Abstract
Cadmium (Cd) is a ubiquitous environmental pollutant that has been associated with male reproductive toxicity in both humans and animal models. The underlying mechanism of this response, however, is still uncharacterized. To address this issue, we employed a recently developed and optimized three-dimensional primary Sertoli cell-gonocyte coculture system and examined the time- and dose-dependent effects of Cd on morphological alterations, cell viability, activation of stress signaling pathway proteins, and the disruption of the ubiquitin proteasome system (UPS). Our results demonstrated that Cd exposure lead to time- and dose-dependent morphological changes that are associated with the induction of apoptosis. In response to Cd, we also saw a disruption of the UPS as evaluated through the accumulation of high–molecular weight polyubiquitinated proteins (HMW-polyUb) as well as alterations in proteasome activity. Robust activation of cellular stress response, measured through the increased phosphorylation of stress-activated protein kinase/c-jun N-terminal kinase and p38, paralleled the accumulation of HMW-polyUb. In addition, p53, a key regulatory protein, was upregulated and underwent increased ubiquitination in response to Cd. To further characterize the role of the UPS in Cd cellular response, we compared the above changes with two classic proteasomal inhibitors, lactacystin, and MG132. The stress response and the accumulation of HWM-polyUb induced by Cd were consistent with the response seen with MG132 but not with lactacystin. In addition, Cd treatment resulted in a dose- and time-dependent effect on proteasome activity, but the overall Cd-induced proteasomal inhibition was unique as compared to MG132 and lactacystin. Taken together, our studies further characterize Cd-induced in vitro testicular toxicity and highlight the potential role of the UPS in this response.
Keywords: cadmium, Sertoli cell-gonocyte coculture, ubiquitin proteasome system, stress signaling, male reproductive toxicity
Cadmium (Cd) is a ubiquitous environmental pollutant that has been associated with male reproductive toxicity in both humans and animal studies (Foote, 1999; Gennart et al., 1992; Laskey et al., 1984, 1986). Three thousand tons of Cd are imported or produced annually in the United States with approximately 90% of this being Cd oxide, commonly used in batteries, pigments, plastics, synthetic products, and a variety of other materials. Exposure to Cd is most common within the workplace but also occurs through water and food contamination and cigarette smoke (Satarug et al., 2004). Cd has a long biological half-life (15–20 years) and accumulates over time within the blood, kidneys, liver, and reproductive organs (Henson and Chedrese, 2004). Chronic exposure to Cd has been shown to cause reproductive impairment in male mammals, including azoospermia in hamsters (Wlodarczyk et al., 1995), failure of spermiation and low sperm production in rats (Hew et al., 1993; NTP, 1995), and abnormal sperm head morphology in mice (Mukherjee et al., 1988). A most recent single dose (sc) of Cd chloride treatment study in rat found that Cd-induced apoptosis at low doses of Cd (0.13 and 0.15 mg/100 g body weight [BW]) significantly reduced serum testosterone (T) level at doses of 0.20 and 0.3 mg/100 g BW (Sen Gupta et al., 2004). Numerous studies have reported that Cd also has potent estrogen-like activity both in vitro and in vivo (Derfoul et al., 2003; Fridman et al., 2004; Henson and Chedrese, 2004; Johnson et al., 2003). A single ip dose of Cd (5 μg/kg BW) increased uterine wet weight, promoted growth and development of the mammary glands, and induced hormone-regulated genes in ovariectomized rats (Johnson et al., 2003). These results suggest that Cd's estrogenic potential may play a central role in its ability to disrupt tissue development and function, including within both male and female reproductive systems. Although these studies demonstrate Cd's link to male reproductive toxicity, the underlying mechanism of this response has yet to be fully characterized.
Several studies suggest a role for the ubiquitin proteasome system (UPS) in modulating metal-induced toxicity (Figueiredo-Pereira et al., 1998; Yen et al., 2005). Cd exposure was shown to activate ubiquitin-dependent proteolysis pathway in yeast, and mutants deficient in specific ubiquitin-conjugating enzymes are hypersensitive to Cd (Jungmann et al., 1993). The UPS is a highly conserved pathway that plays an important role in the selective degradation of specific cellular proteins (Marx, 2002). The UPS acts through posttranslational modifications of key transcriptional regulators, impacting various cellular events including cell cycle progression, signal transduction, transcriptional regulation, apoptosis, and DNA repair (DiAntonio et al., 2001; Pagano et al., 1995). Biochemical data indicate that the activity of the UPS is high during spermatogenesis (Rajapurohitam et al., 2002), which is most likely related to the high requirement for massive breakdown of cytoplasmatic and nuclear proteins during this process (Baarends et al., 1999; Dickins et al., 2002; Sutovsky et al., 2001a). In addition, defective sperm are found to be ubiquitin tagged and are related to the elimination during mammalian spermatogenesis (Sutovsky et al., 2001b).
Considering the potential role of the UPS in both metal-induced toxicity and the regulation of key cellular events critical to reproductive function, it is evident that further characterization of these processes are needed to help clarify the mechanistic response to environmental Cd exposure.
In our previous studies, we reported the establishment and characterization of a novel three-dimensional primary Sertoli cell-gonocyte coculture (SGC) system (Yu et al., 2005). Here, we further employ this in vitro SGC system to examine whether low levels of Cd affect the development of neonatal testis and to define the role of the UPS in this mechanistic response. To address these questions, we investigated the time- and dose-dependent effect of Cd on morphological alterations, cell viability, the activation of stress signaling proteins, and the disruption of the UPS. The cell cycle regulatory protein, p53, was also evaluated due to its key role within these responses as well as it being regulated by the UPS. We monitored the UPS through the measurement of high–molecular weight polyubiquitinated proteins (HMW-polyUb) accumulation as well as proteasomal activity. To fully understand this response, we compared these measurements with impacts observed using two classic proteasomal inhibitors, lactacystin and MG132. Our results demonstrated that Cd exposure leads to time- and dose-dependent morphological changes as well as a correlated induction of apoptosis. In addition, the accumulation of HMW-polyUb paralleled the robust activation of the stress response as indicated by the phosphorylation of stress-activated protein kinase (SAPK)/c-jun N-terminal kinase (JNK) and p38. Both the accumulation of HWM-polyUb and the activation of the stress response observed with Cd are similar to the response seen with MG132 but not with lactacystin. Cd treatment also leads to a time- and dose-dependent effect on proteasome activity. This inhibition of the proteasome was different, however, compared to MG132 and lactacystin. Taken together, our studies suggest that UPS dysfunction plays a key role in the underlying mechanism of Cd-induced testicular toxicity.
