Abstract
Introduction:
Nitrosation of nicotine or its metabolites in the human body could lead to formation of the 2 carcinogenic tobacco-specific nitrosamines—N′-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK).
Methods:
We investigated the possibility of endogenous formation of NNN in people who had stopped smoking and used the 21-mg nicotine patch for 6 months. We quantified urinary biomarkers of exposure to NNN—the sum of NNN and its pyridine-N-glucuronide, referred to as total NNN. Also measured were NNK metabolites—the sum of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its N- and O-glucuronides, referred to as total NNAL.
Results:
The average decline of urinary total NNN was less drastic than that of total NNAL: 22% of baseline total NNN and 7.3% of baseline total NNAL were detected in urine 24 weeks after smoking cessation and patch use (p = .02). The average ratio of total NNN to total NNAL in the same urine samples increased from 0.14 in baseline urine to 0.38 after 24 weeks of nicotine patch use.
Discussion:
Overall, these results demonstrate that endogenous formation of NNN may occur in nicotine patch users. However, the levels of urinary total NNN during patch use were generally extremely low. Moreover, in 10 of 20 subjects analyzed here, the rate of decline in total NNN was similar to that in total NNAL, indicating that endogenous formation of NNN is virtually nonexistent in these subjects. Supplementation with ascorbic acid could be a simple approach to block possible NNN formation in nicotine patch users.
Introduction
Cigarette smoking is responsible for an estimated 3 million annual deaths worldwide and causes approximately 30% of all cancer deaths in developed countries (Peto et al., 1996; World Health Organization [WHO], 1997). More than 1 billion smokers and hundreds of millions of smokeless tobacco users worldwide are at risk for tobacco-induced cancer (Hatsukami & Severson, 1999; Pershagen, 1996; WHO, 1997). Complete cessation of tobacco use in any form is the only way to reduce tobacco-related cancer risk in these people. However, tobacco use is highly addictive, a property attributed to the alkaloid nicotine, the only known addictive component of tobacco (U.S. Department of Health and Human Services, 1988). Nicotine replacement therapy (NRT) products are designed to aid smoking cessation by reducing withdrawal symptoms, thereby eliminating exposure to high levels of toxicants and carcinogens abundant in tobacco and cigarette smoke (Schnoll & Lerman, 2006). A number of NRT products are currently used for this purpose, including nicotine patches, gum, lozenges, and others.
Objective evaluation of the potential health effects of these products is indisputably necessary, especially in cases of long-term use. One of our concerns is the possibility of endogenous nitrosation of nicotine or its metabolites in humans, which could lead to formation in NRT users of the two most carcinogenic of the commonly occurring tobacco-specific nitrosamines—N′-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK; Caldwell, Greene, Plowchalk, & deBethizy, 1991; Carmella, Borukhova, Desai, & Hecht, 1997; Hecht et al., 1978; Hecht, Hochalter, Villalta, & Murphy, 2000). NNN and NNK are believed to play an important role in the induction by tobacco products of cancers of the lung, esophagus, oral cavity, and pancreas (reviewed in Hecht, 1998) and are classified by the International Agency for Research on Cancer (2008) as carcinogenic to humans.
We have demonstrated that nicotine-derived nitrosamines are virtually absent in NRT products (Stepanov, Jensen, Hatsukami, & Hecht, 2006). However, it is possible that NNN and NNK could be formed endogenously in people who use these products, leading to their continuous exposure to these powerful carcinogens—an unacceptable risk, particularly in the case of long-term use. Extensive studies have shown that endogenous formation of N-nitrosamines commonly occurs in humans through the reaction of dietary precursors with nitrosating agents supplied by diet, reduction of dietary nitrate, and endogenously produced nitric oxide (Bartsch, Ohshima, Pignatelli, & Calmels, 1989; Marletta, 1988; Mirvish, 1995; Shepard, Schlatter, & Lutz, 1987). It has been demonstrated that NNN is formed endogenously in F344 rats treated with nicotine or nornicotine and sodium nitrite (Carmella et al., 1997; Porubin, Hecht, Li, Gonta, & Stepanov, 2007). However, a study of smokers who had stopped smoking showed no difference in the levels of NNK metabolites—the sum of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its N- and O-glucuronides, referred to as total NNAL—in nicotine patch users compared with those who did not use the nicotine patch, providing no evidence that NNK was formed endogenously from nicotine (Hecht et al., 1999) even though its secondary amine precursor is a nicotine metabolite (Hecht et al., 2000).
