Abstract
Introduction:
The time of day in which craving, withdrawal, and other tobacco abstinence symptoms are assessed may moderate the influences of abstinence or medication on those symptoms.
Methods:
Participants were 209 smokers participating in a 4-week crossover study assessing symptoms due to smoking versus abstinence and while using nicotine (21 mg) versus placebo patch when abstinent. None was trying to quit permanently during the study. Abstinence was verified daily by a carbon monoxide level of less than 5 ppm. Participants completed craving (two measures), total withdrawal, and positive affect (PA) and negative affect forms three times per day: in the morning, upon arrival at the clinic in the afternoon, and in the evening. All comparisons of the effects of time of day, abstinence, and nicotine patch treatment were within subjects.
Results:
Results showed a main effect of time of day on all measures while smoking, wherein PA was higher and the other four measures lower, during afternoon versus morning or evening ratings. Time of day interacted with abstinence on both craving measures, but not the other measures, such that abstinence increased craving less in the morning versus the other times. Time of day also interacted with nicotine (vs. placebo) patch effects in alleviating negative mood to a greater degree during evening versus morning or afternoon ratings.
Discussion:
The data suggest that, compared with traditional single assessments of symptoms at midday, assessments at several times of the day may reveal greater overall levels of symptoms and perhaps greater effects of abstinence and nicotine replacement on select abstinence symptoms.
Introduction
In most clinical studies of smoking cessation, symptoms of abstinence such as craving, withdrawal, and negative affect (NA) are measured once per clinic visit, typically sometime in the middle of the day. However, more frequent assessments at other times of day may more reliably capture the severity of symptoms due to abstinence and the magnitude of relief due to medication, especially early in the quit attempt (Shiffman, Paty, Gnys, Elash, & Kassel, 1995; Shiffman, West, & Gilbert, 2004).
Variability in tobacco abstinence symptoms due to time of day has been examined in a few studies but is not generally a topic receiving substantial research attention. In one of the more comprehensive looks at this question, Teneggi et al. (2002) conducted an inpatient study of 24 smokers participating in three conditions of 3 days duration each: ad libitum smoking, enforced abstinence while receiving nicotine patch, and enforced abstinence while receiving placebo patch. Craving measures during either abstinence condition followed a circadian pattern, with symptoms lowest in the morning and increasing gradually over the day, but no such circadian pattern in craving was seen during the 3-day period of ad libitum smoking. Craving throughout the day was lower with nicotine versus placebo patch, but there was no differential efficacy of nicotine patch in relieving craving as a function of time of day. Therefore, time of day influenced severity of craving due to abstinence but not during smoking, and time of day did not affect craving relief from nicotine patch. In contrast to craving, responses to a withdrawal measure showed no circadian pattern during any condition. Because participants resided in an inpatient setting, variability in responding due to environmental variation was effectively controlled, but it is not clear that this time-of-day effect would be observed in a typical clinical study of smokers smoking and abstaining while in the natural environment.
Other research on smokers in the natural environment similarly suggests time-of-day effects on abstinence symptoms but not on relief of those symptoms by medications. Using sophisticated electronic self-monitoring of symptoms, Shiffman et al. (1995) showed a curvilinear pattern of subjective arousal in 25 dependent smokers across the day (low in morning and night, high in midday). Reductions in arousal due to smoking abstinence were greater at times when prequit arousal was high (i.e., midday) but not when it was low. In other research by this group involving 244 abstaining smokers, 24-hr nicotine (21-mg) patch relieved craving and withdrawal equally in the morning or later in the day compared with 16-hr nicotine (15-mg) patch (Shiffman et al., 2000). This finding was contrary to expectations that the 21-mg patch would be particularly effective for morning craving. However, effects of abstinence per se, and full relief due to nicotine, were not examined given that there was no placebo patch condition. Moreover, the comparison between patch conditions was between subjects rather than within subjects, increasing random variance.
Finally, in a completely within-subjects study using more traditional paper-and-pencil measures, Leischow et al. (1997) found differential efficacy between various nicotine replacement therapies (NRTs) on relieving urges to smoke during 2-day periods of use in 18 smokers. Yet the level of efficacy of each NRT was similar between morning (6:30–8:00 a.m.) and late afternoon (5:00–6:30 p.m.) assessments. Lack of midday assessment may have missed an important time of day during which abstinence relief due to NRT may differ.
