, emigration or the end of the study on 31 December 2004. Age-standardized incidence ratios (SIRs) were calculated for the whole follow-up period, divided into 5-yr periods. SIRs were calculated as the ratio of the observed to the expected number of cases. The expected number of cases was calculated specifically for age (in 5-yr groups), gender, time period and geographic region, which represents specific standardized incidence rates. Sibling risks (defined as SIRs) were calculated by considering multiple sibships. In families, where at least two siblings were affected, each was counted as an index-patient. Sibling risks were calculated in male and female siblings affected with the disease in question, compared with males and females whose siblings were not affected by this condition, and they were adjusted for dependence between the sibling pairs [25]. Index-patients without a sibling were excluded. Assuming a Poisson distribution 95% CIs were calculated.
Ethical considerations
This study was approved by the Ethics Committee of the Karolinska Institute, Stockholm, Sweden. Serial numbers were used for all data linkages in order to ensure the anonymity of all individuals.
Results
Table 1 shows the number of hospitalized cases for the inflammatory diseases RA, SLE and AS. The number of cases of RA, SLE and AS varied with gender. Hospitalizations for RA, SLE and AS are referred to below only as the name of the inflammatory disease.
Table 1.
Number of cases of inflammatory diseases in the 0- to 72-yr-old population—males and females, Sweden, 1973–2004
| Males | Females | All | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| AS | 2469 (34.6) | 1040 (7.4) | 3509 (16.6) |
| SLE | 434 (6.1) | 2330 (16.6) | 2764 (13.1) |
| RA | 4232 (59.3) | 10656 (76.0) | 14888 (70.3) |
| All | 7135 (100.0) | 14026 (100.0) | 21161 (100.0) |
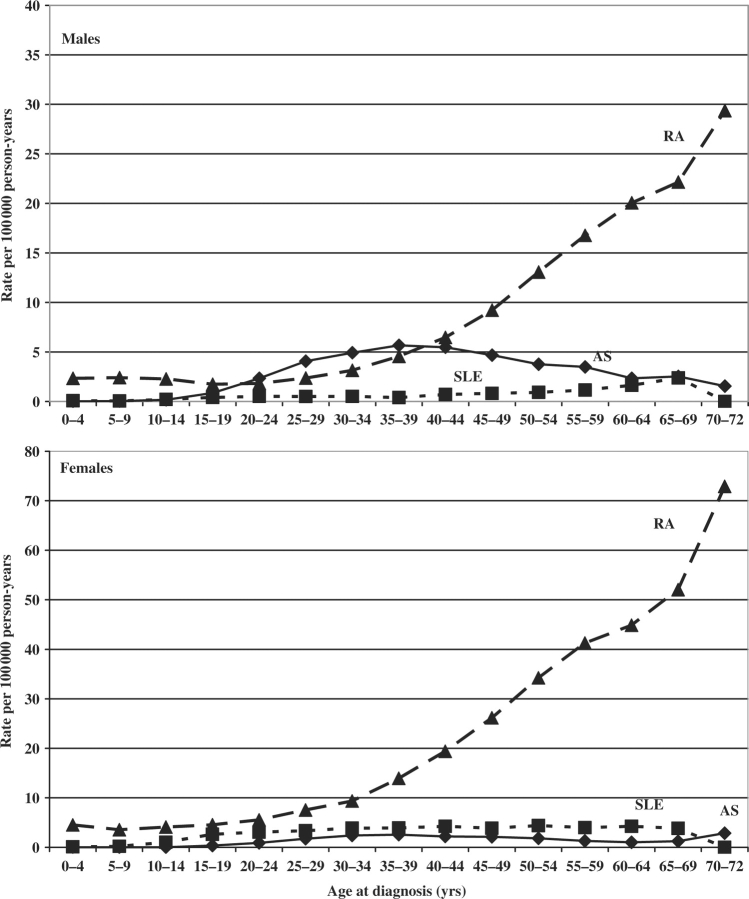
Figure 1 shows age-specific incidence rates for RA, SLE and AS. The rates of RA increased substantially after the age of 35–40 yrs.
Fig. 1.
Age-specific incidence rates of inflammatory diseases in the 0- to 72-yr-old population. Males and females, Sweden, 1973–2004.
Table 2 shows the SIRs for the inflammatory diseases in males and females by age at diagnosis. For males, the overall significant SIRs were 4.72, 4.35 and 4.14 for RA, SLE and AS, respectively, if a sibling was affected by any inflammatory disease. The corresponding significant SIRs for females were 4.12, 3.73 and 4.73. For SLE, the SIR was particularly high among males under age 30 (SIR = 12.29, 95% CI = 3.44, 36.00).