METHODS AND MATERIALS
SGC and treatment of Cd.
The SGC was followed as previously described (Yu et al., 2005). Briefly, male pups were obtained by mating Sprague-Dawley rats (Harlan, Kent, OH). Testes were dissected from 5-day-old rats, and a cell suspension containing primarily Sertoli cells and type A spermatogonia was isolated with the multiple digestion steps. Cells were resuspended in hormone- and serum-free Eagle's Minimal Essential Medium (Life Technologies, Inc., Gaithersburg, MD) containing 0.1mM nonessential amino acids, 1mM sodium pyruvate, 3mM sodium lactate, 1% ITS+™ premix (a culture supplement containing insulin, transferin, selenium, linoleic acid, and bovine serum albumin; BD Biosciences, Bedford, MA). Cells were plated in 35-mm tissue culture–treated dishes at 2.4 × 106 density in serum-free medium. Immediately after seeding, an ice-cold extracellular matrix (ECM) medium (5 μg/ml, BD Biosciences) was applied to these dishes at a final concentration of 200 μg/ml. Serial dilutions were prepared from a stock solution of Cd and added directly to the culture medium 48 h after the addition of ECM overlay. The final concentrations tested were 0.5, 2.5, 5, 10, 20, and 40μM. Separate treatments with MG132 and lactacystin were conducted for 24 h from stock solutions of these agents in dimethyl sulfoxide, and final concentration was 2.5μM.
Morphology and viability.
All cultures were viewed with a Nikon inverted microscope equipped with phase-contrast optics (Nikon, Tokyo, Japan) at intervals during culture to assess their general appearance. Resultant images were captured and digitized using a Coolsnap Camera (Roper Scientific, Inc., Duluth, GA). The digitized image was processed using Adobe Photoshop. Neutral red (NR) assay was used to determine viability of the cultured cells after treatment as previously reported (Borenfreund and Puerner, 1985). Briefly, cells were treated with different concentrations of Cd. The media with treatment was removed, and fresh media containing 50 μg/ml NR was added to the dish. After incubation for 3 h at 37°C, 5% CO2, the cells were washed with PBS and NR was eluted with 1% acetic acid/50% ethanol solution. Finally, 200 μl of the resulted NR solution was added to 96 plates and measured at 490 nm.
Assessment of apoptosis.
For the evaluation of the apoptotic morphological changes, cells were fixed and stained with Hoechst 33342 (0.1 mg/ml in PBS) after Cd treatment. The stained cocultures were viewed with appropriate filter under fluorescent microscope. Images were captured and digitized using Spot Camera (Diagnostic Instrument, Inc.) equipped with MetaMorph software. Apoptosis-associated end points were further determined in cell extracts by measuring functional activities associated with caspase-3/7 using caspase-specific fluorogenic substrates. The activity of caspase-3/7 was measured with a fluorometric assay, using N-acetyl-Asp-Glu-Val-Asp-AMC (7-amino-4-methylcoumarin) as the specific substrates as previously reported (Nicholson et al., 1995; Shi et al., 2000). Briefly, 10 μg of cell extract was added in duplicate in 96-well plate format. Reaction buffer containing the fluorogenic substrate enzyme-catalyzed release of 7-amino-4-methyl coumarin (AMC) was added to initiate the reaction which was incubated at 37°C for 2 h and enzyme-catalyzed release of AMC measured by a fluorescence microplate reader at excitation 360 nm and emission 460 nm. Fluorescent units were converted to p mol of AMC released per μg of protein and incubation time (h) using a standard curve generated with known serial dilutions of AMC.
Western blot analysis and immunoprecipitation.
At the appropriate time points, cultured cells were rinsed twice with ice-cold PBS. Cell lysis buffer (Cell Signaling Technology, Inc., Beverly, MA) was added to each dish, and cells were scraped with a rubber policeman. Harvested cells were then sonicated at 40 W for 15 s. Resultant cell lysates were centrifuged at 16000 × g for 15 min at 4°C. Supernatant fractions were collected, and the concentration of protein was determined with a commercially available kit (Protein Assay kit, Bio-Rad Laboratories, Hercules, CA) with bovine serum albumin as a standard. All samples were subsequently stored at −80°C until assayed.
Western blot analysis for the selected proteins was performed according to the previously described method (Yu et al., 2001, 2005). Briefly, equal amounts of protein (20 μg) were separated on Criterion™ 10–20% Tris-HCl precast gels (Bio-Rad Laboratories) according to the manufacturer's protocol. Proteins were subsequently transferred onto polyvinylidene difluoride nylon membranes (Bio-Rad Laboratories) for immunoblot analyses. Membranes were rinsed briefly in Tris-buffered saline (TBS), pH 7.6, blocked with 5% nonfat dried milk in TBS with 0.1% Tween-20 (T-TBS) for 20 min and rinsed again with T-TBS. Membranes were then incubated overnight with primary antibody and for 1.5 h with a secondary antibody. Following antibody incubation, the membrane was washed four times for 5 min with T-TBS. The primary antibodies included phospho-SAPK/JNK, phospho-p38 mitogen-activated protein kinase (MARP), phospho-serine/threonine protein kinase (AKT), phospho-p53 (Cell Signaling Technology, Inc.), and ubiquitin (Santa Cruz Biotechnology). Furthermore, β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) was used as an internal standard for the protein loading. After hybridization with secondary antibodies conjugated to horseradish peroxidase, the immunocomplex was detected with the enhanced chemiluminescence detection reagent (GE life Science, Piscataway, NJ) and exposed to X-ray films. Quantification of band intensities was achieved using the NIH ImageJ (http://rsb.info.nih.gov/ij/index.html).
To immunoprecipitate p53 proteins, whole cell lysates containing 100 μg of total proteins were incubated with 1 μg/ml of p53 polyclonal antibody (Santa Cruz Biotechnology) at 4°C overnight on a rotating shaker. The immunoprecipitate was washed three times at 4°C with the RIPI buffer (0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride). The immunoprecipitated proteins were released from beads by boiling and were loaded onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated proteins were blotted onto the nitrocellulose membrane and probed with either a p53 monoclonal antibody or an ubiquitin polyclonal antibody.
Fluorogenic peptide substrate assay for proteasome activity.