In sum, evidence suggests that absolute symptom levels and the change in symptoms due to abstinence, but perhaps not relief obtained from medication, may differ across assessments obtained at different times of the day. Also, such time-of-day effects may vary between symptom measures, being more reliable for craving than for withdrawal or other symptoms. However, additional research on time-of-day effects on abstinence symptoms and medication effects may be warranted. Although the within-subject studies discussed previously controlled for individual variability, they necessarily involved relatively small samples, given the extended duration of assessments across conditions. Other research used large samples but did not include a placebo condition and compared treatment effects between subjects rather than within subjects to better control error variance.
In the present study, we examined time-of-day effects on tobacco abstinence symptoms and on NRT versus placebo relief of symptoms using a large outpatient sample in a fully within-subjects design. We used traditional paper-and-pencil measures that might be practical for most clinical studies of smokers in the natural environment. We reasoned that, if time-of-day effects are observed, routine assessment of symptoms at different times of day may be warranted in most clinical studies. At the very least, timing of such assessments in clinical studies should be controlled to reduce confounding variance in responses between groups or across days and weeks with variance in responses due to the particular time of day. We examined the main effects of time of day on symptoms while smoking and the interaction of time of day with abstinence and with nicotine versus placebo patch treatment while abstinent.
Methods
Participants
The present study was described in ads and flyers as an “evaluation of the effects of the nicotine patch in smokers” but also as “not a treatment study.” Prospective participants were screened briefly by telephone and then again in person for smoking history, health, and intention to quit permanently. Eligible participants were required to have smoked at least 10 cigarettes/day for at least 2 years, provide an expired-air carbon monoxide (CO) reading of at least 10 ppm, and not currently be in the process of quitting. Characteristics of the 209 smokers who completed the study are presented in Table 1. Included are mean scores for the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991), a common self-report measure of dependence predictive of cessation outcome (Baker et al., 2007). (Also shown are subgroups of these 209 who were used in specific analyses of abstinence effects and nicotine versus placebo patch effects; see below.) Aside from these 209 participants, 21 dropped out prior to completing all 4 weeks of assessments and 19 more were removed due to failure to follow directions or, less commonly, due to adverse responses to the patch.
Table 1.
Characteristics of all participants and by analytic subgroup
| Subgroup |
|||
| Characteristics | All participants (N = 209) | Abstinence versus smoking (n = 90) | Nicotine versus placebo patch (n = 79) |
| Mean age (years) | 30.77 (0.82) | 30.43 (1.30) | 29.99 (1.39) |
| Gender (percent male) | 51 | 56 | 56 |
| Mean cigarettes per day | 17.11 (0.35) | 16.39 (0.51) | 16.20 (0.54) |
| Mean FTND score (0–10) | 4.72 (0.10) | 4.54 (0.14) | 4.50 (0.14) |
| Mean years smoking | 14.00 (0.75) | 12.81 (1.17) | 12.37 (1.25) |
| Mean number of prior quit attempts | 1.80 (0.15) | 2.17 (0.26) | 2.20 (0.30) |
| Mean longest prior quit attempt (weeks) | 17.62 (3.79) | 17.04 (3.70) | 16.81 (4.10) |
Note. Mean values are given with SE in parentheses. FTND, Fagerström Test for Nicotine Dependence.
Self-report measures
Craving, withdrawal, positive affect (PA), and NA were assessed at every afternoon clinic visit and at two other times of the day in the natural environment, once in the morning and once in the evening (see Procedures section). Every item in the following measures was rated on a Visual Analog Scale of 0 (not at all) to 100 (extremely).
Craving was assessed with the four-item version of the Questionnaire on Smoking Urges (QSU), a widely used clinical and laboratory measure of urges to smoke (Tiffany & Drobes, 1991). The four items in this version, which are described by Carter and Tiffany (2001), are “Nothing would be better than smoking a cigarette right now,” “I have an urge for a cigarette,” “All I want now is a cigarette,” and “I crave a cigarette right now.” This version of the QSU is internally consistent, with a mean Cronbach’s α of .96 (SD = 0.01) in this study.
Nicotine withdrawal was assessed with the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes, Gust, Skoog, Keenan, & Fenwick, 1991), using the following six items: depressed mood/sad, irritable/angry/frustrated, anxious/nervous, difficulty concentrating, restless/impatient, and drowsiness. In the present study, the mean Cronbach’s α for the MNWS was .85 (SD = 0.02). Items were averaged across symptoms to obtain a total withdrawal score, although secondary analyses were conducted on the individual symptoms. A seventh MNWS item, urge to smoke, was excluded from the total withdrawal score because its greater lability can distort apparent changes in total withdrawal. Instead, we used it as a second, separate measure of craving (labeled here as “MNWS craving item”).
