Abstract
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental contaminants. Traditionally, much of the research has focused on the carcinogenic potential of specific PAHs, such as benzo(a)pyrene, but recent studies using sensitive fish models have shown that exposure to PAHs alters normal fish development. Some PAHs can induce a teratogenic phenotype similar to that caused by planar halogenated aromatic hydrocarbons, such as dioxin. Consequently, mechanism of action is often equated between the two classes of compounds. Unlike dioxins, however, the developmental toxicity of PAH mixtures is not necessarily additive. This is likely related to their multiple mechanisms of toxicity and their rapid biotransformation by CYP1 enzymes to metabolites with a wide array of structures and potential toxicities. This has important implications for risk assessment and management as the current approach for complex mixtures of PAHs usually assumes concentration addition. In this review we discuss our current knowledge of teratogenicity caused by single PAH compounds and by mixtures and the importance of these latest findings for adequately assessing risk of PAHs to humans and wildlife. Throughout, we place particular emphasis on research on the early life stages of fish, which has proven to be a sensitive and rapid developmental model to elucidate effects of hydrocarbon mixtures.
Keywords: PAHs, DLCs, developmental toxicity, synergism, AHR, CYP1A, risk assessment
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are environmental contaminants derived from petroleum and generated by the incomplete combustion of organic compounds. As a class, PAHs enter the environment through natural sources such as oil seeps and forest fires and through a variety of anthropogenic activities. These include the burning of fossil fuels and wood, production of coke and charcoal, metal smelting, petroleum refining, and petroleum spills (Douben, 2003; Latimer and Zheng, 2003). The United Nations Environmental Program has identified PAHs as a class of pollutants of global concern as they pose a real and potential risk to human health and the environment. In the United States, temporal trends suggest that environmental contamination by PAHs is steadily climbing, tracking patterns in urbanization, and combustion of fossil fuels, particularly by automobiles (Van Metre and Mahler, 2005; Van Metre et al., 2000). This is in contrast to reports of downward trends of environmental contamination by the planar halogenated aromatic hydrocarbons, i.e., the dioxin-like compounds (DLCs). PAHs comprise an important class of contaminants that invariably occur as mixtures at United States Environmental Protection Agency Superfund hazardous waste sites and other polluted environments. Widespread PAH contamination at these sites, coupled with their known (i.e., carcinogenic) and suspected toxicity, underlies their increased hazard ranking (from 10th to 7th) over the past decade on the U.S. Agency for Toxic Substances and Disease Registry's priority list (ATSDR, 1997, 2005). These data suggest that PAHs pose a significant threat to human health and ecological integrity and a potentially greater risk than that posed by exposure to the less prevalent DLCs (Van Metre and Mahler, 2005). Nonetheless, with the exception of their well-established role in carcinogenesis, the adverse developmental effects of PAH exposure in sensitive fish species have received attention only recently (Barron et al., 2004; Billiard et al., 2006; Hawkins et al., 2002; Incardona et al., 2006; Wassenberg and Di Giulio, 2004a,b).
Risk Assessment Models
While it is clear that some PAHs are toxic and that emissions are increasing, risk assessment of this class of environmental contaminants can be complicated. Because most environmental exposures are from complex mixtures of PAHs, we focus more on the issues and impacts associated with combinations of PAHs and less so on individual compounds. Current models used to estimate risks of mixtures assume dose or concentration additivity (Barron et al., 2004; Basu et al., 2001). Historically, additivity borrows from the concept of toxic equivalency factors (TEFs) which were developed to assess the risk of DLC mixtures to humans and wildlife with similar modes of action (Ontario Ministry of the Environment, 1985; Safe, 1993; Van den Berg et al., 1998). For additivity, exposure-response curves for chemically related compounds should be parallel and exhibit a similar range of response and efficacy (i.e., similar maxima). Nevertheless, these prerequisites are not always met, even for DLC congeners (Parrott et al., 1995; Van den Berg et al., 2006).
One criteria for applying the TEF approach to DLCs is the assumption that effects are mediated by binding to the aryl hydrocarbon receptor (AHR) (Van den Berg et al., 1998, 2006), a ligand-activated cytosolic transcription factor found in vertebrate species from fish to humans. Binding of DLCs to the AHR initiates nuclear translocation and heterodimerization with the aryl hydrocarbon nuclear translocator (ARNT). The heterodimer binds to dioxin-responsive elements (DREs) to regulate expression of a number of genes of the AHR battery or regulon, including phase I cytochrome P450 (CYP) enzymes, such as CYP1A1/2 and CYP1B1, the aryl hydrocarbon receptor repressor (AHRR), and phase II conjugation enzymes (Nebert and Dalton, 2006).
While some PAHs do bind to the AHR, there is uncertainty as to whether toxic equivalencies (TEQ = ∑(TEF × measured concentrations)) can or should be used to predict the toxicity of complex mixtures. PAHs do not meet the criteria for TEFs developed for DLCs (i.e., structural similarity to polychlorinated dibenzo-p-dioxins/dibenzo furans (PCDD/Fs), persistent, and bioaccumulative) (Van den Berg et al., 1998, 2006). Further, studies reviewed herein will demonstrate that the role of the AHR in PAH toxicity is very complex. An exception where a TEQ approach might be justified would be for carcinogenic PAHs which require CYP-mediated bioactivation or toxification (Incardona et al., 2006). However, PAH-type AHR agonists are rapidly metabolized by the enzymes that they induce, including CYPs such as CYP1A. Certainly, using the induction of CYP1A catalytic activity as a surrogate toxic end point to derive TEFs for PAH mixtures is problematic if not altogether flawed (Whyte et al., 2000). While CYP1A induction is not a toxic end point, the potency of DLCs for inducing CYP1A enzymes generally predicts their toxicity to developing organisms (Guiney et al., 1997; Heid et al., 2001; Safe, 1990, 1993). Thus, measurement of CYP1A activity as a biomarker of exposure is oftentimes used as a surrogate biomarker of effect or as an “early warning system” (Payne et al., 1987). In the strictest sense and as with any biomarker of exposure, CYP1A induction is not necessarily indicative of PAH toxic action. As we caution in this review, equating CYP1A induction with effect is not always acceptable for PAHs that cause developmental (i.e., noncarcinogenic) toxicity. For some PAHs, toxicity may be mediated by CYP1A metabolism while others may exert their toxicity independent of this activity and may actually have their toxicity reduced by this activity.
The idea for this review began with our observations that some PAHs mimicked dioxin, causing developmental and cardiovascular toxicity in the exquisitely sensitive fish early life stages (ELS) model. However, these PAH effects could not be predicted from AHR binding or CYP1A-induction potencies. Furthermore, studies indicated that unlike DLCs, the potencies of PAHs in mixtures were not always additive and that the TEQ approach fell short for these combinations. In this review, we present evidence to suggest that multiple mechanisms underscore PAH developmental effects. It follows then that the toxic potency of a given PAH mixture will be driven by its composition (including both AHR- and non-AHR–binding PAHs). Thus, current models for assessing the risk of PAHs during fish and vertebrate development are inadequate, and a new paradigm for characterizing hazard is required.
In making this case, we explore alternate mechanisms of PAH toxicity as a critical step toward reducing the uncertainty inherent in assessing the risk of a given PAH mixture. A National Institute of Environmental Health Sciences workshop (2002) on the role of environmental agents as important risk factors for cardiovascular disease emphasized the advantages of using alternative, nonmammalian models for the study of chemically induced cardiovascular malformations. While sensitivity varies among humans and across vertebrate taxa, the cardiotoxic effects of DLCs and some PAHs are remarkably similar, likely because the functional properties of the AHR and developmental signaling pathways are well conserved across vertebrate taxa (Hahn, 2002; Karchner et al., 2006; Nasevicius and Ekker, 2000). There are many benefits to using embryonic stages of fish, most notably a transparent chorion which allows for direct, noninvasive observation during embryogenesis. These characteristics make embryonic fish an excellent developmental model for elucidating mechanisms of chemically induced vascular defects in vertebrates (Antkiewicz et al., 2006; Carney et al., 2006b; Goldstone and Stegeman, 2006). Topics discussed herein include: (1) cardiovascular toxicity of AHR agonists during development, (2) chemically resistant populations, (3) altered CYP1A activity studies, (4) possible mechanisms of PAH developmental toxicity, and (5) implications for risk assessment.