Depending on the conditions of the reaction of nicotine with sodium nitrite, NNN is formed in up to 10 times higher yield than NNK (Hecht et al., 1978). Moreover, nicotine in smokers and nicotine patch users is metabolized to nornicotine (Benowitz, Jacob, Fong, & Gupta, 1994; Hukkanen, Jacob, & Benowitz, 2005), which, as a secondary amine, can be nitrosated to form NNN at a far greater rate than nicotine (Mirvish, Sams, & Hecht, 1977). Rose, Levin, and Benowitz (1993) demonstrated that subjects using the nicotine patch concentrate nicotine and cotinine in their saliva, and it is possible that nornicotine also could be concentrated in saliva. After saliva containing nornicotine and nitrite is swallowed, the stomach provides favorable conditions for nitrosation (Mirvish, 1975; Mirvish et al., 1977). Therefore, in the present study we further investigated the possibility of endogenous formation of NNN in humans by quantifying urinary biomarkers of exposure to this carcinogen—the sum of unchanged NNN and its pyridine-N-glucuronide, referred to as total NNN (Stepanov & Hecht, 2005)—in people who had stopped smoking and used the nicotine patch for 6 months. Figure 1 outlines the hypothesized pathway of endogenous NNN formation in nicotine patch users. Total NNAL also was measured.
Figure 1.
Hypothesized pathways of endogenous NNN formation in nicotine patch users.
Methods
Subjects and study design
The study was approved by the appropriate institutional review boards. Smokers were recruited through newspaper and television advertisements and participated in initial telephone screening, followed by an in-person history and physical examination to confirm the absence of any exclusionary medications or medical conditions. Eligible participants were those who smoked at least 10 cigarettes/day for the past year.
At the initial visit, participants completed questionnaires regarding their smoking history and nicotine dependence level (Fagerström Test for Nicotine Dependence) and provided an exhaled carbon monoxide sample for biochemical confirmation of smoking status and a urine sample. Following a prequit counseling visit, participants started gradual reduction of the number of cigarettes per day over the course of 2 weeks (nicotine fading), until their quit date. Starting with the quit day, participants used the 21-mg nicotine patch daily for 24 weeks and provided spot urine samples 4, 8, 16, and 24 weeks after the quit day. A follow-up urine sample was collected 28 weeks after the quit day. Self-reported smoking was assessed at each visit and biochemically verified with carbon monoxide (<10 ppm). Of 215 initially recruited subjects, 70 attended five or six sessions. The present analysis includes 20 participants with biochemically confirmed abstinence from smoking.
A total of 10 nonsmoking volunteers recruited at the Masonic Cancer Center, University of Minnesota, provided spot urine samples. These samples were analyzed to generate negative reference data.
Urine collection and analyses
Urine was collected into polypropylene containers and stored at −20 °C until analysis. Total NNN and total NNAL were analyzed essentially as previously described (Hecht et al., 1999; Porubin et al., 2007; Stepanov & Hecht, 2005). Negative control samples (water blanks) were added to each set of urine samples. If a urine sample collected after the quit date had elevated levels of total NNN or total NNAL, it was analyzed for anatabine to validate abstinence from smoking (Jacob, Yu, Liang, Shulgin, & Benowitz, 1993).
Data analyses
We used SigmaPlot 2001 version 7.101 to determine the relationship of baseline urinary total NNN to total NNAL and to compare the mean levels of total NNN and total NNAL at various timepoints of the study.
Results
Of the 20 people for whom we report data, 11 completed the program (six timepoints) and the remaining 9 completed 24 weeks of nicotine patch use after their quit date but did not provide the follow-up urine sample. Average participant age was 44 years (SD = 8, range = 26–61); 19 (95%) were White and 13 (65%) were male. The average baseline smoking level was 22 cigarettes/day (SD = 11).
Table 1 summarizes urine levels of total NNN and total NNAL at various timepoints during the study. Mean levels of total NNN and total NNAL in baseline urine were 0.12 pmol/ml (SD = 0.10, range = 0.007–0.35) and 1.1 pmol/ml (SD = 0.80, range = 0.095–2.9), respectively, and these values were correlated (r = .44, p = .046).