PA and NA were assessed using the Diener and Emmons (1984) Mood Form, consisting of nine items that yield PA and NA scores. PA scale items are “happy,” “joyful,” “pleased,” and “enjoyment/fun,” whereas NA scale items are “depressed/blue,” “unhappy,” “frustrated,” “worried/anxious,” and “angry/hostile.” Mean Cronbach’s α values in the present study were .94 (SD = 0.01) for PA and .87 (SD = 0.02) for NA.
Procedures
This analysis is based on data from all participants in a project aimed primarily at examining the influence of quitting motivation on ability to quit temporarily during short-term use of nicotine (21 mg) versus placebo patch. (See Perkins, Lerman, Stitzer, et al., 2008, for results of analyses aimed at that issue.) Our focus in this analysis was the influence of time of day of assessment on symptom levels during weeklong periods while smoking, while abstinent, and while using nicotine versus placebo patch when abstinent (i.e., relief by NRT). Because these analyses were fully within subjects (see below), variability between subjects in quitting motivation was controlled.
Participants engaged in 4 weeks of assessment, consisting of two 2-week phases, each involving a week of ad libitum smoking (baseline: weeks 1 and 3) followed by a week of trying to quit while using nicotine (21 mg) or placebo patch (quit assessment: weeks 2 and 4). Order of nicotine and placebo patch was counterbalanced between participants. Responses during 5-day treatment periods were thought to be particularly clinically significant because abstinence symptoms often peak within the first few days after quitting (Hatsukami, McBride, Pirie, Hellerstedt, & Lando, 1991; Perkins et al., 1996).
Participants came to the clinic 3 days/week during each ad libitum smoking baseline week (e.g., Monday, Wednesday, and Friday) and on all five weekdays (Monday to Friday) during each patch week. CO levels were obtained at each visit. Each of the self-report measures (craving, withdrawal, PA and NA) was assessed three times on each of these days: in the morning, upon arrival at the clinic in the afternoon, and in the evening. Participants were given small booklets to use for morning and evening assessments, and part of their payment for participation was contingent on completing these measures and turning them in at the next clinic visit. Participants were given a time window of a few hours in which to complete a given booklet, and they recorded on each booklet the time of completion. Mean times of completion for morning, afternoon, and evening assessments were 9:34 a.m. (SD = 87 min), 1:38 p.m. (SD = 117 min), and 8:13 p.m. (SD = 110 min), respectively.
During the in-person screening session prior to week 1, all participants provided written informed consent for participation after the nature and consequences of the study were explained. All also agreed in writing that they would try hard to quit during the two patch weeks (weeks 2 and 4), which were presented to participants as simulated brief quit attempts to evaluate medication effects. Both NicoDerm CQ (21-mg) patches and placebo patches matched in size and appearance were obtained from 1-800-patches (Salt Lake City, UT). Patch placement was checked at each clinic visit, and participants also received part of their payment contingent on compliance with patch use. Abstinence was defined as a CO level of less than 5 ppm and self-report of no smoking at all in the past 24 hr. The strict CO cutoff, approximately half that commonly used in clinical trials of cessation (8 ppm), was used because pilot participants often had CO levels of 8 ppm or less but freely admitted to having smoked in the past 24 hr. Research shows that some abstinence symptoms are not always elevated significantly at abstinence durations of less than 24 hr (e.g., Hatsukami, Fletcher, Morgan, Keenan, & Amble, 1989). Similar research on short-term abstinence also has used a CO cutoff of 5 ppm (Alessi, Badger, & Higgins, 2004).
After the end of study week 2, the end of the first week of trying to quit while using a patch, participants were instructed to resume smoking regularly during week 3, the ad libitum smoking week prior to the second patch condition. This week constituted a washout and return to baseline conditions, so that each patch effect could be assessed independently after a period of ad libitum smoking. No subject refused to resume smoking after the first abstinence assessment period during week 2. Participants monitored cigarette use during all weeks using a simple form kept with their cigarette pack and shown to be reliable (Perkins et al., 1996). Ad libitum smoking did not vary between the weeks preceding the placebo week (CO = 21.9 ppm, SD = 0.6; cigarettes/day = 15.5, SD = 0.4) or the nicotine week (CO = 22.8 ppm, SD = 0.7; cigarettes/day = 15.8, SD = 0.4). The same procedures and assessments from week 2 were repeated during the second patch week, study week 4.