DLCs AND PAHs ARE CARDIOTERATOGENS DURING EARLY VERTEBRATE DEVELOPMENT
In vertebrates, the cardiovascular system is one of the most sensitive targets of dioxin action. Cardiac dysfunction in fish ELSs is also a recently discovered subset of effects following chronic PAH exposure (Billiard et al., 2006; Incardona et al., 2004, 2006; Wassenberg and Di Giulio, 2004b; Wassenberg et al., 2005). For that reason, mechanisms underlying PAH teratogenicity are often compared to those of DLCs (Billiard et al., 1999, 2006; Incardona et al., 2005). The role of the AHR pathway in mediating dioxin toxicity has been the subject of extensive study, and a complete review is beyond the scope of this manuscript. However, it is instructive to briefly discuss the role of the AHR pathway in mediating teratogenesis by DLCs in vertebrates because of the apparent similarities in toxicity of some PAHs.
The Cardiovasculature is a Sensitive Target of Dioxin Toxicity
Although the majority of studies have examined cardiac effects of dioxin exposure in birds and fish, a recent study showed that the fetal murine heart is especially vulnerable to dioxin toxicity when challenged in utero (Thackaberry et al., 2005b). Specifically, reduced heart somatic index and inhibition of cardiomyocyte proliferation were observed. In the same study, postnatal or lactational exposure of pups exposed in utero showed increased heart-to-body weight and decreased heart rate (i.e., bradycardia; Thackaberry et al., 2005b). This proposed compensatory cardiac hypertrophy was also observed in an earlier study in dioxin-treated mice (Lin et al., 2001). Similarly, altered heart size and decreased myocyte number or growth have been demonstrated in fish and birds—in these species dioxin exposure also increases vascular apoptosis and permeability (Antkiewicz et al., 2005; Guiney et al., 2000; Hill et al., 2004; Hornung et al., 1999; Invitski et al., 2001; Rifkind, 2006; Toomey et al., 2001; Walker and Catron, 2000). Thus, the suite of dioxin-induced cardiovascular defects are fairly similar across vertebrate taxa (Thackaberry et al., 2005b). These effects are supported mechanistically by microarray studies in hearts of dioxin-exposed zebra fish embryos (Danio rerio) and fetal mice where expression of cardiac-specific genes, such as those encoding for cell cycle regulation and proliferation, are downregulated following exposure (Carney et al., 2006a; Thackaberry et al., 2005a). Cardiovascular gene expression profiles of dioxin-exposed zebra fish embryos were also consistent with cardiomyopathy observed in these fish (Goldstone and Stegeman, 2006; Handley-Goldstone et al., 2005).
Fish appear to be the most sensitive of all vertebrate classes to dioxin-induced teratogenicity (Peterson et al., 1993). The relative sensitivity of fish to dioxin-induced ELS mortality (Elonen et al., 1998) is in the range of the most sensitive avian and mammalian species. Accordingly, our understanding of mechanisms underlying dioxin toxicity has been advanced using fish ELS as model developmental systems (Goldstone and Stegeman, 2006). Exposure of salmonid ELS to dioxin causes an overt toxicity syndrome called blue sac disease (BSD), characterized by increased rates of mortality, pericardial and yolk sac edemas, regional ischemia, subcutaneous hemorrhages, craniofacial deformities, and arrested growth (Spitsbergen et al., 1991). Signs of toxicity closely resembling BSD have been described in several fish species including rainbow trout (Oncorhynchus mykiss), zebra fish, Japanese medaka (Oryzias latipes), and killifish (Fundulus heteroclitus) exposed to DLC-type AHR agonists (Chen and Cooper, 1999; Elonen et al., 1998; Helder, 1981; Toomey et al., 2001; Walker and Peterson, 1991; Wannemacher et al., 1992). Several studies have shown that fish embryonic vasculature is the primary physiologic target for dioxin-induced embryotoxicity (Belair et al., 2001; Dong et al., 2002; Guiney et al., 1997; Henry et al., 1997; Hornung et al., 1999). In embryonic fish, pericardial edema is secondary to inhibition of angiogenesis, reduced blood flow, and circulatory failure (Bello et al., 2004; Hornung et al., 1999). In birds, inhibition of blood vessel formation (Ivnitski et al., 2001) is associated with both reduced expression and sensitivity to vascular endothelial growth factor (VEGF) (Ivnitski-Steele et al., 2004, 2005). Similar toxicological profiles across taxa are a strong argument that embryonic stages of zebra fish are an appropriate model of effects in higher vertebrates.
For a more comprehensive review of dioxin-induced cardiovascular toxicity, the reader is referred to Carney et al. (2006b) and Goldstone and Stegeman (2006).
Role of the AHR Pathway in Developmental Toxicity of DLCs
The AhR.
Perturbation or atypical modulation of the AHR signaling pathway can disrupt normal cardiovascular development during the ELS of vertebrates (Carney et al., 2006b; Goldstone and Stegeman, 2006). There are well-established positive relationships among the AHR-binding affinity of DLCs, their potency for CYP1A induction, and their toxicity to vertebrate models, including mammals, birds, and fishes (Guiney et al., 1997; Heid et al., 2001; Safe, 1990, 1993). In mammals, activated AHR is mandatory for acute and teratogenic response to dioxin as evidenced by the resistance of receptor null mice to toxicity (Fernandez-Salguero et al., 1996; Gonzalez and Fernandez-Salguero, 1998; Mimura et al., 1997; Peters et al., 1999; Rifkind 2006; Walisser et al., 2004). Genetic ablation of the Ahr is also associated with aberrant vascular patterning or phenotypes in nontreated mice, underscoring the importance of this pathway in normal vascular development (Lahvis et al., 2000; Lund et al., 2003; McMillan and Bradfield, 2007; Rifkind, 2006; Walisser et al., 2004).
Recent advances using morpholino antisense technology have confirmed the obligatory role of the AHR pathway in dioxin-induced ELS toxicity by embryonic knockdown of target gene expression in zebra fish (see below). However, there are some distinct differences compared to mammalian AHR and unique challenges, particularly when it comes to elucidating a physiological or developmental role for this pathway in fish.
Unlike mammals which have only one AHR (AHR1), fish have several AHR isoforms belonging to two clades (AHR1 and AHR2) which originated from an independent duplication event prior to divergence of teleosts and separation of piscine and mammalian lines (Hahn et al., 1997; Karchner et al., 2005). Thus, it has been suggested that AHR function in fish is shared or divided among several AHRs (Hahn, 2001; Karchner et al., 2005). Although zebra fish AHR1(A) more closely resembles the single mammalian AHR protein (Karchner et al., 1999), this receptor does not bind DLC (dioxin) or PAH benzo-(a)pyrene (BaP) ligands and, furthermore, exhibits negligible transactivation activity in vitro (Andreasen et al., 2002b; Karchner et al., 2005). However, recent data suggest that PAHs can act via AHR1A to induce CYP1A in vivo; i.e., knock down of zebra fish embryo ahr1a partially protected against pyrene-induced toxicity and reduced induction of CYP1A in hepatic tissue (Incardona et al., 2006). Additionally, a functional AHR protein, AHR1B, has been characterized and is expressed early in zebra fish development, prompting speculation that it plays more of an endogenous or physiological role in ELS of fish (Karchner et al., 2005). On the other hand, AHR2 protein categorically mediates toxic outcome following dioxin insult in fish (Andreasen et al., 2002a; Antkiewicz et al., 2006; Karchner et al., 2005; Prasch et al., 2003).