Two different proteasome activities were measured as previously reported (Bobba et al., 2002; Canu et al., 2000; Rodgers and Dean, 2003), using fluorogenic substrates, Suc-Leu-Leu-Val-Tyr-AMC (Suc-LLVY-AMC, 50μM) and Z-Leu-Leu-Glu-AMC (Z-LLE-AMC, 200μM). These substrates are used to measure chymotryptic and peptidylglutamyl-peptide hydrolyzing (PGPH) activities associated with the proteasome. Hydrolysis of these substrates was independent of the ubiquitin system. Lysates (25 μg) were incubated at 37°C with the fluorogenic substrates in 100 μl of 50mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 8, and 5mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, for 4 h, respectively. Enzyme-catalyzed release of AMC was measured by a fluorescence microplate reader at excitation 360 nm and emission 460 nm. Fluorescent units were converted to p mol of AMC released using a standard curve generated with known serial dilutions of AMC.
Statistical analysis.
The results of quantitative analysis of cell viability, proteasome activities and Western blot bands’ densitometric quantification are the mean ± SEM. Statistical significance was determined using one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparison tests. A P value less than 0.05 denoted the presence of a statistically significant difference.
RESULTS
Cd-induced Time- and Dose-dependent Apoptotic Morphological Alterations and Cytotoxicity
With the ECM overlay at 200 μg/ml, Sertoli cells rapidly attached to the plate. The gonocytes, easily distinguished by their nuclear size and cytoplasmic density, adhered to the underling Sertoli cells 2 h after ECM overlay as described previously (Yu et al., 2005). In the control, the SGC with overlay of ECM at 200 μg/ml formed testicular-like three-dimensional structure 24 h after plating (Fig. 1A). In response to Cd treatment, we observed a dose-dependent disruption in cell morphology (Figs. 1B–D). An increase in the number of the round-up gonocytes was observed beginning at the low Cd concentration of 5μM (Fig. 1B) and was significantly present at 20μM Cd (Fig. 1D). Approximately 90% of the cells became rounded with 40μM of Cd (not shown), but the rounded cells still adhered to the dish. The disruption of the SGC structure in response to MG132 (2.5μM) began 8 h after treatment (Fig. 1E) and was significant at 24 h (Fig. 1F). There were a few morphological changes in treatment with 2.5μM lactacystin at 8 h (Fig. 1G), with no further morphological changes observed at the 24-h time point (Fig. 1H).
FIG. 1.

Dose-dependent morphological changes after exposure to Cd (A–D) and classical proteasomal inhibitors, MG132 (E, F) and lactacystin (G, H). Sertoli cells and gonocytes were isolated by the sequential enzymatic digestion and cocultured for 48 h. Cells were treated with the Cd chloride for 24 h at 0 (A), 5 (B), 10 (C) and 20 (D) μM, MG132 (2.5μM) for 8 (E) or 24 h (F) or treated with lactacystin (2.5μM) for 8 (G) or 24 h (H). Dose-dependent disruptions of morphology by Cd were observed (A–D). An increase in the number of the detached gonocytes as shown round-up, and disconnection between Sertoli cell and gonocytes at 24 h was observed even at low concentration of 5μM. MG132 (2.5μM) induced increase in the number of detachment of gonocyte both at 8 and 24 h (E–F) after treatment, while lactacystin of 2.5μM (G–H) only induced a fewer number increase of detached gonocyte at 8 h but no further morphological changes at the 24-h time point.
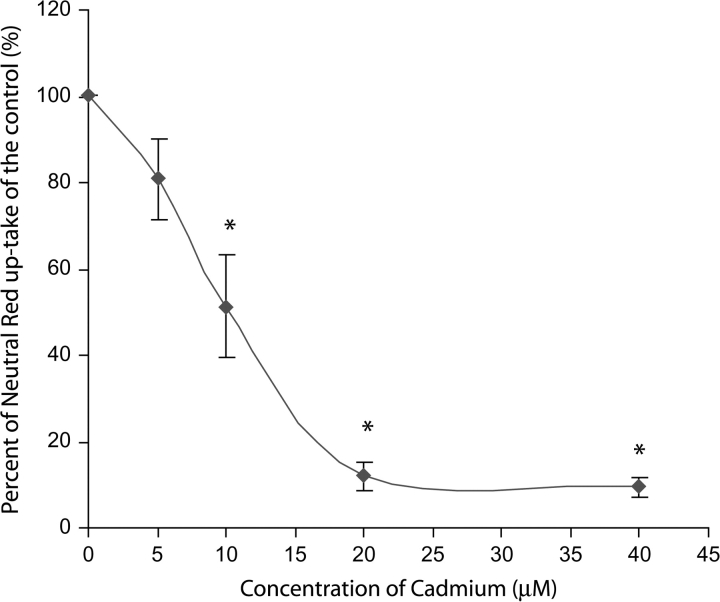
In the morphological examinations of cells with Hoechst 33342 nuclear staining, we observed significant and consistent dose-dependent morphological changes as indicated by the heavily condensed nuclei, nuclear shrinkage, and subsequent chromatin condensation into the periphery of the nuclei (Fig. 2). MG132 (2.5μM) induced morphological alterations indicative of apoptosis at 8 and 24 h, while lactacystin did not induce obvious changes at 2.5μM (data not shown). Cell viability, measured by NR Assay (Fig. 3), illustrates a dose-dependent cytotoxicity in response to Cd with approximately 80% cell viability at 5μM Cd and an LC50 approximately at 10μM Cd.
FIG. 2.
Cd-induced dose-dependent apoptotic morphological alteration (A–D). For the evaluation of the apoptotic morphological changes, cells were fixed and stained with Hoechst 33342 (0.1 mg/ml in PBS) 24 h after Cd treatment. The stained cocultures were viewed with appropriate filters under fluorescent microscope. The image was captured and digitized with a Spot camera (Diagnostic Instrument, Inc.) equipped with MetaMorph software.
FIG. 3.
Dose-dependent decrease in cell viability in response to Cd treatment within Sertoli cell-gonocyte. Sertoli cells and gonocytes were isolated by the sequential enzymatic digestion and cocultured for 48 h. Cells were treated with the Cd chloride (0–40μM) for 24 h. Cytotoxicity assessments were conducted using the NR dye uptake assay which determines cell viability by assessing lysosomal accumulation of NR dye. Data are presented as mean ± SE, n ≥ 3. Cd treatment leads to a dose-dependent decrease in cell viability with an LC50 approximately at 10μM.
Activation of Caspase-3/7-like Activity after Cd Treatment
The caspase family of proteases plays a crucial role in apoptotic germ cell death. To examine the apoptotic mechanism involved in Cd-induced cell death within the primary neonatal SGCs, we measured caspase-3/7-like activity (Fig. 4). A dose-dependent increase in caspase-3/7-like activity 24 h after treatment was observed in response to Cd. At 24 h, significant increase in caspase-3/7-like activity was seen at 5μM and above. MG132 (2.5μM) significantly induced caspase-3-like activity at both 8 and 24 h after treatment, while lactacystin (2.5μM) did not activate the caspase-3-like activity.