Data analyses
Analyses were within subjects and addressed three main questions of interest:
Does the severity of symptoms while smoking vary by time of day?
Does the severity of symptoms due to abstinence vary by time of day?
Does the relief of symptoms due to nicotine versus placebo patch during abstinence vary by time of day?
We used repeated measures linear mixed-effects models with Residual Maximum Likelihood (REML) estimation to address each of these questions for each of the five dependent measures. All models incorporated a direct product covariance structure to accommodate the repeated measures on two different scales, with compound symmetric covariance between days and unstructured covariance between times of day. The within-subjects factors in the analyses were time of day (all three questions), smoking versus abstinence (Question 2), and nicotine versus placebo patch while abstinent (Question 3). Analyses of Question 1 involved all 209 participants and both smoking weeks (weeks 1 and 3). Analyses of Question 2 involved the 90 participants who were abstinent at least 1 day during the placebo patch condition and compared responses on those days to responses during the preceding smoking week. Analyses of Question 3 involved the 79 participants who were abstinent on at least 1 day during each nicotine and placebo patch week (weeks 2 and 4 only). This approach controlled for abstinence status in the comparison of effects due to nicotine versus placebo patch.
All analyses were performed using SAS/STAT version 9.13 for Windows. Significant effects involving time of day were followed up with simple comparisons between timepoints. The text describes the significance of those comparisons, which are not shown in the figures. The figures show the significance of comparisons between conditions at all particular timepoints.
Results
Results of models for the three questions of interest are shown in Table 2.
Table 2.
Results of mixed-effects models examining the main effects of time of day, as well as main effects and interactions with time of day for abstinence and for nicotine patch use (vs. placebo) while abstinent
| Measure | Time of day (N = 209) | Abstinence (n = 90) | Abstinence × time of day (n = 90) | Nicotine (n = 79) | Nicotine × time of day (n = 79) |
| QSU craving | F(2, 373) = 46.75*** | F(1, 439) = 26.26*** | F(2, 513) = 22.23*** | F(1, 409) = 62.30*** | F(2, 490) = 0.76 |
| MNWS Visual Analog Scale craving item | F(2, 372) = 27.44*** | F(1, 432) = 23.17*** | F(2, 515) = 13.04*** | F(1, 404) = 31.70*** | F(2, 480) = 1.05 |
| MNWS total withdrawal | F(2, 335) = 7.13*** | F(1, 398) = 7.32** | F(2, 519) = 0.10 | F(1, 385) = 1.49 | F(2, 496) = 0.48 |
| NA | F(2, 346) = 7.11*** | F(1, 382) = 4.24* | F(2, 513) = 0.23 | F(1, 382) = 2.43 | F(2, 487) = 3.10* |
| PA | F(2, 342) = 16.76*** | F(1, 389) = 0.98 | F(2, 520) = 2.49 | F(1, 357) = 6.51* | F(2, 488) = 1.16 |
*p < .05; **p < .01; ***p < .001.
Main effect of time of day
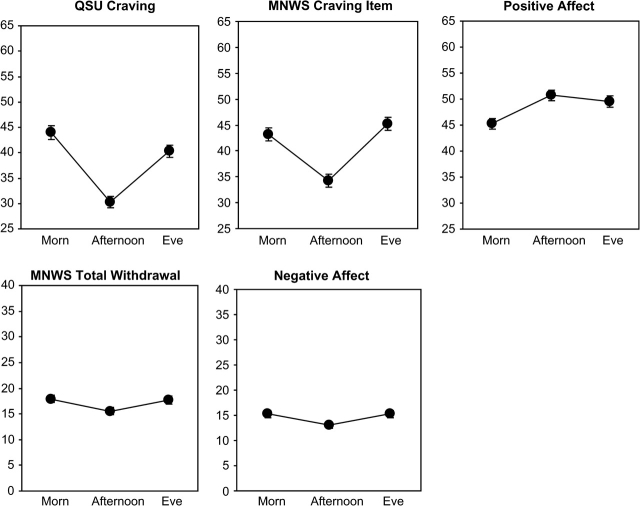
Results showed a main effect of time of day on each measure (see Table 2). As shown in Figure 1, except for PA, each measure was lower during afternoon versus morning or evening ratings (i.e., a “V”-shaped effect of time of day). In analyses of the MNWS symptoms taken individually, only difficulty concentrating and drowsiness showed the V-pattern across time of day. Comparisons between afternoon versus morning or evening ratings were significant for drowsiness, and the comparison between afternoon and evening ratings was significant for difficulty concentrating (results not shown). For PA, afternoon and evening ratings did not differ but were higher than the morning rating (Figure 1).