Using morpholinos to knock down translation of AHR2 protein in developing zebra fish prevents CYP1A expression and toxicity typical of dioxin exposure (Carney et al., 2004; as reviewed in Carney et al., 2006b). We have also shown that ahr2 knockdown protects zebra fish from PCB126, a potent dioxin-like AHR agonist (Billiard et al., 2006). Along with AHR2, ARNT1 protein is obligatory for cardiac toxicity in dioxin-exposed zebra fish embryos (Antkiewicz et al., 2006; Prasch et al., 2006). AHR·ARNT cooperativity in developmental signaling and dioxin toxicity has also been shown in mutant mice homozygous for a lower expressing, or hypomorphic, Arnt allele (Walisser et al., 2004). Because AHR2 morphants are normal compared to noninjected embryos, one might conclude that there is no functional role of this receptor during fish development (Billiard et al., 2006; Dong et al., 2004; Incardona et al., 2006; Prasch et al., 2003; Teraoka et al., 2003). However, the fact that an AHR2 morphant phenotype has not been observed in zebra fish studies could be more a consequence of rapid degradation of the antisense morpholino in vivo or that residual AHR expression in knockdown embryos is capable of supporting normal development (Carney et al., 2006b; Karchner et al., 2005).
CYP1A.
While fish studies are consistent with the literature showing that dioxin toxicity to mammals is AHR dependent, the role of CYP1A induction and activity in toxicity is ambiguous for vertebrates in general. Roles for CYP-mediated toxicity, including generation of reactive oxygen species (ROS) and/or reactive metabolites, are discussed in detail below (see “Possible mechanisms of developmental PAH toxicity” section). Of the CYP enzymes, CYP1A is maximally induced following AHR activation by environmental contaminants in fish as well as in mammals (Goldstone and Stegeman, 2006; Jönsson et al., 2007a,b; Timme-Laragy et al., 2007; Wang et al., 2006). For these reasons, we gave most weight to the CYP1A subfamily in this review. However, by definition, AHR-mediated toxicity depends on one or more of the AHR-regulated gene targets. Thus, the toxicological implication of other CYP1s, particularly for rapidly metabolized PAHs, is also discussed where appropriate.
What is the toxicological significance of CYP1A activity? Recent evidence calls for a review of the classically held paradigm that CYP1A induction is simply a biomarker of exposure or adverse effect (Rifkind, 2006). While induction of CYP1A in mammals is tightly linked to AHR activation, it is not necessarily predictive of the expression of other, and currently unknown, AHR-regulated genes that mediate toxic response to DLCs (Brunnberg et al., 2006). In mammalian studies, there is no evidence that cyp1a1 or 1b1 null mice are protected against dioxin-induced developmental toxicity. In contrast, CYP1A2 sequestered dioxin in hepatic tissue of pregnant mice or dams, thus protecting against embryotoxicity and teratogenesis (Dragin et al., 2006; Ma and Lu, 2007).
Guiney et al. (1997) provided the first mechanistic evidence in fish that CYP1A could be the downstream target of dioxin-activated AHR and that this induction might mediate developmental toxicity. Dose-response curves for CYP1A induction in the vascular endothelium of lake trout sac fry were correlated with dose-response curves for mortality, and overt signs of toxicity were preceded by CYP1A induction (Guiney et al., 2000). Dioxin-induced apoptosis was also correlated with embryotoxicity and colocalized with CYP1A expression in medaka and killifish embryos (Cantrell et al., 1998; Toomey et al., 2001). Recently, Jönsson et al. (2007b) demonstrated similar dose-response curves for CYP1A, 1B1, 1C1, and CYP1C2 expression and abnormalities (e.g., pericardial edema) in zebra fish embryos exposed to PCB126.
Most studies report that chemical inhibition of CYP1A activity, or morpholino knock down of CYP1A protein, is protective in the case of DLCs (Billiard et al., 2006; Cantrell et al., 1996; Dong et al., 2004; Teraoka et al., 2003; Wassenberg and Di Giulio, 2004b). As a result, it has been hypothesized that CYP1A enzyme activity causes developmental toxicity of DLCs (Billiard et al., 2006; Cantrell et al., 1996; Dong et al., 2004; Teraoka et al., 2003; Wassenberg and Di Giulio, 2004b). However, several recent studies suggest the opposite, that dioxin activation of the AHR pathway causes teratogenicity by a CYP1A-independent mechanism in zebra fish (Antkiewicz et al., 2006; Carney et al., 2004). Regarding the two morpholino studies that were at odds with respect to CYP1A-mediated teratogenesis, Carney et al. (2004) suggested that signs of TCDD toxicity are not manifested until 96 hours post-fertilization (hpf) in zebra fish and that this explained the “lack of response” at 72 hpf observed by Teraoka et al. (2003). While AHR activation seems to be a prerequisite for dioxin toxicity, the identity of the trigger gene or genes regulating teratogenicity remains unknown (Carney et al., 2004). This brings us back to the original question first proposed almost two decades ago: What gene targets downstream of the AHR/ARNT pathway elicit dioxin toxicity? This question, still unanswered, is the target of a concerted effort currently underway in the field of toxicogenomics (e.g., Antkiewicz et al., 2005; Carney et al., 2006a; Goldstone and Stegeman, 2006; Handley-Goldstone et al., 2005).
PAHs Mimic Developmental and Cardiotoxic Effects of Dioxin but Mixture Toxicity is Nonadditive
As with dioxin, chronic exposure of ELS of fish species to some PAHs leads to CYP1A induction, edemas, hemorrhaging, cardiac dysfunction, mutations, heritable changes in progeny, morphological deformities, neuronal cell death, anemia, reduced growth, and increased mortality rates (Barron et al., 2003, 2004; Billiard et al., 2006; Brinkworth et al., 2003; Colavecchia et al., 2004, 2006; Incardona et al., 2004, 2005; Marty et al., 1997; Wassenberg and Di Giulio, 2004a; Wassenberg et al., 2005). However, evidence for PAH-induced developmental and cardiovascular toxicity is not restricted to fish. The Nebert laboratory first demonstrated that BaP was teratogenic to mice (as reviewed in Nebert, 1989). Since then, several studies have demonstrated that this class of compounds are developmental toxicants in both humans and laboratory species (as reviewed by Ramesh et al., 2004). Crude oil has also been shown to induce developmental effects in rats and birds, effects that were attributed to the PAH fraction of petroleum hydrocarbon mixtures (Feuston et al., 1997; Hoffman and Gay, 1981). In their studies with ducks, Hoffman and Gay (1981) observed that addition of individual PAHs to crude oil greatly enhanced its embryotoxic effects. Thus, additive or synergistic effects could account for the developmental toxicity of crude oil even when individual PAHs are present at low concentrations (Hoffman and Gay, 1981). More recently, prenatal exposure to of rats and human, PAHs has been associated with adverse effects on fetal growth and/or the developing vasculature (Choi et al., 2006; Sanyal and Li, 2007). Further, studies have indicated that PAHs are a significant risk factor for pathogenesis of cardiovascular disease in humans (as reviewed in Korashy and El-Kadi, 2006).
Because some PAHs are agonists for the AHR and moderately induce CYP1A (Billiard et al., 2002; Table 1), a commonly held assumption is that PAH teratogenicity is also AHR mediated. If this hypothesis is correct, we would expect that those compounds with low or negligible binding affinity to the AHR would be less toxic than those PAHs with moderate to high affinity for the AHR protein. In addition, toxic interactions among PAH-type AHR agonists should be strictly additive, and the potency of complex PAH mixtures could be estimated using the TEQ approach for dioxin-like AHR agonists (Walker et al., 1996; Zabel et al., 1995).
TABLE 1.