FIG. 4.
Dose- and time-dependent increase in caspase-3/7-like activity in response to Cd treatment within SGCs. Sertoli cells and gonocytes were isolated by the sequential enzymatic digestion and cocultured for 48 h. Cells were treated with 1–40μM Cd chloride, MG132 (2.5μM) or lactacystin (2.5μM) for 24 h. The activity of caspase-3/7 was measured by a fluorometric assay at 8 and 24 h after treatment, with N-acetyl-Asp-Glu-Val-Asp-AMC (7-amino-4-methylcoumarin) as the specific substrates. Fluorescent units were converted to p mol of AMC released per 10 μg of protein and incubation time (h) using a standard curve generated with known serial dilutions of AMC. Data are presented as mean ± SE, n ≥ 3. Cd treatment leads to a dose- and time-dependent increase in caspase-3/7-like activity. The Cd response was similar to the broad-range proteasomal inhibitor, MG132 versus the specific proteasomal inhibitor lactacystin.
Cd-induced Accumulation of HMW-polyUb in SGC
Recent studies have suggested a role for the UPS in mediating metal toxicity (Figueiredo-Pereira and Cohen, 1999; Figueiredo-Pereira et al., 1998). We explored whether Cd exposure perturbed UPS function within the SGC. Cd treatment resulted in a dose- and time-dependent upregulation of HMW-polyUb proteins (Fig. 5). The HMW-polyUb levels in the 10 and 20μM Cd treatments were already highest at 8-h time point (See Fig. 5). In a time-dependent manner, MG132 increased the level of HMW-polyUb proteins at all time points observed. Lactacystin increased these proteins both at the 4- and 8-h time points and returned to the control level at the 24-h time point.
FIG. 5.
Accumulation of HMW-polyUb in SGC in response to Cd treatment. Sertoli cells and gonocytes were isolated by the sequential enzymatic digestion and cocultured for 48 h. Cells were treated with 0–40μM Cd chloride, MG132 (2.5μM) or lactacystin (2.5μM) for 24 h and harvested. Cell extracts were prepared and subjected to Western blot analysis of HMW-polyUb (A) as described in the “Materials and Methods” section. Quantification of resulting band intensities of HMW-polyUb was achieved using the “NIH J-Image” software (B). Data are presented as arbitrary units after internal standard correction with β-actin. Each data point represents the mean percent ± SE of three separate experiments. Statistical significance was determined by ANOVA followed by Tukey-Kramer multiple comparison (*p < 0.05) as compared with the control for each time point. Cd treatment resulted in a dose-dependent increase in HMW-polyUb.
Cd-induced Changes of Proteasome Activity in SGCs
In order to determine whether the accumulation of HMW-polyUb by Cd is due to the alteration of proteasomal activity as observed with MG132, we also measured proteasomal activity through the fluorogenic substrates Suc-LLVY-AMC and Z-LLE-AMC. The substrates Suc-LLVY-AMC and Z-LLE-AMC are associated with the chymotryptic and PGPH of the proteasome, respectively. Using this method, we found that the inhibition of the proteasomal activity varied toward the two substrates. There were no dose-dependent alterations in the chymotrypsin-like proteasomal activity following Cd treatments (Fig. 6A). MG132 significantly inhibited the chymotrypsin-like activity of the proteasome throughout the 24-h treatment. Lactacystin also significantly inhibited the chymotrypsin-like activity at both 4 and 8 h but returned to the level comparable to the control by 24 h. Treatment with Cd at both 10 and 20μM activated the PGPH-like activity of the proteasome at the early time point (4 h) and then decreased significantly after 24 h (Fig. 6B). MG132 significantly decreased PGPH-like activity only at 8 h after treatment, while no significant changes of PGPH-like activity in lactacystin-treated cells were observed (Fig. 6B).
FIG. 6.
Chymotryptic-like activities (A) and PGPH (B) of the proteasome in the SGCs. Cells were treated with the Cd chloride from 0 to 20μM or treated with MG132 (2.5μM) or lactacystin (2.5μM) for 4, 8 and 24 h and harvested. Aliquots of 25 μg protein extract were incubated in 100 μl reaction buffer containing fluorogenic substrates Suc-LLVY-AMC and Z-LLE-AMC. These substrates are used to measure chymotryptic (A) and PGPH (B) activities associated with the proteasome. The assay was incubated at 37°C, and the fluorescence was monitored with spectrofluorimeter. Fluorescent units were converted to p mol of AMC released per 10 μg of protein and incubation time (h) using a standard curve generated with known serial dilutions of AMC. Data are presented as mean percent ± SE, n ≥ 3.
Cd-induced Upregulation of Phosphorylation of SAPK/JNK and p38 MAPK
Mitogen-activated protein kinases (MAPKs) are a family of serine/threonine protein kinases that are involved in many cellular pathways such as cell proliferation, differentiation, movement, and death and have also activated in response to cellular stress. Treatment with Cd resulted in a time- and dose-dependent upregulation of the phosphorylated forms of SAPK/JNK (p-SAPK/JNK, Figs. 7A, B) and p38 (p-p38, Fig. 7C). Significant upregulation of p-SAPK was observed at 10μM Cd (24 h), while significant upregulation of p-p38 was observed at the higher doses of 20 and 40μM, 4 and 8 h after Cd treatment. At the 24-h treatment, p38 was significantly activated at 10μM. MG132 (2.5μM) also upregulated p-SAPK and p-JNK throughout the observation period, while lactacystin only upregulated p-SAPK/JNK at 4 and 8 h.
FIG. 7.

Dose- and time-dependent inductions of SAPK/JNK and p38 MAPK in SGC. Sertoli cells and gonocytes were isolated by the sequential enzymatic digestion and cocultured for 48 h. Cells were treated with the Cd chloride from 0 to 40μM or treated with MG132 (2.5μM) or lactacystin (2.5μM) for 4, 8 and 24 h and harvested. Cell extracts prepared and subjected to Western blot analysis of the phosphorylation status of SAPK/JNK and p38 as described in the “Materials and Methods” section. Quantification of resulting band intensities was achieved using the “NIH J-Image” software. Quantitative analysis of the phosphorylation of both bands associated with SAPK/JNK (p46/54) is shown in (A) and (B) and p38 in (C). Data are presented as arbitrary units after internal standard correction with β-actin. Each data point represents the mean ± SE of three separate experiments. Statistical significance was determined by ANOVA followed by Tukey-Kramer multiple comparison (*p < 0.05) as compared with the control for each time point.