Figure 1.
Fitted mean (±SEM) craving (QSU-4 item; MNWS craving item), withdrawal (MNWS total), and PA and NA (Diener and Emmons Mood Form) during smoking weeks, as a function of time of day (morning, afternoon, evening).
Time of day and abstinence effects
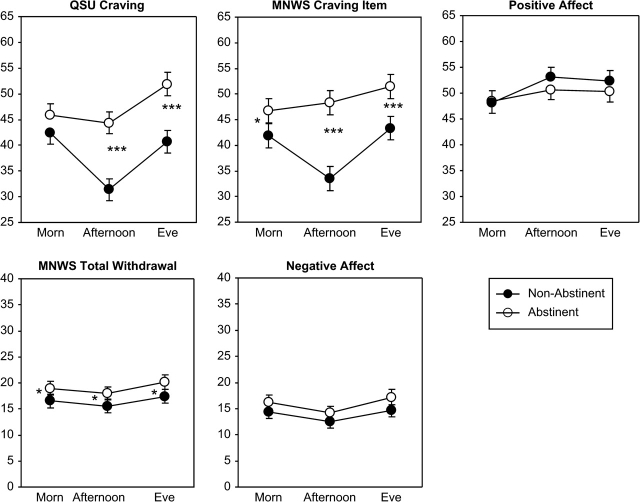
The main effect of smoking abstinence was significant for each measure, except PA (see Table 2). As shown in Figure 2, responses to these measures were significantly higher on the abstinence days during the placebo patch week compared with the preceding smoking week. The main effect of abstinence was significant for all the individual MNWS items except depressed mood/sad and drowsiness (not shown). Time of day interacted with abstinence effects for QSU craving and the MNWS craving item (see Table 2). For the other individual MNWS items, time of day interacted with abstinence only for the MNWS drowsiness item, as the increase in drowsiness rating from afternoon to evening was greater during abstinence versus smoking (not shown). As also shown in Figure 2, the difference between smoking and abstinence for both craving measures was greater during afternoon and evening compared with morning. Effects of abstinence on the other measures were not influenced by time of day of assessment.
Figure 2.
Fitted mean (±SEM) craving, withdrawal, and PA and NA due to abstinence, by time of day. Comparisons are between responses on abstinent days while using placebo patch and responses on smoking days during the preceding week. Participants were those who abstained at least 1 day while on placebo patch (n = 90). *p < .05; **p < .01; ***p < .001 for the difference at each timepoint between smoking and abstinence.
Time of day and nicotine versus placebo patch effects
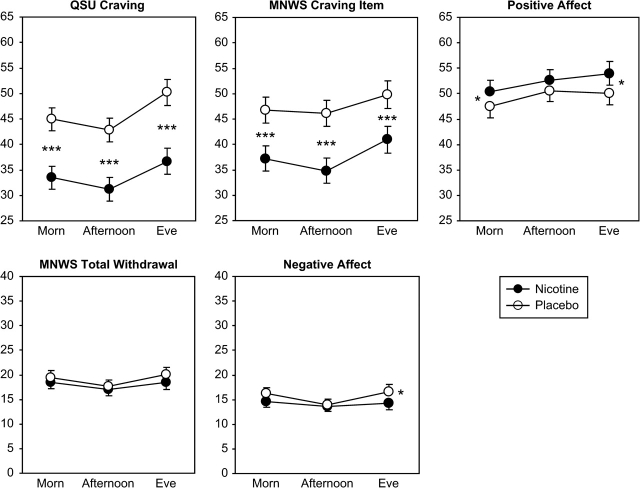
The main effect of nicotine versus placebo patch while abstinent was significant for QSU craving, MNWS craving item, and PA (see Table 2) but not for total MNWS or NA. Nicotine (vs. placebo) patch reduced both craving measures and increased PA, as shown in Figure 3. For the other individual MNWS items, only irritable/angry/frustrated and restless/impatient showed a significant main effect of nicotine (not shown). Time of day interacted with nicotine patch in alleviating NA, as nicotine (vs. placebo) patch reduced NA modestly, but significantly, during evening ratings but not during morning or afternoon ratings (see Figure 3). However, time of day did not influence any other effects of the nicotine patch, including relief of the individual MNWS items.