Table 1.
Levels of total NNN, total NNAL, and total NNN as a percent of total NNAL in the urine of 20 subjects who stopped smoking and used the nicotine patch
| Total NNN, pmol/ml urine |
Total NNAL, pmol/ml urine |
Total NNN as % of total NNAL |
||||||||||||||||
| weeks on patch |
weeks on patch |
weeks on patch |
||||||||||||||||
| Subject | BL | 4 | 8 | 16 | 24 | 28 | BL | 4 | 8 | 16 | 24 | 28 | BL | 4 | 8 | 16 | 24 | 28 |
| 1 | 0.146 | 0.010 | 0.002 | 0.005 | 0.004 | 0.008 | 1.06 | 0.40 | 0.06 | 0.03 | 0.03 | 0.04 | 14 | 3 | 3 | 17 | 12 | 23 |
| 2 | 0.072 | 0.007 | 0.006 | 0.014 | 0.010 | NA | 0.92 | 0.06 | 0.05 | 0.02 | 0.01 | LOQ | 8 | 12 | 12 | 58 | 170 | NA |
| 3 | 0.227 | 0.016 | 0.021 | 0.017 | 0.004 | S | 0.93 | 0.17 | 0.06 | 0.05 | 0.03 | S | 25 | 9 | 34 | 32 | 12 | NA |
| 4 | 0.068 | 0.009 | 0.006 | 0.005 | NA | 0.003 | 0.42 | 0.08 | 0.05 | 0.01 | LOQ | 0.01 | 16 | 11 | 13 | 56 | NA | 60 |
| 5 | 0.236 | 0.022 | 0.016 | 0.006 | 0.005 | 0.006 | 1.28 | 0.13 | 0.05 | 0.01 | 0.02 | 0.01 | 19 | 17 | 30 | 43 | 29 | 100 |
| 6 | 0.059 | 0.061 | 0.062 | 0.020 | 0.009 | 0.061 | 0.17 | 0.10 | 0.02 | 0.01 | 0.01 | 0.03 | 35 | 62 | 410 | 330 | 110 | 230 |
| 7 | 0.064 | 0.017 | NA | 0.024 | 0.013 | 0.003 | 0.24 | LOQ | 0.04 | 0.02 | 0.03 | LOQ | 27 | NA | NA | 160 | 42 | NA |
| 8 | 0.163 | 0.033 | 0.012 | 0.007 | 0.002 | 0.003 | 0.75 | 0.05 | 0.05 | 0.03 | 0.05 | 0.03 | 21 | 65 | 26 | 27 | 4 | 11 |
| 9 | 0.154 | 0.015 | 0.017 | 0.016 | LOQ | 0.009 | 2.38 | 0.12 | 0.08 | 0.04 | 0.23 | NA | 6 | 13 | 22 | 46 | NA | NA |
| 10 | 0.015 | 0.002 | 0.001 | 0.005 | 0.002 | LOQ | 0.45 | 0.27 | 0.16 | 0.16 | 0.05 | 0.07 | 3 | 1 | 1 | 3 | 4 | 0 |
| 11 | 0.049 | 0.003 | LOQ | 0.016 | NA | 0.009 | 1.70 | NA | 0.07 | 0.05 | NA | NA | 3 | NA | NA | 33 | NA | NA |
| 12 | 0.347 | 0.073 | 0.419 | 0.034 | NA | NA | 1.69 | NA | LOQ | 0.01 | NA | NA | 21 | NA | NA | 310 | NA | NA |
| 13 | 0.030 | LOQ | 0.029 | 0.007 | 0.016 | NA | 0.53 | 0.07 | 0.03 | LOQ | 0.08 | NA | 6 | NA | 85 | NA | 19 | NA |
| 14 | 0.084 | LOQ | 0.023 | LOQ | 0.021 | NA | 2.30 | 0.16 | 0.05 | 0.09 | 0.06 | NA | 4 | NA | 49 | NA | 34 | NA |
| 15 | 0.188 | 0.046 | NA | S | 0.015 | NA | 2.95 | 0.35 | 0.13 | S | 0.17 | NA | 7 | 13 | NA | NA | 9 | NA |
| 16 | 0.007 | 0.006 | 0.012 | LOQ | 0.007 | NA | 0.10 | 0.13 | 0.03 | LOQ | LOQ | NA | 7 | 5 | 48 | NA | NA | NA |
| 17 | 0.067 | 0.013 | 0.010 | LOQ | 0.004 | NA | 1.37 | 0.22 | LOQ | 0.07 | 0.09 | NA | 5 | 6 | NA | NA | 5 | NA |