Cd-induced Upregulation of p53 and Its Ubiquitination in the SGC
The expression of p53 and the activation of p53 through phosphorylation at serine-15 in response to Cd were examined (Fig. 8A). A significant increase of the total p53 and phosphor-p53 was evident at 24 h, 20μM. Similarly, MG132 (2.5μM) increased the p53 and phosphor-p53 in a time-dependent manner with peak levels occurring at 24 h, a time of peak apoptosis. Lactacystin increased p53 to a lesser extend at the 4- and 8-h time points and returned to the control level by the 24-h time point. By using immunoprecipitation, we first precipitated with p53 antibody, then probed with either anti-ubiquitin antibody or anti-p53 antibody, found a significant increase in ubiquitinated p53 proteins in Cd, MG132, and lactacystin-treated cells (Fig. 8B), and the specific band around 50 kDa was further confirmed by using the p53 monoclonal antibody (Fig. 8C).
FIG. 8.

Cd-induced upregulation of p53 (A, B) and its ubiquitination (C, D) in the SGCs. Sertoli cells and gonocytes were isolated by the sequential enzymatic digestion and cocultured for 48 h. Cells were treated with the Cd chloride from 0 to 20μM or treated with MG132 (2.5μM) or lactacystin (2.5μM) for 24 h and harvested. Cell extracts prepared and subjected to Western blot analysis of p53 (both phosphorylated and total p53, A, B) as well as immunoprecipitation analysis (C, D) as described in the “Materials and Methods” section. To immunoprecipitate p53, whole cell lysates containing 100 μg of total proteins were incubated with 1 μg/ml of p53 polyclonal antibody for 24 h. The separated proteins were blotted onto the nitrocellulose membranes and probed either ubiquitin polyclonal antibody (C) or p53 (D) monoclonal antibody. Figures are the representatives of the three independent experiments.
DISCUSSION
In vitro models for testicular toxicity provide important tools for investigating specific mechanisms of toxicity (Yu et al., 2005). We applied this in vitro model to further characterize the mechanism of Cd-induced testicular toxicity. To this end, we investigated the time- and dose-dependent effect of Cd on morphological alterations, disruption of the UPS, and activation of stress signaling proteins. Our results demonstrated that Cd exposure leads to time- and dose-dependent morphological changes, a response that is supported by the induction of apoptosis. Cd exposure also resulted in a dose-dependent accumulation of HMW-polyUb paralleling the activation of cellular stress responses. Cd appears to act more like a nonspecific proteasomal inhibitor, demonstrating similar cellular responses to MG132 as compared to the specific proteasomal inhibitor, lactacystin. Treatment of Cd also leads to a time- and dose-dependent effect on proteasomal activity; however, this response was distinctly different from both MG132 and lactacystin. Taken together, our studies demonstrate that Cd exposure leads to UPS dysfunction and cellular stress, suggesting an important role for this mechanistic response in testicular toxicity.
The UPS pathway has emerged as an important mechanism for regulating a variety of cellular processes including DNA repair, cell cycle control, oncogenesis, abnormal protein catabolism, antigen processing, ribosome biogenesis, transcription, viral infection, neural and muscular degeneration, and stress response (Ben-Neriah, 2002; DiAntonio et al., 2001; Pagano et al., 1995; Schulman et al., 2000). Biochemical data indicate that the UPS pathway (Ub, E1, E2, E3, and deubiquitination enzymes) is highly active in the testis due to the specific requirements associated with the proliferation and massive breakdown of cytoplasmatic and nuclear proteins during the last phase of spermatogenesis (Kon et al., 1999; Rajapurohitam et al., 2002; Sutovsky et al., 2001c). Spermatogenesis, the specific process of proliferation and differentiation of germ cells, is a highly regulated process where defective sperms are ubiquitin tagged and eliminated (Sutovsky et al., 2001b). Although the molecular mechanism of ubiquitination of sperm is not clear, the assay for detecting ubiquitin-tagged sperm has been proposed as a biomarker for male infertility (Rawe et al., 2002; Sutovsky, 2003; Sutovsky et al., 2001a, 2003). In spermatogenesis, there appears to be a special requirement for certain components of the UPS, as exemplified in humans and mice by the mutation of USP9Y and HR6B, respectively (Baarends et al., 1999; Ng et al., 2002). Both genes encode for proteins that take part in the UPS and are ubiquitously expressed, but their mutation generates no apparent phenotype other than male infertility (Roest et al., 1996). A recent study found that a deletion within a gene encoding for one of the key deubiquitin enzymes, ubiquitin carboxyterminal hydrolase-1 (Uch-L1), caused shrinking of seminiferous tubules, decreasing total number of cells and enlargement of remaining cells in seminiferous tubules in 25-week-old mice (Kwon et al., 2003, 2004). Immunohistochemical studies have shown that Uch-L1 is localized in both spermatogonia and Sertoli cells in the testis (Kon et al., 1999). Clearly, the UPS is a critical pathway within the testis and is important for normal spermatogenesis. Disruption of this pathway, therefore, by environmental toxicants such as Cd may play a significant role in the underlying mechanism of testicular toxicity.
Our study demonstrates the time- and dose-dependent disruption of the UPS through the observed accumulation of HMW-polyUb as well as proteasomal inhibition, suggesting that the UPS plays a primary role in the mechanistic response to Cd (Figs. 5 and 6). Alternate studies have similarly linked metals with the accumulation of HMW-polyUb and proteasomal inhibition (Araya et al., 2002; Figueiredo-Pereira and Cohen, 1999; Kirkpatrick et al., 2003). The accumulation of HMW-polyUb that we observed in our study correlated with the response seen with the nonspecific, broad-range proteasomal inhibitor MG132 but not with the specific inhibitor lactacystin (Fig. 5). Supporting this observation, as with the HMW-polyUb, both Cd and MG132 demonstrated a similar, time-dependent increase in the caspase-3/7-like activity or apoptotic response. In the case of lactacystin, the cells were able to recover by 24 h and demonstrated very little caspase-3/7 activity (Fig. 4). The changes in caspase-3 activity support the observed morphological alterations (Figs. 1 and 2).