Table 2.
SIRs for inflammatory diseases in siblings by age at diagnosis—Males and females, Sweden, 1973–2004
| AS |
SLE |
RA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Age at diagnosis (yrs) | O | SIR | 95% CI | O | SIR | 95% CI | O | SIR | 95% CI |
| Males | |||||||||
| <30 | 20 | 5.01 | 2.16, 10.95 | 7 | 12.29 | 3.44, 36.00 | 19 | 5.36 | 2.28, 11.86 |
| 30–39 | 38 | 4.40 | 2.20, 8.55 | 1 | 1.69 | 0.00, 13.70 | 33 | 5.58 | 2.71, 11.09 |
| 40–49 | 24 | 3.09 | 1.40, 6.51 | 6 | 6.20 | 1.58, 19.20 | 62 | 5.57 | 3.02, 10.10 |
| 50–59 | 13 | 4.22 | 1.58, 10.23 | 0 | 50 | 3.95 | 2.07, 7.36 | ||
| ≥60 | 4 | 9.02 | 1.66, 33.00 | 1 | 2.43 | 0.00, 19.71 | 15 | 3.22 | 1.27, 7.54 |
| All | 99 | 4.14 | 2.38, 7.13 | 15 | 4.35 | 1.71, 10.16 | 179 | 4.72 | 2.87, 7.73 |
| Females | |||||||||
| <30 | 7 | 4.48 | 1.25, 13.11 | 13 | 3.77 | 1.41, 9.13 | 52 | 5.93 | 3.13, 11.00 |
| 30–39 | 19 | 5.32 | 2.26, 11.76 | 26 | 5.16 | 2.38, 10.70 | 96 | 5.74 | 3.29, 9.92 |
| 40–49 | 14 | 4.64 | 1.79, 11.05 | 16 | 3.03 | 1.22, 6.98 | 131 | 4.15 | 2.45, 6.96 |
| 50–59 | 6 | 4.32 | 1.10, 13.37 | 9 | 2.55 | 0.82, 6.88 | 100 | 3.20 | 1.84, 5.51 |
| ≤60 | 0 | 4 | 4.20 | 0.77, 15.36 | 28 | 2.70 | 1.27, 5.53 | ||
| All | 46 | 4.73 | 2.45, 8.93 | 68 | 3.73 | 2.05, 6.68 | 407 | 4.12 | 2.64, 6.43 |
Bold type: 95% CI does not include 1.00. O: observed number of sibling cases.
Table 3 shows the concordant and discordant associations between RA, SLE and AS. For concordance, i.e. the same subtype of inflammatory disease, there were significant positive associations between siblings. The concordant significant SIRs for RA, SLE and AS were 5.12, 17.02 and 17.14, respectively. AS was only associated with AS. In contrast, RA and SLE were associated with both RA and SLE, although the concordant significant SIRs were higher than the discordant significant SIRs.
Table 3.
SIRs calculated for concordant and discordant associations in siblings—Sweden, 1973–2004
| AS |
SLE |
RA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Subtype | O | SIR | 95% CI | O | SIR | 95% CI | O | SIR | 95% CI |
| AS | 102 | 17.14 | 9.88, 29.44 | 3 | 0.79 | 0.11, 3.30 | 40 | 1.67 | 0.85, 3.23 |
| SLE | 3 | 0.80 | 0.11, 3.37 | 43 | 17.02 | 8.71, 32.45 | 37 | 2.39 | 1.19, 4.67 |
| RA | 38 | 1.74 | 0.87, 3.39 | 33 | 2.32 | 1.13, 4.61 | 515 | 5.12 | 3.31, 7.89 |
| All | 143 | 4.54 | 2.71, 7.57 | 79 | 3.84 | 2.15, 6.77 | 592 | 4.23 | 2.76, 6.49 |
Bold type: 95% CI does not include 1.00. O: observed number of sibling cases.
Discussion
The main findings of the present study are the strong concordant associations between RA, SLE and AS in siblings. The significant SIRs were 5.12, 17.02 and 17.14, respectively. There were also discordant associations between RA and SLE, whereas AS was only associated with AS.
Our study is in agreement with previous ones showing that the development of RA, SLE and AS is strongly influenced by genetic factors [16, 18–22, 26–28].