Figure 3.
Fitted mean (±SEM) craving, withdrawal, and PA and NA due to nicotine versus placebo patch use on abstinent days, by time of day. Comparisons are between responses to nicotine patch versus placebo patch while abstinent. Participants were those who were abstinent at least 1 day during the nicotine patch week and 1 day during the placebo patch week (n = 79). *p < .05; **p < .01; ***p < .001 for the difference at each timepoint between nicotine patch and placebo patch.
Discussion
Results of these analyses indicate that craving, withdrawal, and other symptoms varied by time of day while participants were smoking, generally being lower during afternoon versus morning or evening (i.e. “V”-shaped; see Figure 1). The magnitude of the difference between timepoints was approximately 20%–25% for the craving measures and 10% for the other measures. For craving, this time-of-day effect was almost as large as the overall effect of abstinence or nicotine replacement (Figures 2 and 3), suggesting that the time-of-day effect is clinically significant. For NA, the smaller time-of-day effect was nevertheless similar to that due to abstinence and greater than that due to nicotine replacement. For PA, the time-of-day effect was similar to that due to nicotine replacement and greater than that due to abstinence.
Perhaps more important, time of day interacted with abstinence to influence craving and with nicotine patch to influence NA, although the influences of abstinence or nicotine patch treatment on other symptoms were not altered by time of day. Abstinence increased craving less in the morning than at the other times, as craving dropped from the morning to the afternoon while participants were smoking but remained elevated in the afternoon and increased further in the evening when participants were abstinent (see Figure 2). Thus, the interaction may reflect the acute influences of midday smoking in reducing craving from the higher levels in the morning that resulted from the typical overnight decrease in blood nicotine when participants were asleep, whereas such elevated craving in the morning persisted across time when participants remained abstinent. Yet the presence of smoking cues or something else other than acute smoking or nicotine deprivation influenced the general rise in craving from afternoon to evening, since this rise occurred whether participants were abstinent or smoking and whether they were using nicotine versus placebo patch while abstinent (see Figure 3). Time of day also interacted with nicotine patch effects in alleviating NA but in a pattern different from that seen with craving. Nicotine relieved NA to a modestly, but significantly, greater degree during the evening versus morning or afternoon (see Figure 3). So, contrary to craving, the rise in NA from afternoon to evening occurred only in the absence of nicotine exposure, suggesting that nicotine per se eliminated this time-of-day effect on NA during abstinence.
Our findings were generally consistent with prior studies, noted in the introduction, showing a robust time-of-day influence on craving but less so on other symptoms and only modestly in the relief of those symptoms due to nicotine versus placebo patch during abstinence. On the other hand, time-of-day effects in the present study were more pronounced during smoking compared with abstinence (see Figures 1 and 2). This pattern is the reverse of that observed by Teneggi et al. (2002), who studied smokers during brief enforced abstinence within an inpatient setting; by contrast, we studied smokers during voluntary simulated quit attempts in an outpatient setting. Therefore, environmental stimuli associated with different times of the day that are present in outpatient studies but generally absent in inpatient studies could contribute to variation in symptoms across time while smoking. These stimuli could include particular locations or people and not just explicit smoking cues (Conklin, 2006; Conklin, Robin, Perkins, Salkeld, & McClernon, 2008).
The present findings suggest that, compared with a traditional single assessment of symptoms midday in the clinic, clinical studies of smoking cessation may benefit from symptom assessments at other times of the day, such as in the morning and evening (Shiffman et al., 2004). Such assessments may better gauge the dynamic and systematic changes in symptoms across the day, more accurately capture overall levels of symptoms and the influence of abstinence on some symptoms, and perhaps detect greater effects of medication on a few symptoms (e.g., NA). These added assessments do not need to be burdensome for either participants or researchers, as we were able to determine these effects using simple and short paper-and-pencil forms totaling 20 individual Visual Analog Scale items and taking less than 3 min to complete per occasion, on average. Where added daily assessments are impractical, our results suggest that afternoon or evening may be the best single time of day to examine symptoms in clinical trials, given that effects due to abstinence may be most pronounced then, compared with the morning. This observation also may have relevance for the timing of sessions in lab-based studies aimed specifically at understanding abstinence effects on these symptoms.
Strengths of the present study include (a) the large sample size, even for the subanalyses of abstinence effects and nicotine patch effects; (b) the fully within-subject design of each comparison, which increased statistical power by reducing random variance; and (c) the stringent CO cutoff of 5 ppm to verify abstinence during patch weeks (weeks 2 and 4), as in other studies of daily assessments of abstinence (e.g., Alessi et al., 2004), so that we could be certain that participants had not smoked in the preceding 24 hr.