Within our study, the resultant accumulation of HWM-polyUb in response to Cd exposure paralleled the activation of cellular stress responses (Fig. 7). Previous studies have demonstrated that the disruption of the UPS, as observed with lactacystin and MG132, leads to significant activation of stress signaling, as well as alterations to other cellular pathways (Lopez Salon et al., 2000; Yang and Yu, 2003). In addition, oxidative stress has been implicated in Cd-induced toxicity both in vivo or in vitro studies (Dong et al., 1998; Jurczuk et al., 2004; Shaikh et al., 1999; Yang et al., 1997). Cd specifically has been linked with oxidative stress and the accumulation of ubiquitinated proteins within neuronal cells (Figueiredo-Pereira et al., 1998). A key component of the stress response is the MAPK family which is comprised of vital signal transducers differentially activated in response to a wide variety of extracellular stimuli (Chuang et al., 2000; Figueiredo-Pereira et al., 1998; Garrington and Johnson, 1999; Nebreda and Porras, 2000). Major subfamilies of MAPKs have been described including extracellular signal–regulated kinase, JNK, and p38. In this study, Cd treatments significantly increased level of p-SAPK/JNK in SGC cells even at low concentration (10μM) at all time points (Fig. 7). However, a significant increase in p-p38 MAPK was only observed at 24 h at Cd concentrations of 10 and 20μM. This response paralleled the accumulation of HMW-polyUb and induction of apoptosis. The upregulation of p-SAPK/JNK and p-p38 has previously been associated with low-level Cd exposure and subsequent impacts on cell cycle progression and mitotic arrest (Ding and Templeton, 2000; Iryo et al., 2000). To date, the relationship between MAPK activation and the accumulation of HMW-polyUb induced by Cd is unclear. Our study links these two critical responses of Cd-induced toxicity, cellular stress, and UPS disruption, as indicated by the accumulation of HWM-polyUb and the parallel activation of stress responses.
One pivotal target that responds to stress is the tumor suppressor, p53. Normally, the cellular level of p53 is low due to a short protein half-life. In response to a variety of stimuli, however, it is increased steeply by stabilization. This balance between p53 production and degradation is maintained by the UPS and its components (Li et al., 2002; Maki et al., 1996). Impact to the UPS through classical proteasomal inhibitors have been associated with alterations in p53 regulation leading to apoptosis and cell cycle alterations (Chen et al., 2000). In addition, Cd has previously been associated with increased phosphorylation of p53 (Matsuoka and Igisu, 2001). These characteristics highlighted p53 as a possible key component of the mechanistic response to Cd. Our study demonstrates a significant upregulation of p53 and its ubiquitination at the critical dose of 20μM (Fig. 8). The results seen with p53 in this study further suggest that both the UPS and the proteins involved in stress responses are critical mechanisms of Cd exposure and testicular toxicity.
In summary, the results of the present study demonstrate unequivocally that low doses (10μM) of Cd lead to an accumulation of HMW-polyUb in conjunction with cytotoxicity. In addition, the accumulation of HMW-polyUb parallels the stress response. Cd exhibited similar properties to the nonspecific proteasomal inhibitor, MG132, resulting in the activation of stress proteins and apoptosis. However, this response was seen at a higher dose with Cd as compared to MG132. This suggests that Cd-induced cytotoxicity may be only partially associated with proteasomal-like inhibition resulting in the accumulation of HMW-polyUb conjugates. Cd-induced alterations to the UPS, however, provide an integrative mechanism that can explain the critical events leading to alterations in key oxidative stress signaling pathways, disruption in cell cycle regulation, and ultimately in spermatogenesis. The UPS might represent a novel and critical key pathway for male reproductive toxicity. Characterization of this pathway and understanding its role in metal toxicity will improve our ability to prevent the damage caused by both metals and other toxicants that are mediated through the UPS.
FUNDING
USEPA-NIEHS UW Center for Child Environmental Health Risks Research (EPA R826886 and NIEHS 1PO1ES09601), the NIEHS (R01-ES1063), UW NIEHS Center for Ecogenetics and Environmental Health (5 P30 ES07033), Colgate-Palmolive Grants for Alternative Research, Society of Toxicology and the Johns Hopkins Center for Alternatives to Animal Testing.
References
- Araya J, Maruyama M, Inoue A, Fujita T, Kawahara J, Sassa K, Hayashi R, Kawagishi Y, Yamashita N, Sugiyama E, et al. Inhibition of proteasome activity is involved in cobalt-induced apoptosis in human alveolar macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;283:L849–L858. doi: 10.1152/ajplung.00422.2001. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Hoogerbrugge JW, Roest HP, Ooms M, Vreeburg J, Hoeijmakers JH, Grootegoed JA. Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev. Biol. 1999;207:322–333. doi: 10.1006/dbio.1998.9155. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y. Regulatory functions of ubiquitination in the immune system. Nat. Immunol. 2002;3:20–26. doi: 10.1038/ni0102-20. [DOI] [PubMed] [Google Scholar]
- Bobba A, Canu N, Atlante A, Petragallo V, Calissano P, Marra E. Proteasome inhibitors prevent cytochrome c release during apoptosis but not in excitotoxic death of cerebellar granule neurons. FEBS Lett. 2002;515:8–12. doi: 10.1016/s0014-5793(02)02231-7. [DOI] [PubMed] [Google Scholar]
- Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985;24:119–124. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Canu N, Barbato C, Ciotti MT, Serafino A, Dus L, Calissano P. Proteasome involvement and accumulation of ubiquitinated proteins in cerebellar granule neurons undergoing apoptosis. J. Neurosci. 2000;20:589–599. doi: 10.1523/JNEUROSCI.20-02-00589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Chang D, Goh M, Klibanov S, Ljungman M. Role of p53 in cell cycle regulation and apoptosis following exposure to proteasome inhibitors. Cell Growth Differ. 2000;11:239–246. [PubMed] [Google Scholar]
- Chuang SM, Wang IC, Yang JL. Roles of JNK, p38 and ERK mitogen-activated protein kinases in the growth inhibition and apoptosis induced by cadmium. Carcinogenesis. 2000;21:1423–1432. [PubMed] [Google Scholar]