The novel contribution of our study is that the large-scale approach allowed us to objectively quantify the clinical risk of RA, SLE and AS in first-degree relatives (siblings). However, studies of environmental factors suggest that genes alone are not sufficient to explain the development of these inflammatory diseases [29, 30]. For example, environmental factors may trigger SLE in genetically susceptible individuals [31]. SLE has also been associated with genetic polymorphism in TNF [32], DNA methylation [33], differential gene expression [19, 34] and low levels of mannan-binding lectin [35]. Moreover, in SLE there is a familial aggregation of both SLE and RA [36, 37], which is in accord with the results of the present study showing discordant associations between RA and SLE. Also, the rare condition ‘rhupus’ shows that RA and SLE share the same features [37, 38] and there are patients that present clinical and serological evidence of both RA and SLE [39].
A key strength of this study is that the study population included the entire Swedish population, which allowed us to present risk estimates by gender, age and types of the three inflammatory diseases. Because of the national registration number assigned to each individual in Sweden, it was possible to trace the records of every individual for the whole follow-up period and calculate the exact risk time. An additional strength is that the use of hospital diagnoses eliminated potential recall bias. Finally, the data in the Swedish Hospital Discharge Register are nearly 100% complete. In 2001, the main diagnosis was missing in only 0.9% of the hospitalized cases [23].
The present study also has limitations. Data on out-patients are not available in nationwide registers, such as the registers used in this study. However, previous studies from Sweden show that ∼75% of all RA patients have been hospitalized at least once [40–42]. The hospitalization rate in Sweden seems to be higher among SLE patients than among RA and AS patients, according to data from the present study (Table 1). We calculated the ratios between the numbers of open-care patients and hospitalized patients based on data from central registers in Stockholm County during 2005. The ratios were ∼5.8 : 1, 3.8 : 1, and 3.2 : 1, for RA, SLE, and AS, respectively, which indicates that the hospitalization rates are higher among patients with SLE and AS than among patients with RA. We also lacked information on environmental risk factors, such as smoking. Smoking has been suggested to trigger RA according to new data supporting a gene–environment interaction [29, 43]. Additionally, we were not able to test for the validity of the diagnoses because our data were based on the entire population. However, we only used main diagnoses recorded in the hospital registers, which increases the probability that the diagnoses are valid. Finally, our research database does not include data RF-positive or -negative RA.
Conclusions
The results of this study confirm that RA, SLE and AS have a strong genetic component. The large-scale approach allowed us to objectively quantify the clinical risk of these inflammatory diseases in siblings, which constitutes useful knowledge for clinicians and geneticists. In addition, the analysis of concordant and discordant associations between RA, SLE and AS showed that RA and SLE also correlate with one another, whereas AS showed no correlation with RA or SLE. These additional results may be useful in future studies aimed at finding specific genes associated with these diseases.

Acknowledgements
Funding: The National Institutes of Health (R01 HD052848-01 A1), The Swedish Research Council (K2005-27X-15428-01A), the Swedish Council for Working Life and Social Research (2006-0386 and 2007-1754), The Swedish Research Council Formas (2006-4255-6596-99 and 2007-1352) and the Stockholm County Council.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–90. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 2.Hopkinson ND, Doherty M, Powell RJ. The prevalence and incidence of systemic lupus erythematosus in Nottingham, UK, 1989–1990. Br J Rheumatol. 1993;32:110–5. doi: 10.1093/rheumatology/32.2.110. [DOI] [PubMed] [Google Scholar]