Key Pharmacokinetic and Toxicological Differences between Planar Halogenated DLCs and PAHs
| DLCs | PAHs |
| Potent AHR agonists | Weak to moderate AHR agonists |
| Potent CYP1A inducers | Weak to moderate CYP1A inducers |
| Poor substrates for CYP1A | Excellent substrates for CYP1A |
| Slowly metabolized | Rapidly metabolized |
| Long half-life | Short half-life |
| Generate ROS by uncoupling CYP1A | Metabolized to reactive intermediates |
| All cardiotoxic | Some are cardiotoxic |
However, data regarding the role of the AHR pathway in causing PAH cardiotoxicity during development do not support this hypothesis. A recent study suggests that cardiovascular toxicity caused by exposure of fish ELS to weathered crude oil is independent of both AHR1 (AHR1A) and AHR2 (Incardona et al., 2005). Further, CYP1A activity is protective against toxicity caused by exposure to some PAHs. Another confounding factor is that PAH-type AHR agonists and CYP1A inhibitors typically co-occur in environmental mixtures. We have observed nonadditive, synergistic cardiovascular interactions between PAH-type AHR agonists and CYP1A inhibitors. Our recent work with a sediment extract from a PAH-polluted Superfund site also suggests that the interactive effects of PAH mixtures may drive the developmental toxicity of these compounds (Wassenberg and Di Giulio, 2004b; Wassenberg et al., 2005). This is a departure from responses observed with dioxin-like AHR agonists where CYP1A inhibition generally reduced toxicity or had no effect (Cantrell et al., 1996; Dong et al., 2002).
Although there is a strong, positive relationship between the ability of PAHs to bind to the AHR and to induce CYP1A (Billiard et al., 2002; Bols et al., 1999; Piskorska-Pliszczynska et al., 1986), the relationships between AHR binding and CYP1A activity and their roles in PAH toxicity are far less clear. The most potent AHR agonists are not necessarily toxic (Billiard, 2002; Billiard et al., 2002), and PAHs do not always behave in an additive fashion (Basu et al., 2001; Hodson et al., 2007; Wassenberg and Di Giulio, 2004b; Wassenberg et al., 2005). In studies with rainbow trout embryos, the rank order of potency of PAHs for CYP1A induction (Basu et al., 2001) did not predict the potency of these same compounds for causing BSD (Billiard, 2002). Surprisingly, the most potent CYP1A inducer, benzo[k]fluoranthene (BkF), was nontoxic to trout ELS. Similarly, Wassenberg and Di Giulio (2004b) demonstrated that BaP by itself was virtually nontoxic to larval killifish, despite its high potency for inducing CYP1A. In contrast, alkyl-PAHs such as retene (7-isopropyl-1-methylphenanthrene) are weaker AHR agonists than B[k]F but far more toxic to fish ELS (Billiard et al., 1999). Overall, AHR-binding affinity underestimates PAH potency for causing dioxin-like teratogenesis to fish ELS (Barron et al., 2004; Billiard et al., 2002). Thus, it appears that potency of PAHs for binding to the AHR and activating the cyp1a gene alone is not enough to predict toxicity and that CYP1A induction equivalency factors for PAHs are neither additive nor appropriate for estimating toxicities of mixtures of PAHs (Basu et al., 2001).
A key difference between the embryotoxicities of PAHs and DLCs is the rapid metabolism and excretion of PAHs (Table 1), a process based largely on CYP1 enzyme activity (Brown et al., 2002; Niimi and Palazzo, 1986). An altered capacity to metabolize and excrete these compounds could be a factor in resistance to PAH toxicity that has been observed in some populations of fish chronically exposed to contaminated environments. In the following section, we summarize our studies of a Fundulus population inhabiting an estuary polluted by PAHs from an adjacent U.S. Superfund site. This population has adapted to pollutants present in its environment over many generations of exposure, providing a case study in evolutionary ecotoxicology and a powerful model for understanding developmental toxicity through examination of mechanisms underlying resistance.
INSIGHTS GAINED FROM CHEMICALLY RESISTANT POPULATIONS
Multiple populations of fish have been discovered inhabiting sites highly contaminated with DLCs, PAHs, and mixtures thereof (reviewed by Wirgin and Waldman, 2004). These populations are defined “resistant” by the criteria that:
They survive chronic exposure to levels of contaminants in their habitats that would prove fatal to most naïve fish and
They are resistant to the toxic effects of laboratory exposure to DLCs and PAHs (e.g., Meyer et al., 2002, 2003a; Meyer and Di Giulio, 2003; Nacci et al., 1999, 2002; Ownby et al., 2002; Prince and Cooper, 1995; Roark et al., 2005; Wirgin and Waldman, 2004).
Several groups of investigators have asked what biochemical or molecular changes are responsible for the resistant phenotype of these fish (reviewed by Hahn, 1998, 2001; Wirgin and Waldman, 1998, 2004). These studies are relevant to this review in that a better understanding of mechanisms of adaptation to AHR agonists should yield insight into the basic mechanisms of toxicity of AHR agonists. A common characteristic of the populations studied has been altered CYP1A mRNA and protein expression and activity (Arzuaga and Elskus, 2002; Bello et al., 2001; Courtenay et al., 1999; Elskus et al., 1999; Meyer et al., 2002, 2003b). While “basal” or “constitutive” expression is perhaps a misleading term to describe activity in chronically exposed populations, CYP1A expression in wild fish from contaminated sites is only two- to threefold higher than in reference site fish, rather than markedly higher as would be expected based on the level of contaminant exposure. More strikingly, sensitivity to CYP1A induction is markedly reduced or undetectable in fish from these resistant populations after laboratory exposure to prototypical inducers. Furthermore, lack of CYP1A induction has been observed in several other populations that have not been directly tested for toxicity resistance (Brammell et al., 2004; Förlin and Celander, 1995; Monosson and Stegeman, 1991), suggesting that recalcitrance to CYP1A induction is common to fish populations chronically exposed to AHR agonists.
These results, consistent across several species of fish at multiple sites, suggest that low CYP1A inducibility might be an adaptive response, a hypothesis advanced by several researchers (Elskus, 2001; Elskus et al., 1999; Nacci et al., 2002). For example, extracts of sediments from the contaminated Elizabeth River are highly teratogenic to killifish embryos reared from parents from relatively uncontaminated reference sites. The predominant effects of exposures to these extracts are cardiovascular defects. In contrast, embryos from parents of killifish indigenous to the Elizabeth River site are remarkably resistant to these effects. Previous studies suggested that downregulation of AHR-mediated gene transcription and upregulation of antioxidant defenses were associated with the resistance exhibited by the Elizabeth River population (Meyer et al., 2002, 2003a). In a related study, Nacci et al. (2002) showed that the Elizabeth River killifish exhibited low CYP1A inducibility and produced few DNA adducts after laboratory exposure to BaP.
These studies provide correlative evidence suggesting that low CYP1A inducibility is protective in the context of ELS toxicity. The hypothesis that high CYP1A activity mediates toxicity of high concentrations of DLCs and PAHs also appeared mechanistically attractive based on the capacity of CYP1A to activate PAHs to reactive metabolites and generate ROS in the presence of DLCs, as discussed elsewhere in this article. Nonetheless, while the correlation between resistance and altered CYP1A expression is widespread, making a causal link theoretically plausible, no cause-and-effect relationship has been demonstrated. Furthermore, one of the resistant populations exhibited refractory CYP1A induction in response to exposure to DLCs but not PAHs (Courtenay et al., 1999), and in hepatocytes from another fish population, CYP1A was highly refractory to induction by DLCs, but only less refractory to induction by PAHs (Bello et al., 2001). These results suggest that the modes of toxicity of DLCs and PAHs are not identical.