The study also had a number of limitations. First, we used traditional paper-and-pencil measures of abstinence symptoms for practical reasons, given our large sample size and the desire to demonstrate that assessments could be done with modest burden to participants. Electronic assessments can be cumbersome for participants and time consuming and expensive to analyze, making them less practical for regular use in clinical studies. However, the use of such electronic diary assessments or similar means may be more valid than written self-report forms (Stone, Shiffman, Schwartz, Broderick, & Hufford, 2002), perhaps leading to different findings. We had participants record the time at which they completed the forms and found that these times roughly corresponded to the desired times for the three assessments per day (i.e., morning, afternoon, and evening). However, this self-report information did not verify that the forms were completed at those stated times. If the actual times of day varied much more than these recorded times of day indicate, such random variability should obscure, rather than exaggerate, our time-of-day effects. Therefore, our results may underestimate the magnitude of the effects of time of day on abstinence symptoms.
Second, and similarly, some of our specific findings may be biased by our procedures, and different approaches to assessment may reveal a pattern of effects due to time of day that differ from the results seen here. For example, the V-shape we observed across time of day may be a function of us having obtained ratings at only three timepoints per day; more frequent assessment may reveal that the influence of time of day is closer to U-shaped, J-shaped, or even more complex.
Third, because the afternoon assessment always took place during the clinic visit, whereas the morning and evening assessments took place in the subjects’ natural environments, time of day may have been confounded with the location of the assessment. Therefore, the differences due to afternoon versus morning or evening could be due to participants having completed the measures in a controlled environment (clinic) in the afternoon versus in the presence of potentially varying environmental stimuli that could influence symptoms in the morning or evening. Although a possible contributor to the difference between afternoon and evening ratings, as discussed, it seems unlikely as the primary cause of the difference between morning and afternoon. Time-of-day effects were more pronounced during smoking than during abstinence (see Figures 1 and 2), even though the location of assessments remained the same for each time of day between the two conditions. Future research should control for assessment location in order to isolate effects due to time of day per se (Conklin et al., 2008). Nevertheless, we would argue that, whether due to time of day or varying location, this variability in symptom level over the day warrants more frequent symptom assessments in short-term studies of smoking, abstinence, and medication responses.
Fourth, this study used a sample of smokers not intending to quit permanently during the patch weeks, thus limiting our ability to generalize the findings with regard to abstinence and nicotine patch effects by time of day (see Figures 2 and 3). For example, nicotine patch did not significantly reduce total withdrawal, consistent with other studies of briefly abstinent smokers not trying to quit permanently (Teneggi et al., 2002; see also Perkins, Stitzer, & Lerman, 2006) but contrary to most clinical trial results (Jorenby, Keehn, & Fiore, 1995). Moreover, analyses of abstinence effects by time of day were limited to the participants who self-selected to abstinence during the patch weeks, as with all cessation studies, including all outpatient and some inpatient studies of enforced abstinence (since not all are able to abstain even when paid to do so; e.g., Juliano, Donny, Houtsmuller, & Stitzer, 2006). Yet the time-of-day effects were strongest while participants were smoking, suggesting good generalizability of our findings regarding the main effects of time of day to symptom levels in the general population of smokers while they smoke (see Figure 1). Future studies should explore the possibility of individual differences in the degree to which time of day affects symptom reports during smoking and abstinence, as well as in response to cessation medications (e.g., Cinciripini et al., 2004; Perkins, Lerman, Grottenthaler, et al., 2008).
In conclusion, assessment of tobacco abstinence symptoms just once at midday may not adequately reflect the dynamic but systematic changes in symptoms throughout the day. Time of day influences craving, withdrawal, and affect while smoking and may influence craving due to abstinence and relief of NA by nicotine patch while abstinent. These effects, as well as the modest burden of these measures, suggest that clinical research on abstinence symptoms may generate richer findings by increasing the frequency of symptom assessments each day.
Funding
National Institutes of Health (P50 CA/DA84718).
Declaration of Interests
Caryn Lerman has served as a consultant for GlaxoSmithKline, Pfizer, and Astra Zeneca. She has research funding, unrelated to the present study, from Pfizer and Astra Zeneca. No other authors have any potential conflicts of interest to report.