- 3.Nightingale AL, Farmer RD, de Vries CS. Systemic lupus erythematosus prevalence in the UK: methodological issues when using the general practice research database to estimate frequency of chronic relapsing-remitting disease. Pharmacoepidem Dr S. 2007;16:144–51. doi: 10.1002/pds.1253. [DOI] [PubMed] [Google Scholar]
- 4.Hanly JG, Fisk JD, McCurdy G, Fougere L, Douglas JA. Neuropsychiatric syndromes in patients with systemic lupus erythematosus and rheumatoid arthritis. J Rheumatol. 2005;32:1459–6. [PubMed] [Google Scholar]
- 5.Brey RL, Holliday SL, Saklad AR, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58:1214–20. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 6.Ainiala H, Loukkola J, Peltola J, Korpela M, Hietaharju A. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology. 2001;57:496–500. doi: 10.1212/wnl.57.3.496. [DOI] [PubMed] [Google Scholar]
- 7.Sanna G, Bertolaccini ML, Cuadrado MJ, et al. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol. 2003;30:985–92. [PubMed] [Google Scholar]
- 8.Kozora E, Ellison MC, Waxmonsky JA, Wamboldt FS, Patterson TL. Major life stress, coping styles, and social support in relation to psychological distress in patients with systemic lupus erythematosus. Lupus. 2005;14:363–72. doi: 10.1191/0961203305lu2094oa. [DOI] [PubMed] [Google Scholar]
- 9.Martindale J, Smith J, Sutton CJ, Grennan D, Goodacre L, Goodacre JA. Disease and psychological status in ankylosing spondylitis. Rheumatology. 2006;45:1288–93. doi: 10.1093/rheumatology/kel115. [DOI] [PubMed] [Google Scholar]
- 10.Ariza-Ariza R, Hernandez-Cruz B, Navarro-Sarabia F. Physical function and health-related quality of life of Spanish patients with ankylosing spondylitis. Arthritis Rheum. 2003;49:483–7. doi: 10.1002/art.11197. [DOI] [PubMed] [Google Scholar]
- 11.Bostan EE, Borman P, Bodur H, Barca N. Functional disability and quality of life in patients with ankylosing spondylitis. Rheumatol Int. 2003;23:121–6. doi: 10.1007/s00296-002-0261-4. [DOI] [PubMed] [Google Scholar]
- 12.Kobelt G, Andlin-Sobocki P, Maksymowych WP. Costs and quality of life of patients with ankylosing spondylitis in Canada. J Rheumatol. 2006;33:289–95. [PubMed] [Google Scholar]
- 13.Chistiakov DA, Chistiakov AP. Is FCRL3 a new general autoimmunity gene? Hum Immunol. 2007;68:375–83. doi: 10.1016/j.humimm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–50. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 15.Worthington J. Investigating the genetic basis of susceptibility to rheumatoid arthritis. J Autoimmun. 2005;25(Suppl):16–20. doi: 10.1016/j.jaut.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Newton JL, Harney SM, Wordsworth BP, Brown MA. A review of the MHC genetics of rheumatoid arthritis. Genes Immun. 2004;5:151–7. doi: 10.1038/sj.gene.6364045. [DOI] [PubMed] [Google Scholar]
- 17.Orozco G, Rueda B, Martin J. Genetic basis of rheumatoid arthritis. Biomed Pharmacother. 2006;60:656–62. doi: 10.1016/j.biopha.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Thabet MM, Wesoly J, Slagboom PE, Toes RE, Huizinga TW. FCRL3 promoter 169 CC homozygosity is associated with susceptibility to rheumatoid arthritis in Dutch Caucasians. Ann Rheum Dis. 2007;66:803–6. doi: 10.1136/ard.2006.064949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnan S, Chowdhury B, Tsokos GC. Autoimmunity in systemic lupus erythematosus: integrating genes and biology. Semin Immunol. 2006;18:230–43. doi: 10.1016/j.smim.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Akahoshi M, Nakashima H, Shirakawa T. Roles of genetic variations in signalling/immunoregulatory molecules in susceptibility to systemic lupus erythematosus. Semin Immunol. 2006;18:224–9. doi: 10.1016/j.smim.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Reveille JD. Genetic studies in the rheumatic diseases: present status and implications for the future. J Rheumatol Suppl. 2005;72:10–3. [PubMed] [Google Scholar]
- 22.Reveille JD. The genetic basis of ankylosing spondylitis. Curr Opin Rheumatol. 2006;18:332–41. doi: 10.1097/01.bor.0000231899.81677.04. [DOI] [PubMed] [Google Scholar]
- 23.Rosen M, Hakulinen T. Use of disease registers. In: Ahrens W, Pigeot I, editors. Handbook of epidemiology. Berlin: Springer-Verlag; 2005. [Google Scholar]
- 24.The National Board of Health and Welfare. The Swedish hospital discharge register and the cause of death register (1961–2001). [(21 August 2007, date last accessed).]; http://www.sos.se/epc/english/ParEng.htm.