| 18 | 0.299 | 0.157 | 0.050 | 0.022 | 0.012 | NA | 0.99 | 0.11 | 0.04 | 0.02 | 0.02 | NA | 30 | 150 | 130 | 110 | 71 | NA |
| 19 | 0.039 | 0.005 | 0.020 | 0.001 | 0.007 | NA | 0.85 | 0.11 | LOQ | LOQ | 0.04 | NA | 5 | 5 | NA | NA | 16 | NA |
| 20 | 0.024 | 0.002 | 0.003 | 0.002 | 0.016 | NA | 0.18 | 0.08 | 0.03 | 0.03 | 0.05 | NA | 13 | 2 | 10 | 8 | 36 | NA |
| Mean | 0.117 | 0.028 | 0.042 | 0.013 | 0.009 | 0.013 | 1.07 | 0.15 | 0.06 | 0.04 | 0.06 | 0.03 | 14 | 25 | 63 | 88 | 38 | 70 |
| SD | 0.099 | 0.039 | 0.099 | 0.009 | 0.006 | 0.020 | 0.80 | 0.10 | 0.04 | 0.04 | 0.06 | 0.02 | 10 | 39 | 110 | 110 | 46 | 85 |
| Median | 0.070 | 0.014 | 0.016 | 0.011 | 0.008 | 0.007 | 0.93 | 0.12 | 0.05 | 0.03 | 0.05 | 0.03 | 10 | 11 | 28 | 45 | 19 | 42 |
Note. BL, baseline (before cessation of smoking); NA, not analyzed; LOQ, below the limit of quantitation (∼0.001 pmol/ml urine for NNN and ∼0.03 pmol/ml urine for NNAL); S, smoking at this time point according to anatabine analysis.
We found a considerable initial decline in total NNN and total NNAL levels after cessation of smoking: 4 weeks after the quit date, mean total NNN and mean total NNAL dropped to 0.028 pmol/ml urine (SD = 0.039) and 0.15 pmol/ml urine (SD = 0.10), respectively. Urinary excretion of total NNN in Subject 6 was the same at baseline and after 4, 8, and 28 weeks of nicotine patch use. Subject 13 had similar total NNN at baseline and after 8 weeks of patch use. After 8 weeks of being on the patch, Subjects 12 and 16 had higher total NNN compared with baseline. Anatabine, a tobacco alkaloid structurally related to nicotine, is not likely to be present in foods or to have other sources of exposure and is not present in nicotine-containing medications. It can be used to validate abstinence or measure the extent of tobacco use in persons undergoing NRT (Jacob et al., 2002). Therefore, samples with elevated levels of total NNN or total NNAL were analyzed for anatabine and compared with baseline anatabine levels in the same subjects. Two samples, denoted by “S” in Table 1, had anatabine levels comparable with those in the baseline urine and were not used in further analyses.
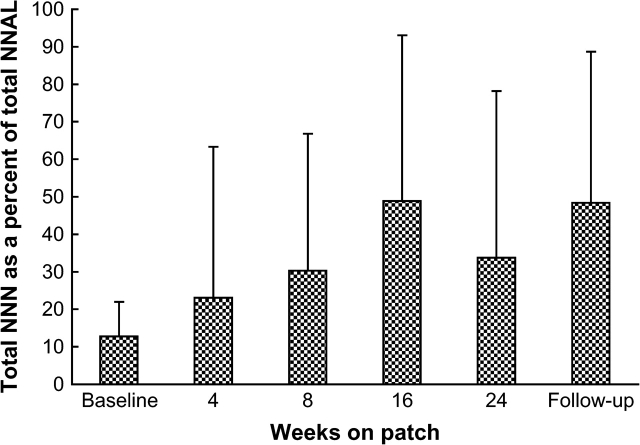
The amount of total NNN was calculated as the percentage of total NNAL for each subject at each timepoint to compare the relative amounts of the analytes (see Table 1). Mean values for total NNN expressed as the percentage of total NNAL also were determined after exclusion of Subjects 6, 12, 13, and 16 (Figure 2).