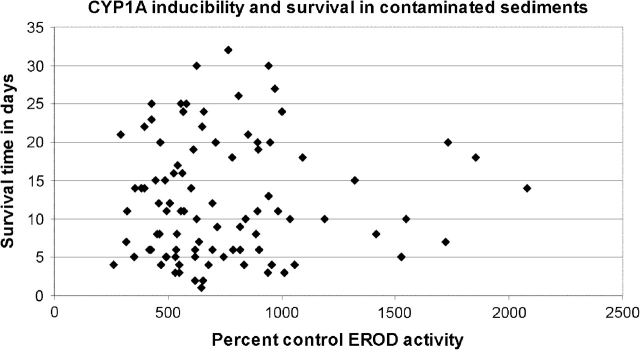
There is currently no direct evidence to support the hypothesis that a lack of CYP1A inducibility in resistant fish populations is protective in the context of exposure to high levels of PAHs. For killifish embryos from a reference site (Kings Creek), we observed no correlation between CYP1A inducibility and their subsequent survival when exposed to Elizabeth River environmental samples contaminated with lethal concentrations of PAHs (Fig. 1, adapted from Meyer et al., 2002). Additionally, killifish from this site on the Elizabeth River were highly resistant to the toxicity of those sediments, and resistance was more heritable than the lack of CYP1A inducibility (Meyer et al., 2002, 2003b). These data suggest that co-occurrence of low inducibility and chemical resistance in Elizabeth River fish does not reflect a causal relationship, i.e., resistance was likely conferred by some other mechanism or combination of mechanisms.
FIG. 1.
Lack of correlation between Ethoxyresorufin-O-deethylase (EROD) induction in embryos and posthatch survival in Elizabeth River sediment pore water for King's Creek larvae (adapted from Meyer et al., 2002). Larvae previously characterized for EROD induction after exposure to the inducer 3-methylcholanthrene as embryos were exposed to a 1:10 dilution of Elizabeth River sediment pore water in individual glass scintillation vials (i.e., one part sediment pore water, nine parts clean artificial sea water) 2 days posthatch. Spearman's rank order correlation coefficient: r = − 0.0008, p = 0.9228.
In summary, there is a common co-occurrence of poor CYP1A inducibility and resistance to embryotoxicity in wild fish populations inhabiting sites highly contaminated with AHR agonists. However, current evidence from experiments with indigenous fish populations does not support the hypothesis that lack of CYP1A induction confers protection in PAH-contaminated environments. It may be that altered expression of other AHR-regulated genes provides protection to these resistant fish populations. In this case, lack of CYP1A inducibility may not be a toxicologically protective adaptation but rather a marker of AHR pathway inhibition. While regulation of other AHR pathway genes has thus far received less attention in resistant fish populations, mRNA inducibility of at least one additional AHR-regulated gene, AHRR, is diminished in at least two resistant populations (Meyer et al., 2003b; Roy et al., 2006). mRNA expression levels in the absence of a laboratory exposure were not significantly different from those observed in a reference population in any of three tested resistant populations (Karchner et al., 2002; Meyer et al., 2003b; Roy et al., 2006).
Wild populations of fish resistant to PAH-induced embryotoxicity are a complicated model system. The resistant phenotype could reflect a number of changes across more than one genetic or biochemical parameter. However, the value of studying such populations is that they demonstrate environmentally relevant responses to DLC and PAH contamination. Whatever the mechanisms of adaptation that operate in these resistant populations, they reflect processes that unequivocally protect against realistic, multigenerational environmental exposures to PAHs. Currently, the evidence obtained from these populations suggests that lack of CYP1A inducibility, while correlated with PAH resistance, is not responsible for PAH resistance. A protective role for altered an AHR pathway, however, appears likely. One possibility is that epigenetic alterations in AHR pathway gene expression are involved. This phenomenon has been observed in various mammalian systems (Jin et al., 2004; Mulero-Navarro et al., 2006; Schnekenburger et al., 2007; Takahashi et al., 1998; Tokizane et al., 2005), although evidence so far does not support a role for promoter region methylation in preventing CYP1A inducibility in resistant fish populations (Arzuaga et al., 2004; Timme-Laragy et al., 2005).
Finally, contaminated sites also contain numerous other known and unknown chemical contaminants which may further complicate observed synergistic cardiovascular interactions between PAH-type AHR agonists and CYP1A inhibitors (reviewed below).
INSIGHTS GAINED FROM ALTERED CYP1A ACTIVITY STUDIES
To directly test the hypothesis that lowered CYP1A activity is protective of PAH-derived toxicity, pharmacological agents, mRNA antisense morpholinos, and CYP1A1 knockout mice have been used to modulate or prevent CYP1A activity in animals exposed to PAHs. Experiments with killifish embryos coexposed to extracts of PAH-contaminated sediments or pure PAHs with a suite of CYP1A inhibitors demonstrated that CYP1A inhibition increased the incidence of heart deformities rather than decreasing embryotoxicity. This suggested that many AHR agonists and CYP1A inhibitors together were synergistic, independent of the mechanism of action or chemical structure of the inhibitor (Wassenberg and Di Giulio, 2004a,b; Wassenberg et al., 2005).
An inherent limitation of studies with CYP1A inhibitors is that they may have other activities in addition to the inhibition of CYP1A. For example, α-naphthoflavone (ANF) has been characterized as a CYP1A inhibitor, an AHR antagonist and an AHR agonist (Merchant and Safe, 1995; Merchant et al., 1992; Testa and Jenner, 1981). Piperonyl butoxide is a nonspecific P450 inhibitor, so in vivo it is likely to inhibit other enzymes in addition to CYP1A (Franklin, 1977; Murray and Reidy, 1990; Testa and Jenner, 1981). A further complication is that the nature of these interactions may change with exposure or dose. Inhibitors will have their own unique subset of sublethal and lethal modes of action which can interact with acute and chronic mechanisms of PAH toxicity. For example, rainbow trout embryos exposed to a range of retene concentrations showed an exposure-dependent increase in the prevalence and severity of signs of BSD (Billiard et al., 1999; Brinkworth et al., 2003). As with killifish, a coexposure to low concentrations of ANF greatly enhanced the toxicity of retene. In contrast, at higher concentrations of ANF, the toxicity of all concentrations of retene decreased so that rates of mortality and BSD were not different from control (Hodson et al., 2007). This exposure-dependent synergism and antagonism of toxicity by one inhibitor of CYP1A activity may be related to exposure-dependent changes in the rate of metabolism and tissue concentrations of specific metabolites of retene (reviewed below).
We have also compared toxicity of PAHs with and without CYP1A inhibitors to wild type versus ahr2a or cyp1a zebra fish morphants. Similar to the results with chemical inhibitors in killifish embryos, knock down of CYP1A protein expression by morpholino injection increased the toxicity of a PAH-type AHR agonist (BNF) and the toxicity of a mixture of BNF with the CYP1A inhibitor, ANF (Billiard et al., 2006). In contrast, cyp1b1 knockdown did not alter synergistic developmental toxicity of PAHs in zebra fish (Timme-Laragy et al., 2007).
Homozygous CYP1A1 knockout mice showed less liver damage and survived the acute effects of injection of the PAH BaP for 3 days longer than did those that were heterozygous for CYP1A1 (Uno et al., 2001). However, these CYP1A1 knockout mice also showed fourfold higher levels of BaP-DNA adducts than did those heterozygous for CYP1A1. That is, the acute lethality of BaP was reduced by a lack of CYP1A1 whereas genotoxicity was actually increased (Uno et al., 2001). In contrast, but more in parallel with what we have observed in fish, in a more recent study this group found that BaP administered in the diet caused lethality in CYP1A1 knockout mice at a dose that was not lethal to CYP1A1-expressing mice (Uno et al., 2004). They proposed that CYP1A1 activity is critical for the detoxification of orally administered BaP in mice, rather than enhancing the toxicity of BaP, as they suggested previously.