Supplementary Material
Acknowledgments
The authors thank David Zheng, Zachary Chakan, and Roy Chengappa for their valuable assistance.
References
- Alessi SM, Badger GJ, Higgins ST. An experimental examination of the initial weeks of abstinence in cigarette smokers. Experimental and Clinical Psychopharmacology. 2004;12:276–287. doi: 10.1037/1064-1297.12.4.276. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim S-Y, et al. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research. 2007;9(Suppl. 4):S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Wetter DW, Tomlinson GE, Tsoh JY, DeMoor CA, Cinciripini LG, et al. The effects of the DRD2 polymorphism on smoking cessation and negative affect: Evidence for a pharmacogenetic effect on mood. Nicotine & Tobacco Research. 2004;6:229–239. doi: 10.1080/14622200410001676396. [DOI] [PubMed] [Google Scholar]
- Conklin CA. Environments as cues to smoke: Implication for human extinction-based research and treatment. Experimental and Clinical Psychopharmacology. 2006;14:12–19. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: The effects of environments on smokers’ cue reactivity. Experimental and Clinical Psychopharmacology. 2008;16:207–214. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E, Emmons RA. The independence of positive and negative affect. Journal of Personality and Social Psychology. 1984;47:1105–1117. doi: 10.1037//0022-3514.47.5.1105. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Fletcher L, Morgan S, Keenan R, Amble P. The effects of varying cigarette deprivation duration on cognitive and performance tasks. Journal of Substance Abuse. 1989;1:407–416. [PubMed] [Google Scholar]
- Hatsukami D, McBride C, Pirie P, Hellerstedt W, Lando H. Effects of nicotine gum on prevalence and severity of withdrawal in female cigarette smokers. Journal of Substance Abuse. 1991;3:427–440. doi: 10.1016/s0899-3289(10)80024-0. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. Archives of General Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Keehn DS, Fiore MC. Comparative efficacy and tolerability of nicotine replacement therapies. CNS Drugs. 1995;3:227–236. [Google Scholar]
- Juliano LM, Donny EC, Houtsmuller EJ, Stitzer ML. Experimental evidence for a causal relationship between smoking lapse and relapse. Journal of Abnormal Psychology. 2006;115:166–173. doi: 10.1037/0021-843X.115.1.166. [DOI] [PubMed] [Google Scholar]
- Leischow SJ, Valente SN, Hill AL, Otte PS, Aickin M, Holden T, et al. Effects of nicotine dose and administration method on withdrawal symptoms and side effects during short-term smoking abstinence. Experimental and Clinical Psychopharmacology. 1997;5:54–64. doi: 10.1037//1064-1297.5.1.54. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, D’Amico D, Fonte C, Wilson A, Stiller RL. Low-dose nicotine nasal spray use and effects during initial smoking cessation. Experimental and Clinical Psychopharmacology. 1996;4:157–165. [Google Scholar]
- Perkins KA, Lerman C, Grottenthaler A, Ciccocioppo M, Milanak M, Conklin CA. Dopamine and opioid gene variants are associated with increased smoking reward and reinforcement due to negative mood. Behavioural Pharmacology. 2008;19:641–649. doi: 10.1097/FBP.0b013e32830c367c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Stitzer ML, Fonte CA, Briski JL, Scott JA, et al. Development of procedures for early human screening of smoking cessation medications. Clinical Pharmacology and Therapeutics. 84:216–221. doi: 10.1038/clpt.2008.30. (2008) [DOI] [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: A proposal for new methodologies. Psychopharmacology. 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Elash CA, Paton SM, Gwaltney CJ, Paty JA, Clark DB, et al. Comparative efficacy of 24-hour and 16-hour transdermal nicotine patches for relief of morning craving. Addiction. 2000;95:1185–1195. doi: 10.1046/j.1360-0443.2000.95811855.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Elash C, Kassel JD. Nicotine withdrawal in chippers and regular smokers—subjective and cognitive effects. Health Psychology. 1995;14:301–309. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West RJ, Gilbert DG. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine & Tobacco Research. 2004;6:599–614. doi: 10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient non-compliance with paper diaries. British Medical Journal. 2002;324:1193–1194. doi: 10.1136/bmj.324.7347.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teneggi V, Tiffany ST, Squassante L, Milleri S, Ziviani L, Bye A. Smokers deprived of cigarettes for 72 h: Effect of nicotine patches on craving and withdrawal. Psychopharmacology. 2002;164:177–187. doi: 10.1007/s00213-002-1176-1. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire of smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.