Figure 2.
Total NNN expressed as a percentage of total NNAL in the urine of 16 participants. Subjects 6, 12, 13, and 16, who at any timepoint while on patch had similar or higher total NNN compared with the baseline, are not included. Bars indicate SD.
Individual values of total NNN and total NNAL during nicotine patch use were compared with the baseline values for each subject. The baseline values were set at 100%, and the percentage of baseline was calculated for each subject at each timepoint after the quit day. The mean urinary excretion of total NNN and total NNAL, expressed as a percentage of the baseline value, is illustrated in Figures 3A (all subjects) and B (Subjects 6, 12, 13, and 16 excluded).
Figure 3.
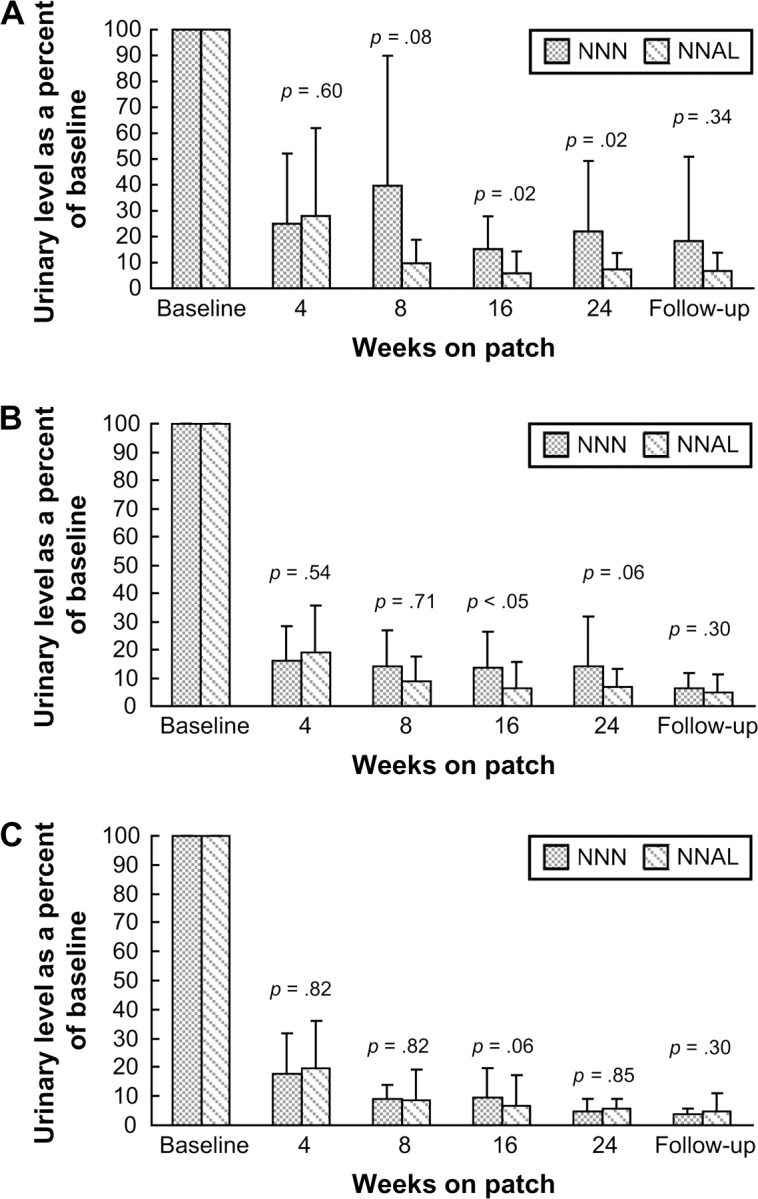
Mean levels of total NNN (dark bars) and total NNAL (striped bars) at various timepoints of the study expressed as a percentage of the baseline value. The levels are compared at each timepoint: (A) in all subjects; (B) Subjects 6, 12, 13, and 16, who at any timepoint while on patch had similar or higher total NNN compared with the baseline, are excluded; (C) only Subjects 1, 3, 4, 5, 8, 9, 10, 15, 17, and 18, who had less than 20% of baseline total NNN in their urine starting with week 8 of patch use. Bars indicate SD; numbers above the bars indicate p value of the paired t test.