- 25.Hemminki K, Vaittinen P, Dong C, Easton D. Sibling risks in cancer: clues to recessive or X-linked genes? Br J Cancer. 2001;84:388–91. doi: 10.1054/bjoc.2000.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaffney PM, Langefeld CD, Graham RR, et al. Fine-mapping chromosome 20 in 230 systemic lupus erythematosus sib pair and multiplex families: evidence for genetic epistasis with chromosome 16q12. Am J Hum Genet. 2006;78:747–58. doi: 10.1086/503686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye D, Pan F, Zhang K, Li X, Xu J, Hao J. A novel single-nucleotide polymorphism of the Fcgamma receptor IIIa gene is associated with genetic susceptibility to systemic lupus erythematosus in Chinese populations: a family-based association study. Clin Exp Dermatol. 2006;31:553–7. doi: 10.1111/j.1365-2230.2006.02133.x. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman KM, Kelly JA, Herring BJ, et al. Evaluation of the genetic association of the PTPN22 R620W polymorphism in familial and sporadic systemic lupus erythematosus. Arthritis Rheum. 2006;54:2533–40. doi: 10.1002/art.21963. [DOI] [PubMed] [Google Scholar]
- 29.Klareskog L, Padyukov L, Ronnelid J, Alfredsson L. Genes, environment and immunity in the development of rheumatoid arthritis. Curr Opin Immunol. 2006;18:650–5. doi: 10.1016/j.coi.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Mohan C. Environment versus genetics in autoimmunity: a geneticist's perspective. Lupus. 2006;15:791–3. doi: 10.1177/0961203306070005. [DOI] [PubMed] [Google Scholar]
- 31.Eroglu GE, Kohler PF. Familial systemic lupus erythematosus: the role of genetic and environmental factors. Ann Rheum Dis. 2002;61:29–31. doi: 10.1136/ard.61.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parks CG, Pandey JP, Dooley MA, et al. Genetic polymorphisms in tumor necrosis factor (TNF)-alpha and TNF-beta in a population-based study of systemic lupus erythematosus: associations and interaction with the interleukin-1alpha-889 C/T polymorphism. Hum Immunol. 2004;65:622–31. doi: 10.1016/j.humimm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Sekigawa I, Kawasaki M, Ogasawara H, et al. DNA methylation: its contribution to systemic lupus erythematosus. Clin Exp Med. 2006;6:99–106. doi: 10.1007/s10238-006-0103-x. [DOI] [PubMed] [Google Scholar]
- 34.Mandel M, Achiron A. Gene expression studies in systemic lupus erythematosus. Lupus. 2006;15:451–6. doi: 10.1191/0961203306lu2332oa. [DOI] [PubMed] [Google Scholar]
- 35.Saevarsdottir S, Kristjansdottir H, Grondal G, Vikingsdottir T, Steinsson K, Valdimarsson H. Mannan-binding lectin and complement C4A in Icelandic multicase families with systemic lupus erythematosus. Ann Rheum Dis. 2006;65:1462–7. doi: 10.1136/ard.2005.046086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alarcon-Segovia D, Alarcon-Riquelme ME, Cardiel MH, et al. Familial aggregation of systemic lupus erythematosus, rheumatoid arthritis, and other autoimmune diseases in 1,177 lupus patients from the GLADEL cohort. Arthritis Rheum. 2005;52:1138–47. doi: 10.1002/art.20999. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Reyna TS, Alarcon-Segovia D. Overlap syndromes in the context of shared autoimmunity. Autoimmunity. 2005;38:219–23. doi: 10.1080/08916930500050145. [DOI] [PubMed] [Google Scholar]
- 38.Amezcua-Guerra LM, Springall R, Marquez-Velasco R, Gomez-Garcia L, Vargas A, Bojalil R. Presence of antibodies against cyclic citrullinated peptides in patients with ‘rhupus’: a cross-sectional study. Arthritis Res Ther. 2006;8:R144. doi: 10.1186/ar2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothfield NF, Lim AA. Systemic lupus erythematosus evolving into rheumatoid arthritis. J Rheumatol. 2006;33:188–90. [PubMed] [Google Scholar]
- 40.Allebeck P, Ahlbom A, Allander E. Increased mortality among persons with rheumatoid arthritis, but where RA does not appear on death certificate: eleven-year follow-up of an epidemiological study. Scand J Rheumatol. 1981;10:301–6. doi: 10.3109/03009748109095320. [DOI] [PubMed] [Google Scholar]
- 41.Bjornadal L, Baecklund E, Yin L, Granath F, Klareskog L, Ekbom A. Decreasing mortality in patients with rheumatoid arthritis: results from a large population based cohort in Sweden, 1964–95. J Rheumatol. 2002;29:906–12. [PubMed] [Google Scholar]
- 42.Ekstrom K, Hjaklgrim H, Brandt L, et al. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 2003;48:963–70. doi: 10.1002/art.10939. [DOI] [PubMed] [Google Scholar]
- 43.Deighton C, Criswell LA. Recent advances in the genetics of rheumatoid arthritis. Curr Rheumatol Rep. 2006;8:394–400. doi: 10.1007/s11926-006-0071-x. [DOI] [PubMed] [Google Scholar]