While they did not look at effects of PAH toxicity, Dubey et al. (2003, 2005) found that CYP1A1 activity was cardioprotective in human cardiac fibroblast and smooth muscle cells because it catalyzes the conversion of estradiol to anti-mitogenic metabolites that act independently of the estrogen receptor. Treatment of these cells with CYP1A1 inhibitors removed the cardioprotective effects of estradiol. Further exploration of cardioprotective effects of CYP1A1 activity on estrogen during development may be fruitful.
The cooccurrence of AHR agonists and CYP1A inhibitors is typical of PAH-contaminated environmental mixtures. Fluoranthene (FL) and the heterocyclic PAHs, carbazole (CB) and dibenzothiophene (DBT), are common components of complex mixtures of PAHs (e.g., coal tar, crude oil). Each has been characterized in vivo and in vitro as a CYP1A inhibitor, and each increases embryotoxicity when combined with PAH-type AHR agonists (Wassenberg and Di Giulio 2004a,b; Wassenberg et al., 2005; Willett et al., 1998, 2001). Hence, synergistic interactions of these compounds with other PAHs may be responsible for the observed embryotoxicity of complex mixtures. FL, CB, and DBT have only recently been characterized as CYP1A inhibitors, and their potential interactions with other oxidative enzymes or their ability to interfere with AHR signaling is not yet known (Wassenberg et al., 2005; Willett et al., 1998, 2001). The synergy that we have observed between these classes of molecules is an important consideration when assessing risks posed by exposure to PAH contamination and also provides additional insights concerning mechanisms underlying greater-than-additive toxicities caused by exposure to PAH mixtures.
While the role of CYP1A activity has not been clearly elucidated in the toxicity of either DLCs or PAHs, there is a clear difference between these two classes of compounds when CYP1A is inhibited during development. Lowered CYP1A activity or knock down of CYP1A protein in embryos dosed with DLCs resulted in either reduced toxicity or had no change in toxicity (Billiard et al., 2006; Cantrell et al., 1996; Carney et al., 2004; Dong et al., 2004; Teraoka et al., 2003; Wassenberg and Di Giulio, 2004b). In contrast for PAHs, inhibition of CYP1A was manifested as either an increase or decrease in fish embryotoxicity, depending on ANF exposure concentration (Hodson et al., 2007; Wassenberg and Di Giulio, 2004b). However, cyp1a knockdown in zebra fish embryos markedly enhanced toxicity of BNF alone and in combination with ANF (Billiard et al., 2006). This disparity suggests that the mechanism of toxicity of the PAH-type AHR agonists differs from that of DLCs and that the relationship among AHR agonism, CYP1A activity, and PAH-mediated embryotoxicity is quite complex.
POSSIBLE MECHANISMS OF DEVELOPMENTAL PAH TOXICITY
Although developmental toxicity has been associated in fish models with exposure to crude oil, retene (C-4 alkyl-phenanthrene), and PAH-contaminated sediments, not all PAHs tested cause this syndrome. For example, in contrast to injected BaP and BkF, waterborne BaP and BkF were potent CYP1A inducers, but nontoxic to fish embryos within the limits of their solubilities (Billiard, 2002; Wassenberg and Di Giulio, 2004b), perhaps because toxicity was limited kinetically by low rates of uptake (see “AhR-mediated toxicity” section below). Conversely, phenanthrene did not induce CYP1A but was embryotoxic without signs of BSD (Hawkins et al., 2002). As discussed above, effects can be potentiated or antagonized by specific mixtures, suggesting nonlinear toxic interactions. In this section, we summarize recent research that points to four hypotheses (Fig. 2) to explain BSD: narcosis, cardiac-mediated toxicity (direct interference with cardiac development), AHR-mediated toxicity (altered transcription of AHR pathway genes during ontogeny), and CYP1A-mediated toxicity (toxicity due to oxygenation of PAHs by CYP1A enzymes).
FIG. 2.
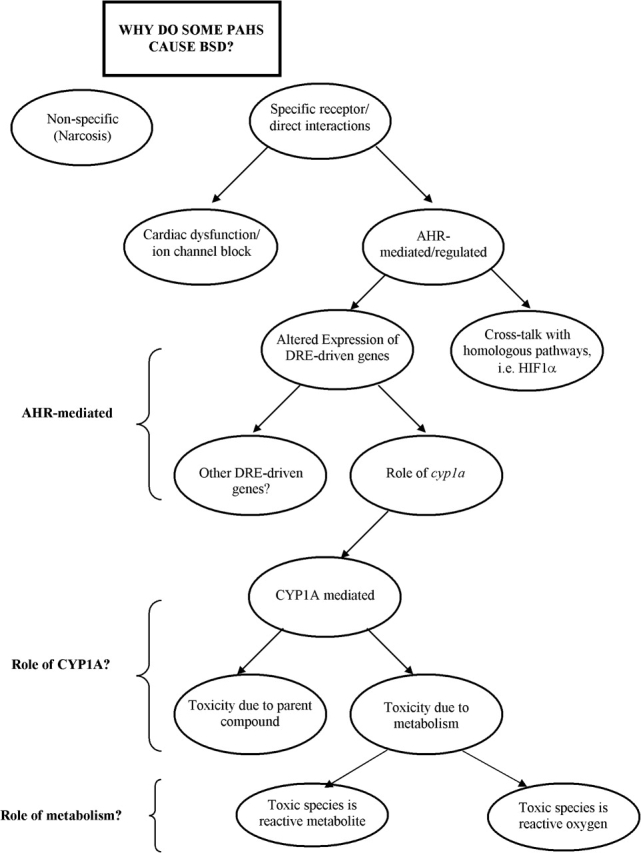
Flow chart of potential mechanisms of developmental toxicity or BSD of PAHs to ELS of fish.
Narcosis
While a narcotic mechanism for PAH toxicity has been proposed for the development of water and sediment quality guidelines (Di Toro et al., 2000), this model has not been thoroughly evaluated for the chronic toxicity of PAHs. Narcosis usually describes chemicals with baseline or minimum toxicity for which the exact biochemical mechanism is unknown (Meador, 2006). The proposed target site of action of narcotics is the lipid membrane bilayer, and thus the potency of narcotic-acting chemicals is directly related to their octanol-water partition coefficients or Kow (Incardona et al., 2004; McCarty and Mackay, 1993). Historically, the strong correlations between water-lipid partitioning and toxicity were interpreted to mean that narcotics dissolve in lipid membranes and interfere with membrane function to cause an anesthetic or narcotic-like effect. However, a review by Campagna et al. (2003) demonstrated that many anesthetics with similar structures and similar affinities for lipid actually caused markedly different biochemical, neurological, and anesthetic effects. Each anesthetic had unique molecular targets and interfered with neuronal functioning in a different way. Hence, in aquatic toxicology, narcosis as a general mechanism most properly describes the relationships among water-lipid partitioning, the kinetics of bioaccumulation, and the acute lethality of organic compounds. As shown by Campagna et al. (2003), narcosis encompasses a wide array of mechanisms, but these are difficult to discern in aquatic toxicology because of the dominating effect of water-lipid partitioning on exposure and measured toxicity. Therefore, it is not surprising that the sublethal toxicity of PAHs which interact with specific receptors can only be approximated from Kow.