Total NNN was detected in 4 of 10 urine samples from nonsmokers reportedly unexposed to secondhand smoke (detection limit = ∼0.001 pmol NNN/ml urine). Average total NNN in these samples was 0.002 pmol/ml urine (SD = 0.001).
Discussion
This is the first study in which urinary biomarkers of the nicotine-derived carcinogen NNN were measured in people who had stopped smoking and used the nicotine patch for 6 months. For the majority of our subjects, urinary total NNN declined considerably after smoking cessation. However, in some subjects, total NNN at one or more timepoints after smoking cessation was comparable with or higher than baseline levels. The overall decline of urinary total NNN was less drastic than that of total NNAL. These results support the hypothesis that endogenous NNN formation occurs in some nicotine patch users.
Urinary total NNN is a relatively new biomarker. To strengthen the reliability of the results obtained here for total NNN, we also measured total NNAL—an established and commonly measured urinary metabolite of the related nicotine-derived carcinogen NNK. Interindividual variation in total NNN and total NNAL observed in the baseline urine samples (see Table 1) was consistent with that reported previously (Carmella, Akerkar, Richie, & Hecht, 1995; Stepanov & Hecht, 2005). The levels of total NNN and correlation between total NNN and total NNAL in baseline urine also were similar to those reported earlier (Stepanov & Hecht, 2005). The initial decrease in urinary total NNN was similar to that observed for total NNAL in our subjects (see Table 1), which was consistent with the results of our previous study that dealt with the excretion of total NNAL after smoking cessation (Hecht et al., 1999). After this initial decrease, however, mean total NNN fluctuated around the value reached 4 weeks after smoking cessation, while the decline in mean total NNAL stopped after 8 weeks of abstinence from smoking. Four subjects had levels of urinary total NNN that were elevated over or comparable with baseline at one or more timepoints after smoking cessation, indicating that endogenous nitrosation of nicotine does take place.
Different rates of decline in urinary total NNN and total NNAL during nicotine patch use are reflected in the ratio of total NNN to total NNAL in the same urine samples (see Table 1). In the baseline urine samples, total NNN was an average 14% of total NNAL, which is consistent with the previously reported ratio between these biomarkers (Stepanov & Hecht, 2005). This value increased in samples collected after smoking cessation, and after 24 weeks of nicotine patch use it averaged 38%. This increase in total NNN to total NNAL ratio also was observed when the four subjects who had elevated urinary total NNN after smoking cessation were not included in the data analysis (see Figure 2). When expressed as a percentage of the baseline level, total NNN in the urine of patch users was more persistent than total NNAL. Thus, 24 weeks after smoking cessation, urinary total NNN in all our subjects was an average 22% of baseline NNN, whereas this value for total NNAL was 7.3% (see Figure 3A); this difference was statistically significant (p = .02). Calculations made without inclusion of Subjects 6, 12, 13, and 16 produced similar results (see Figure 3B); however, the statistical power of this difference decreased (p = .06). We further excluded Subjects 2, 7, 11, 14, 19, and 20, who, at any timepoint starting with 8 weeks of patch use, had in their urine 20% or more of the baseline total NNN. Exclusion of these subjects left 10 (50% of our participants) who demonstrated a decrease in total NNN similar to that of total NNAL over the study period (see Figure 3C). This stratified analysis indicated that endogenous formation of NNN is more or less extensive in some patch users and is virtually nonexistent in others.
The difference in the average rates of decrease of total NNN and total NNAL in the urine of some long-term patch users is consistent with the results of kinetic studies that show that nitrosation of nicotine produces more NNN than NNK (Hecht et al., 1978) and that nornicotine is nitrosated more readily than is nicotine (Mirvish et al., 1977), supporting endogenous NNN formation in patch users via nitrosation of nicotine or metabolically formed nornicotine. Ascorbic acid is an effective inhibitor of endogenous nitrosation (Mirvish, 1986, 1994; Mirvish, Wallcave, Eagen, & Shubik, 1972), and we have demonstrated that it inhibits endogenous NNN formation in rats treated with nornicotine and sodium nitrite (Porubin et al., 2007). Therefore, a simple approach to block possible NNN formation in nicotine patch users could be supplementation with ascorbic acid.