- Derfoul A, Lin FJ, Awumey EM, Kolodzeski T, Hall DJ, Tuan RS. Estrogenic endocrine disruptive components interfere with calcium handling and differentiation of human trophoblast cells. J. Cell Biochem. 2003;89:755–770. doi: 10.1002/jcb.10558. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Haghighi AP, Portman SL, Lee JD, Amaranto AM, Goodman CS. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature. 2001;412:449–452. doi: 10.1038/35086595. [DOI] [PubMed] [Google Scholar]
- Dickins RA, Frew IJ, House CM, O'Bryan MK, Holloway AJ, Haviv I, Traficante N, de Kretser DM, Bowtell DD. The ubiquitin ligase component Siah1a is required for completion of meiosis I in male mice. Mol. Cell Biol. 2002;22:2294–2303. doi: 10.1128/MCB.22.7.2294-2303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Templeton D. Activation of parallel mitogen-activated protein kinase cascades and induction of c-fos by cadmium. Toxicol. Appl. Pharmacol. 2000;162:93–99. doi: 10.1006/taap.1999.8829. [DOI] [PubMed] [Google Scholar]
- Dong W, Simeonova PP, Gallucci R, Matheson J, Flood L, Wang S, Hubbs A, Luster MI. Toxic metals stimulate inflammatory cytokines in hepatocytes through oxidative stress mechanisms. Toxicol. Appl. Pharmacol. 1998;151:359–366. doi: 10.1006/taap.1998.8481. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira ME, Cohen G. The ubiquitin/proteasome pathway: Friend or foe in zinc-, cadmium-, and H2O2-induced neuronal oxidative stress. Mol. Biol. Rep. 1999;26:65–69. doi: 10.1023/a:1006909918866. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira ME, Yakushin S, Cohen G. Disruption of the intracellular sulfhydryl homeostasis by cadmium-induced oxidative stress leads to protein thiolation and ubiquitination in neuronal cells. J. Biol. Chem. 1998;273:12703–12709. doi: 10.1074/jbc.273.21.12703. [DOI] [PubMed] [Google Scholar]
- Foote RH. Cadmium affects testes and semen of rabbits exposed before and after puberty. Reprod. Toxicol. 1999;13:269–277. doi: 10.1016/s0890-6238(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Fridman O, Corro L, Herkovits J. Estradiol uptake, toxicity, metabolism, and adverse effects on cadmium-treated amphibian embryos. Environ. Health Perspect. 2004;112:862–866. doi: 10.1289/ehp.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- Gennart JP, Buchet JP, Roels H, Ghyselen P, Ceulemans E, Lauwerys R. Fertility of male workers exposed to cadmium, lead, or manganese. Am. J. Epidemiol. 1992;135:1208–1219. doi: 10.1093/oxfordjournals.aje.a116227. [DOI] [PubMed] [Google Scholar]
- Henson MC, Chedrese PJ. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp. Biol. Med. (Maywood) 2004;229:383–392. doi: 10.1177/153537020422900506. [DOI] [PubMed] [Google Scholar]
- Hew KW, Ericson WA, Welsh M. A single low cadmium dose causes failure of spermiation in the rat. Toxicol. Appl. Pharmcol. 1993;121:15–21. doi: 10.1006/taap.1993.1123. [DOI] [PubMed] [Google Scholar]
- Iryo Y, Matsuoka M, Wispriyono B, Sugiura T, Igisu H. Involvement of the extracellular signal-regulated protein kinase (ERK) pathway in the induction of apoptosis by cadmium chloride in CCRF-CEM cells. Biochem. Pharmacol. 2000;60:1875–1882. doi: 10.1016/s0006-2952(00)00510-4. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Kenney N, Stoica A, Hilakivi-Clarke L, Singh B, Chepko G, Clarke R, Sholler PF, Lirio AA, Foss C, et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat. Med. 2003;9:1081–1084. doi: 10.1038/nm902. [DOI] [PubMed] [Google Scholar]
- Jungmann J, Reins HA, Schobert C, Jentsch S. Resistance to cadmium mediated by ubiquitin-dependent proteolysis. Nature. 1993;361:369–371. doi: 10.1038/361369a0. [DOI] [PubMed] [Google Scholar]
- Jurczuk M, Brzoska MM, Moniuszko-Jakoniuk J, Galazyn-Sidorczuk M, Kulikowska-Karpinska E. Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem. Toxicol. 2004;42:429–438. doi: 10.1016/j.fct.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick D, Dale K, Catania J, Gandolfi A. Low-level arsenite causis accumulation of ubiquitinated proteins in rabbit renal cortical slices and HERK293 cells. Toxicol. Appl. Pharmacol. 2003;186:101–109. doi: 10.1016/s0041-008x(02)00019-4. [DOI] [PubMed] [Google Scholar]
- Kon Y, Endoh D, Iwanaga T. Expression of protein gene product 9.5, a neuronal ubiquitin C-terminal hydrolase, and its developing change in sertoli cells of mouse testis. Mol. Reprod. Dev. 1999;54:333–341. doi: 10.1002/(SICI)1098-2795(199912)54:4<333::AID-MRD3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kwon J, Kikuchi T, Setsuie R, Ishii Y, Kyuwa S, Yoshikawa Y. Characterization of the testis in congenitally ubiquitin carboxy-terminal hydrolase-1 (Uch-L1) defective (gad) mice. Exp. Anim. 2003;52:1–9. doi: 10.1538/expanim.52.1. [DOI] [PubMed] [Google Scholar]
- Kwon J, Wang YL, Setsuie R, Sekiguchi S, Sakurai M, Sato Y, Lee WW, Ishii Y, Kyuwa S, Noda M, et al. Developmental regulation of ubiquitin C-terminal hydrolase isozyme expression during spermatogenesis in mice. Biol Reprod. 2004;71:515–521. doi: 10.1095/biolreprod.104.027565. [DOI] [PubMed] [Google Scholar]
- Laskey JW, Rehnberg GL, Laws SC, Hein JF. Reproductive effects of low acute doses of cadmium chloride in adult male rats. Toxicol. Appl. Pharmacol. 1984;73:250–255. doi: 10.1016/0041-008x(84)90330-2. [DOI] [PubMed] [Google Scholar]
- Laskey JW, Rehnberg GL, Laws SC, Hein JF. Age-related dose response of selected reproductive parameters to acute cadmium chloride exposure in the male Long-Evans rat. J. Toxicol. Environ. Health. 1986;19:393–401. doi: 10.1080/15287398609530937. [DOI] [PubMed] [Google Scholar]
- Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416:648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- Lopez Salon M, Morelli L, Castano EM, Soto EF, Pasquini JM. Defective ubiquitination of cerebral proteins in Alzheimer's disease. J. Neurosci. Res. 2000;62:302–310. doi: 10.1002/1097-4547(20001015)62:2<302::AID-JNR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Maki CG, Huibregtse JM, Howley PM. In vivo ubiquitination and proteasome-mediated degradation of p53(1) Cancer Res. 1996;56:2649–2654. [PubMed] [Google Scholar]