One test of the narcosis model is the concentration of chemical present in fish that are intoxicated; for lethality, concentrations are typically in the range of 3–7 mmol/kg (wt/wt), the acute critical body residue (McCarty and Mackay, 1993). For example, waterborne phenanthrene was embryotoxic to trout when tissue concentrations exceeded 1 mmol/kg (Billiard, 2002; Hawkins et al., 2002). While phenanthrene was lethal to embryos, it was not a CYP1A inducer and did not cause signs of BSD. Taken together, these data suggested a nonspecific, narcotic mode of action, in contrast to retene, which caused mortality and severe BSD in trout embryos at tissue concentrations that were almost undetectable (<0.01 mmol/kg; Hawkins et al., 2002). However, as discussed in the next section, a study by Incardona et al. (2004) concluded that three-ringed PAHs such as phenanthrene were not narcotic to zebra fish embryos. Rather, embryotoxic effects were secondary to disruption of cardiac conduction. The narcosis model also cannot account for the nonadditive, synergistic cardiovascular interactions between PAH-type AHR agonists and CYP1A inhibitors observed with killifish (Wassenberg and Di Giulio, 2004b). Similarly, when larval trout were coexposed to waterborne phenanthrene and the CYP1A inducer BNF, the toxicity of phenanthrene increased dramatically, while tissue concentrations of phenanthrene decreased to <0.01 mmol//kg. This suggested a switch from a general (narcotic) to a specific mode of action and a potential role in toxicity for metabolites of phenanthrene created by CYP1A oxygenation (Hawkins et al., 2002). Finally, owing to their rapid metabolism, typical bioconcentration factors for most PAHs are <1000. Hence, at waterborne concentrations that are chronically toxic (for e.g., retene: 0.04–0.1μM; Brinkworth et al., 2003), they would not bioaccumulate to concentrations consistent with the critical body residues required for narcosis (Billiard, 2002; Wassenberg and Di Giulio, 2004b).
Cardiac-Mediated Toxicity
While the toxicity of phenanthrene appears to fit the narcosis model (Hawkins et al., 2002), there is also recent evidence that it may act by directly targeting cardiac function. Research by Incardona et al. (2004, 2005) demonstrated that the tricyclic PAHs fluorene, phenanthrene, and DBT were directly cardiotoxic to zebra fish embryos and that this dysfunction was independent of narcosis. The effects mimicked the atrio-ventricular conduction block associated with the silent heart (sih) mutation in zebra fish embryos, and the proposed cause was blockage of cardiac ion channels. This highly specific mode of action seems to be independent of CYP1A oxygenation or induction because knock down of ahr2 expression by morpholinos did not reduce toxicity. Larger PAHs appeared to act by different mechanisms, with chrysene causing strong CYP1A induction but not cardiotoxicity and pyrene causing CYP1A induction and pathology in peripheral blood vessels similar to dioxin (Incardona et al., 2004). Tests with four to five component mixtures containing the same total PAH concentrations, but each with a different mix of PAHs, showed that only the mixture with high proportions of phenanthrene and DBT was cardiotoxic. This was consistent with a specific mode of action of individual compounds rather than a general mode of action associated with the sum of all PAHs. Incardona et al. (2005) also demonstrated that the concentrations of individual tricyclic PAHs found in weathered Alaska North Slope crude oil were sufficient to explain cardiac dysfunction in developing zebra fish exposed to the water-accommodated fraction. However, the overall response of the fish reflected the presence of other compounds having additional effects.
Morpholino knock down of AHR1 (AHR1A; Andreasen et al., 2002a; Karchner et al., 2005) and AHR2 blocked CYP1A induction in zebra fish but did not protect against toxicity of phenanthrene and DBT, suggesting that cardiac dysfunction was independent of the AHR receptor (Incardona et al., 2005). In contrast, pyrene toxicity was localized in the peripheral vasculature and was clearly AHR dependent because toxic effects were decreased or delayed in AHR2 morphants. DBT and phenanthrene are usually classified as weak AHR agonists or noninducers (e.g., Basu et al., 2001; Billiard, 2002; Hawkins et al., 2002; Wassenberg et al., 2005). However, for mixtures of PAHs, there may yet be a role of CYP1A induction in their toxicity. As indicated above, coexposure of larval trout to phenanthrene and to BNF, a strong CYP1A inducer, significantly increased the toxicity of phenanthrene, although the specific effects on the heart were not examined (Hawkins et al., 2002). Taken together, these data suggest multiple mechanisms of toxicity that vary with the structure of PAH and with complex interactions in mixtures of AHR agonists and nonagonists.
Another possible mechanism that has been proposed for dioxin-induced cardiovascular toxicity (reviewed in Goldstone and Stegeman, 2006) that could also apply to PAH is cross-talk of activated AHR with homologous signaling pathways (i.e., hypoxia) through negative regulation or competition for reciprocal transcription/cellular cofactors. A correctly patterned vasculature for oxygen and nutrient transport to developing tissues is essential for normal development and survival of the vertebrate embryo (Weinstein, 1999). Hypoxia-inducible factor-1 (HIF-1), a heterodimeric basic-helix-loop-helix transcription factor composed of two subunits, HIF-1α and HIF-1β (otherwise known as ARNT), mediates changes in gene expression of proangiogenic growth factors. These include VEGF, critical for normal vascular development. Only a few studies have looked at this in fish embryos and while they do not necessarily support a role for this particular interaction in dioxin-exposed zebra fish embryos (Handley, 2003; Handley-Goldstone et al., 2005; Prasch et al., 2004), other nuclear cross-talk scenarios have not been tested, and signaling antagonism during development has not been explored specifically for PAHs. While a possible interaction between developmental signaling pathways has been mentioned here because it underscores cardiotoxic effects similar to dioxin and PAHs, it also highlights a potential role of the AHR pathway discussed below.
AHR-Mediated Toxicity
There is evidence both for and against a role of the AHR in PAH-induced BSD, likely because the mechanisms of toxicity vary considerably among PAHs. For AHR-mediated toxicity, PAHs may act through the AHR without direct involvement of CYP1s. Some four- to six-ringed unsubstituted PAHs that bind strongly to the AHR receptor and are potent CYP1A inducers (e.g., BaP, BkF, chrysenes; Basu et al., 2001; Billiard, 2002; Incardona et al., 2005) were nontoxic to ELS within the limits of their solubility (Billiard, 2002; Incardona et al., 2005; Wassenberg et al., 2005). For these PAHs, AHR binding and gene activation do not seem sufficient by themselves to cause BSD, but they may still be part of the mechanism of toxicity if toxicity involves induction of biotransformation of the PAH to toxic metabolites. In fact, the toxicity of these large PAHs such as BaP may simply be kinetically limited. Sundberg et al. (2006) demonstrated embryotoxicity of BaP in trout larvae exposed at fertilization to injections of single doses of BaP (0.040–8.0 μg/g) in triolein. CYP1A induction was found at concentrations of 3 μg/g or greater and an increased frequency of deformities at 5 μg/g or greater. The toxicity of injected BaP suggests that the amount of BaP that crosses the chorion during waterborne exposures may be insufficient to cause toxicity.
Our recent studies demonstrate that AHR2 zebra fish morphants are protected from synergistic embryotoxicity caused by coexposure to the PAH-type AHR agonist, i.e., BNF and to the CYP1A inhibitor ANF (Billiard et al., 2006). These data provide the first evidence that synergistic, embryotoxic effects of PAHs might be mediated in part by the AHR signaling pathway (Billiard et al., 2006). Recent studies by Incardona et al. (2005, 2006) used morpholinos to block the synthesis of the AHR protein in zebra fish exposed to an array of different PAHs. Developmental defects in zebra fish exposed as embryos to petrogenic PAH mixtures were independent of AHR1 (AHR1A) and AHR2, likely because the tricyclic PAHs (e.g., DBT and phenanthrene) used are weak AHR agonists or non-CYP1A inducers, as indicated above. The authors concluded that cardiotoxicity was an indirect effect stemming from direct impacts on cardiac conduction. In contrast, benz[a]anthracene caused CYP1A induction and embryotoxicity to zebra fish that was first evident as a failure of the developing heart to complete looping, with signs of BSD following cardiotoxicity. Toxicity was antagonized by ahr2 but not cyp1a knockdown, indicating an AHR2-dependent, but CYP1A-independent mechanism, as observed with dioxin (Incardona et al., 2006). The molecular basis for this AHR dependence has not been described.