The absence of a control group in which subjects did not use any NRT product after they quit smoking is the major limitation of the present study. Factors other than endogenous formation, such as low rate of NNN clearance from the body and secondhand smoke exposure, also could contribute to the total NNN levels observed here. However, the kinetics of NNN clearance from the body is unknown, and there are no published studies on total NNN levels in nonsmokers exposed to secondhand smoke. Comparison with a placebo patch group would allow us to address these issues. Also, users of other kinds of NRT products should be studied. In nicotine gum chewers and users of nicotine lozenges, for example, nicotine goes directly to the stomach, where conditions are highly favorable for nitrosation reactions (Mirvish, 1975; Shepard et al., 1987).
In summary, the results of the present study demonstrate that endogenous formation of NNN may occur in some nicotine patch users. However, our findings should not call into question the use of nicotine patch as a smoking cessation product. NRT is an effective tool in the treatment of nicotine dependence. Smoking cessation and use of NRT products significantly decrease exposure to a wide range of carcinogens and toxicants present in cigarette smoke, and the levels of urinary total NNN and total NNAL during patch use were generally extremely low in our study. In the future, supplementation with ascorbic acid could be a simple approach to block possible endogenous NNN formation in nicotine patch users. Similar studies involving other types of NRT products should be conducted.
Funding
Commonwealth of Pennsylvania; National Institutes of Health (CA-81301, DA-013333, and P50CA/DA-84718).
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The authors thank Bradley Lieberman for technical assistance, Katie Wickham for help with anatabine analysis, Peter W. Villalta for help with mass spectrometry techniques, and Bob Carlson for editorial assistance.
References
- Bartsch H, Ohshima H, Pignatelli B, Calmels S. Human exposure to endogenous N-nitroso compounds: Quantitative estimates in subjects at high risk for cancer of the oral cavity, oesophagus, stomach and urinary bladder. Cancer Surveys. 1989;8:335–362. [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, III, Fong I, Gupta S. Nicotine metabolic profile in man: Comparison of cigarette smoking and transdermal nicotine. Journal of Pharmacology and Experimental Therapeutics. 1994;268:296–303. [PubMed] [Google Scholar]
- Caldwell WS, Greene JM, Plowchalk DR, deBethizy JD. The nitrosation of nicotine: A kinetic study. Chemical Research in Toxicology. 1991;4:513–516. doi: 10.1021/tx00023a003. [DOI] [PubMed] [Google Scholar]
- Carmella SG, Akerkar SA, Richie J. P, Jr., Hecht SS. Intraindividual and interindividual differences in metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in smokers’ urine. Cancer Epidemiology, Biomarkers & Prevention. 1995;4:635–642. [PubMed] [Google Scholar]
- Carmella SG, Borukhova A, Desai D, Hecht SS. Evidence for endogenous formation of tobacco-specific nitrosamines in rats treated with tobacco alkaloids and sodium nitrite. Carcinogenesis. 1997;18:587–592. doi: 10.1093/carcin/18.3.587. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Severson HH. Oral spit tobacco: Addiction, prevention and treatment. Nicotine & Tobacco Research. 1999;1:21–44. doi: 10.1080/14622299050011131. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chemical Research in Toxicology. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Chen M, Koch JFD, Miller AT, Murphy SE, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Research. 1999;59:590–596. [PubMed] [Google Scholar]
- Hecht SS, Chen CB, Ornaf RM, Jacobs E, Adams JD, Hoffmann D. Reaction of nicotine and sodium nitrite: Formation of nitrosamines and fragmentation of pyrrolidine ring. Journal of Organic Chemistry. 1978;43:72–76. doi: 10.1021/jo00395a017. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Hochalter JB, Villalta PW, Murphy SE. 2′-Hydroxylation of nicotine by cytochrome P450 2A6 and human liver microsomes: Formation of a lung carcinogen precursor. Proceedings of the National Academy of Sciences. 2000;97:12493–12497. doi: 10.1073/pnas.220207697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacological Reviews. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans: Smokeless tobacco and some tobacco-specific nitrosamines. (Vol. 89) Lyon, France: IARC; 2007. [PMC free article] [PubMed] [Google Scholar]