- Marx J. Cell biology. Ubiquitin lives up to its name. Science. 2002;297:1792–1794. doi: 10.1126/science.297.5588.1792. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Igisu H. Cadmium induces phosphorylation of p53 at serine 15 in MCF-7 cells. Biochem. Biophys. Res. Commun. 2001;282:1120–1125. doi: 10.1006/bbrc.2001.4700. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Gin AK, Sharma A, Talukder G. Relative efficacy of short-term tests in detecting genotoxic effects of cadmium chloride in mice in vivo. Mutat. Res. 1988 doi: 10.1016/0165-1218(88)90173-5. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Porras A. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 2000;25:257–260. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- Ng JM, Vrieling H, Sugasawa K, Ooms MP, Grootegoed JA, Vreeburg JT, Visser P, Beems RB, Gorgels TG, Hanaoka F, et al. Developmental defects and male sterility in mice lacking the ubiquitin-like DNA repair gene mHR23B. Mol. Cell Biol. 2002;22:1233–1245. doi: 10.1128/MCB.22.4.1233-1245.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- NTP. Toxicity studies of cadmium oxide (CAS No. 1306-19-0) administered by inhalation to F344/N rats and B6C3F1 mice. Toxic. Rep. Ser. 1995;39:1–D3. [PubMed] [Google Scholar]
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- Rajapurohitam V, Bedard N, Wing SS. Control of ubiquitination of proteins in rat tissues by ubiquitin conjugating enzymes and isopeptidases. Am. J. Physiol. Endocrinol. Metab. 2002;282:E739–E745. doi: 10.1152/ajpendo.00511.2001. [DOI] [PubMed] [Google Scholar]
- Rawe VY, Olmedo SB, Benmusa A, Shiigi SM, Chemes HE, Sutovsky P. Sperm ubiquitination in patients with dysplasia of the fibrous sheath. Hum. Reprod. 2002;17:2119–2127. doi: 10.1093/humrep/17.8.2119. [DOI] [PubMed] [Google Scholar]
- Rodgers KJ, Dean RT. Assessment of proteasome activity in cell lysates and tissue homogenates using peptide substrates. Int. J. Biochem. Cell Biol. 2003;35:716–727. doi: 10.1016/s1357-2725(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Roest HP, van Klaveren J, de Wit J, van Gurp CG, Koken MH, Vermey M, van Roijen JH, Hoogerbrugge JW, Vreeburg JT, Baarends WM, et al. Inactivation of the HR6B ubiquitin-conjugating DNA repair enzyme in mice causes male sterility associated with chromatin modification. Cell. 1996;86:799–810. doi: 10.1016/s0092-8674(00)80154-3. [DOI] [PubMed] [Google Scholar]
- Satarug S, Ujjin P, Vanavanitkun Y, Baker JR, Moore MR. Influence of body iron store status and cigarette smoking on cadmium body burden of healthy Thai women and men. Toxicol. Lett. 2004;148:177–185. doi: 10.1016/j.toxlet.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Schulman BA, Carrano AC, Jeffrey PD, Bowen Z, Kinnucan ER, Finnin MS, Elledge SJ, Harper JW, Pagano M, Pavletich NP. Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature. 2000;408:381–386. doi: 10.1038/35042620. [DOI] [PubMed] [Google Scholar]
- Sen Gupta R, Kim J, Gomes C, Oh S, Park J, Im WB, Seong JY, Ahn RS, Kwon HB, Soh J. Effect of ascorbic acid supplementation on testicular steroidogenesis and germ cell death in cadmium-treated male rats. Mol. Cell. Endocrinol. 2004;221:57–66. doi: 10.1016/j.mce.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Shaikh ZA, Vu TT, Zaman K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 1999;154:256–263. doi: 10.1006/taap.1998.8586. [DOI] [PubMed] [Google Scholar]
- Shi Y, Melnikov VY, Schrier RW, Edelstein CL. Downregulation of the calpain inhibitor protein calpastatin by caspases during renal ischemia-reperfusion. Am. J. Physiol. Renal. Physiol. 2000;279:F509–F517. doi: 10.1152/ajprenal.2000.279.3.F509. [DOI] [PubMed] [Google Scholar]
- Sutovsky P. Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: Killing three birds with one stone. Microsc. Res. Tech. 2003;61:88–102. doi: 10.1002/jemt.10319. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, McCauley TC, Sutovsky M, Day BN. Early degradation of paternal mitochondria in domestic pig (Sus scrofa) is prevented by selective proteasomal inhibitors lactacystin and MG132. Biol. Reprod. 2003;68:1793–1800. doi: 10.1095/biolreprod.102.012799. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Moreno R, Ramalho-Santos J, Dominko T, Thompson WE, Schatten G. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J. Cell Sci. 2001a;114:1665–1675. doi: 10.1242/jcs.114.9.1665. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Motlik J, Neuber E, Pavlok A, Schatten G, Palecek J, Hyttel P, Adebayo OT, Adwan K, Alberio R, et al. Accumulation of the proteolytic marker peptide ubiquitin in the trophoblast of mammalian blastocysts. Cloning Stem Cells. 2001b;3:157–161. doi: 10.1089/153623001753205115. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Terada Y, Schatten G. Ubiquitin-based sperm assay for the diagnosis of male factor infertility. Hum. Reprod. 2001c;16:250–258. doi: 10.1093/humrep/16.2.250. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk B, Biernacki B, Minta M, Kozaczynski W, Juszkiewicz T. Male golden hamster in male reproductive toxicology testing: Assessment of protective activity of selenium in acute cadmium toxication. Bull. Environ. Contam. Toxicol. 1995;54:907–912. doi: 10.1007/BF00197977. [DOI] [PubMed] [Google Scholar]
- Yang CF, Shen HM, Shen Y, Zhuang ZX, Ong CN. Cadmium-induced oxidative cellular damage in human fetal lung fibroblasts (MRC-5 cells) Environ. Health Perspect. 1997;105:712–716. doi: 10.1289/ehp.97105712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yu X. Regulation of apoptosis: The ubiquitous way. FASEB J. 2003;17:790–799. doi: 10.1096/fj.02-0654rev. [DOI] [PubMed] [Google Scholar]
- Yen JL, Su NY, Kaiser P. The yeast ubiquitin ligase SCFMet30 regulates heavy metal response. Mol. Biol. Cell. 2005;16:1872–1882. doi: 10.1091/mbc.E04-12-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Kubota H, Wang R, Saegusa J, Ogawa Y, Ichihara G, Takeuchi Y, Hisanaga N. Involvement of Bcl-2 family genes and Fas signaling system in primary and secondary male germ cell apoptosis induced by 2-bromopropane in rat. Toxicol. Appl. Pharmacol. 2001;174:35–48. doi: 10.1006/taap.2001.9187. [DOI] [PubMed] [Google Scholar]
- Yu X, Sidhu JS, Hong S, Faustman EM. Essential role of extracellular matrix (ECM) overlay in establishing the functional integrity of primary neonatal rat Sertoli cell/gonocyte co-cultures: An improved in vitro model for assessment of male reproductive toxicity. Toxicol. Sci. 2005;84:378–393. doi: 10.1093/toxsci/kfi085. [DOI] [PubMed] [Google Scholar]