- Jacob P, III, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiology, Biomarkers & Prevention, 2002;11:1668–1673. [PubMed] [Google Scholar]
- Jacob P, III, Yu L, Liang G, Shulgin AT, Benowitz NL. Gas chromatographic-mass spectrometric method for determination of anabasine, anatabine and other tobacco alkaloids in urine of smokers and smokeless tobacco users. Journal of Chromatography B, Biomedical Applications, 1993;619:49–61. doi: 10.1016/0378-4347(93)80445-a. [DOI] [PubMed] [Google Scholar]
- Marletta MA. Mammalian synthesis of nitrite, nitrate, nitric oxide, and N-nitrosating agents. Chemical Research in Toxicology. 1988;1:249–257. doi: 10.1021/tx00005a001. [DOI] [PubMed] [Google Scholar]
- Mirvish SS. Formation of N-nitroso compounds: Chemistry, kinetics, and in vivo occurrence. Toxicology and Applied Pharmacology. 1975;31:325–351. doi: 10.1016/0041-008x(75)90255-0. [DOI] [PubMed] [Google Scholar]
- Mirvish SS. Effects of vitamins C and E on N-nitroso compound formation, carcinogenesis, and cancer. Cancer. 1986;58(Suppl.):1842–1850. doi: 10.1002/1097-0142(19861015)58:8+<1842::aid-cncr2820581410>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Mirvish SS. Experimental evidence for inhibition of N-nitroso compound formation as a factor in the negative correlation between vitamin C consumption and the incidence of certain cancers. Cancer Research. 1994;54(Suppl.):1948s–1951s. [PubMed] [Google Scholar]
- Mirvish SS. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Letters. 1995;17:17–48. doi: 10.1016/0304-3835(95)03786-V. [DOI] [PubMed] [Google Scholar]
- Mirvish SS, Sams J, Hecht SS. Kinetics of nornicotine and anabasine nitrosation in relation to N′-nitrosonornicotine occurrence in tobacco and to tobacco-induced cancer. Journal of the National Cancer Institute. 1977;59:1211–1213. doi: 10.1093/jnci/59.4.1211. [DOI] [PubMed] [Google Scholar]
- Mirvish SS, Wallcave L, Eagen M, Shubik P. Ascorbate-nitrite reaction. Possible means of blocking the formation of carcinogenic N-nitroso compounds. Science. 1972;177:65–68. doi: 10.1126/science.177.4043.65. [DOI] [PubMed] [Google Scholar]
- Pershagen G. Smokeless tobacco. British Medical Bulletin. 1996;52:50–57. doi: 10.1093/oxfordjournals.bmb.a011532. [DOI] [PubMed] [Google Scholar]
- Peto R, Lopez AD, Boreham J, Thun M, Heath C, Jr., Doll R. Mortality from smoking worldwide. British Medical Bulletin. 1996;52:12–21. doi: 10.1093/oxfordjournals.bmb.a011519. [DOI] [PubMed] [Google Scholar]
- Porubin D, Hecht SS, Li Z, Gonta M, Stepanov I. Endogenous formation of N′-nitrosonornicotine in F344 rats in the presence of some antioxidants and grape seed extract. Journal of Agricultural and Food Chemistry. 2007;55:7199–7204. doi: 10.1021/jf0712191. [DOI] [PubMed] [Google Scholar]
- Rose JE, Levin ED, Benowitz N. Saliva nicotine as an index of plasma levels in nicotine patch users. Therapeutic Drug Monitoring. 1993;15:431–435. doi: 10.1097/00007691-199310000-00012. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Lerman C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opinion on Emerging Drugs. 2006;11:429–444. doi: 10.1517/14728214.11.3.429. [DOI] [PubMed] [Google Scholar]
- Shepard SE, Schlatter C, Lutz WK. Assessment of the risk of formation of carcinogenic N-nitroso compounds from dietary precursors in the stomach. Food and Chemical Toxicology. 1987;25:91–108. doi: 10.1016/0278-6915(87)90311-5. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Hecht SS. Tobacco-specific nitrosamines and their pyridine-N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiology, Biomarkers & Prevention. 2005;14:885–891. doi: 10.1158/1055-9965.EPI-04-0753. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine & Tobacco Research. 2006;8:309–313. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. A report of the surgeon general: The health consequences of smoking, nicotine addiction. Washington, DC: US Department of Health and Human Services; 1988. [Google Scholar]
- World Health Organization. Tobacco or health: A global status report. Geneva: World Health Organization; 1997. (pp. 10–48) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.