CYP1-Mediated Toxicity
For some individual PAHs and PAH mixtures, CYP1A enzyme activity plays several roles in embryotoxicity. In zebra fish embryos, CYP1A, 1B1, and 1C1 mRNA are inducible by AHR ligands as early as 24–48 hpf prior to the onset of toxicity (Andreasen et al., 2002b; Timme-Laragy et al., 2007). Immunolocalized CYP1A protein is first detected in zebra fish vasculature and later in cardiac, hepatic, and kidney tissues (Andreasen et al., 2002b). Here, the key process may be CYP1-mediated metabolism of PAHs to toxic intermediates. For example, pyrene caused CYP1A induction, pericardial and yolk sac edema, spinal curvature, and reduced peripheral circulation in zebra fish embryos. Toxicity was antagonized by ahr2 and cyp1a knockdown, and partially antagonized by ahr1a knockdown, indicating a role of CYP1A enzyme activity in the mechanism of toxicity (Incardona et al., 2005, 2006). In contrast, there was a protective role for CYP1A activity in PAH versus DLC teratogenesis and synergism in zebra fish embryos coexposed to PAH-type AHR agonists and CYP1A inhibitors, as shown by increased toxicity after cyp1a knockdown or inhibition (Billiard et al., 2006; Wassenberg and Di Giulio, 2004b; Wassenberg et al., 2005); ahr2 morphants were protected from cardiovascular defects under the same conditions (Billiard et al., 2006).
How do we reconcile these divergent observations? Under normal circumstances, functional CYP1A would confer protection to embryos exposed to PAH-type AHR agonists by metabolizing parent PAHs to reactive intermediates with relatively low toxicity when conjugated via phaseII, metabolism. This model predicts that when CYP1A activity or synthesis of CYP1A protein is stimulated or inhibited, there would be a change in the relative proportions and amounts of toxic metabolites and observed toxicity. In other words, toxicity is a function of the rate at which specific toxic metabolites are produced and excreted. Protection would only be observed if there was an alternate AHR-dependent metabolic pathway for PAHs, assuming that an AHR agonist alters expression of other DRE-driven genes downstream of the AHR/ARNT transcription complex. Hence, AHR knockdown could block alternate pathways of PAH metabolism and protect against synergistic toxicity between BNF (AHR agonist, CYP1A inducer) or ANF (CYP1A inhibitor). Alternatively, PAHs could be metabolized and excreted by an AHR-independent pathway in AHR2 morphants. Otherwise, in the absence of metabolism, PAHs would accumulate to toxic concentrations, as with the narcosis model. Another hypothesis to explain the increased toxicity of PAHs when CYP1A is inhibited is that lowering the rate of metabolism extends the half-life of the PAHs, allowing parent PAH-type AHR agonists to persist longer and behave more like DLCs. While the specific mechanism as to how prolonged agonism confers toxicity has not elucidated, it deserves further investigation (Timme-Laragy et al., 2007).
Some of these hypotheses have been tested by measuring the products of metabolism of retene by trout in the presence and absence of CYP1A inhibitors. In juvenile trout coexposed to retene and ANF, the excretion of retene metabolites in bile was progressively reduced by higher concentrations of ANF (Hodson et al., 2007). In the tissues of larval trout exposed only to retene, there was a broad spectrum of metabolites that included di-hydroxy- and mono-hydroxy derivatives, with very low concentrations of parent retene. With increasing exposure to ANF, there was a progressive reduction in the number and size of metabolite peaks and a corresponding increase in parent retene concentrations. At intermediate exposures to ANF, di-hydroxy metabolites were virtually absent, but there was a higher concentration of mono-hydroxy metabolites, and toxicity increased dramatically. At high exposures of ANF, tissue metabolites of retene were virtually absent (parent retene was the predominant form), and retene toxicity was almost completely antagonized (Hodson et al., 2007). Partial inhibition of CYP1A by ANF seemed to favor the production of the more toxic metabolites of retene while complete inhibition prevented metabolism altogether and eliminated toxicity. These results suggest that CYP1A enzyme activity prevents the accumulation of both parent PAH and the most toxic of the metabolites. Thus, the role of CYP1A enzyme activity in BSD caused by some PAHs may be to prevent the generation of toxic metabolites.
While this discussion has focused on CYP1A, the recent discovery of PAH-inducible CYP1B and CYP1C proteins in fish (e.g., Wang et al., 2006) suggests that PAH metabolism and toxicity following AHR binding by PAHs involves multiple reactions. However, the potential for ANF to modulate PAH metabolism and toxicity by CYP1B and CYP1C are unknown because the tissue distribution, PAH specificity, and metabolites generated by each CYP protein are not yet worked out. These are clearly important research needs if the mechanisms of different PAHs are to be understood.
Similarly, we recently showed that ANF alone acts as a weak AHR2 agonist (Timme-Laragy et al., 2007). Furthermore, when this dose of ANF was combined with BNF—a mixture that we have previously shown to inhibit BNF-induced CYP1A enzyme activity and synergize toxicity (Billiard et al., 2006)—mRNA induction of AHR-mediated cyp1 genes (including cyp1a, cyp1b1, and 1c1) was greatly enhanced and preceded the onset of deformities in zebra fish embryos in contrast to inhibition of CYP1A protein as measured by in vivo activity of ethoxyresorufin-o-deethylase (EROD) (Timme-Laragy et al., 2007).
Based on these data, we propose that the key difference in the role of CYP1A in mediating toxicity between PAH and DLC AHR agonists is that PAHs are much more metabolically labile than DLCs. Thus, CYP1A and other enzymes metabolize the wide variety of PAHs in different ways, resulting both in differential production of toxic metabolites and differential toxicokinetics. For example, while chrysene, pyrene, and benz(a)anthracene caused CYP1A induction, the tissue distribution and intensity of induction varied considerably among the three compounds, implying different rates of uptake and partitioning among tissues (Incardona et al., 2006). Hence, there is a need to understand the toxicokinetics of PAHs and the relative role of metabolites versus parent compounds. Hornung et al. (2007) mimicked maternal transfer of more persistent chemicals, such as DLCs, by acute waterborne exposure of postfertilized medaka eggs to B[a]P. This study demonstrated that multiple embryonic tissues of fish embryos have the ability to metabolize PAHs even prior to cardiac or hepatic development. Redistribution of parent PAH and metabolic intermediates occurs throughout embryonic development. This is coincident with a switch from endogenous feeding as the yolk sac is resorbed and development of the cardiovascular system (Hornung et al., 2007). Mobilization and transfer of sequestered chemical from the yolk could partly explain why the embryonic vasculature is a sensitive, first target. This also confirms other studies showing that the AHR pathway is operational early in fish development (Andreasen et al., 2002b; Jönsson et al., 2007a,b; Timme-Laragy et al., 2007).
One would expect that if metabolism drives PAH toxicity, there should be a clear time dependency of effects. In studies with rainbow trout continuously exposed from eggs to retene, CYP1A activity of embryonic fish increased continually during development as tissue PAH concentrations decreased. These experiments demonstrated that retene was metabolized by CYP1A enzymes and that induction preceded the onset of toxicity (Brinkworth et al., 2003).
Alkyl-Phenanthrene Toxicity
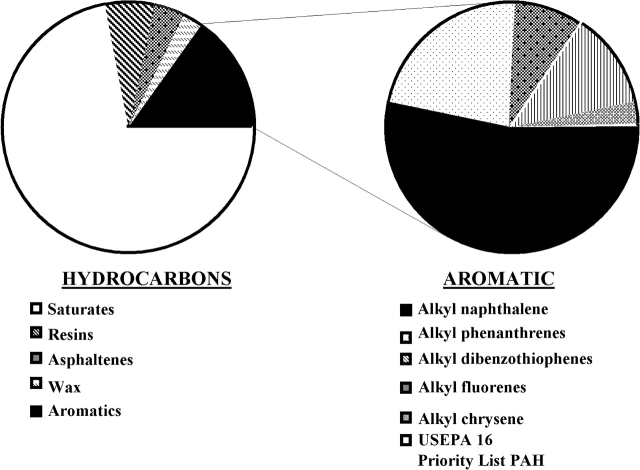
Alkyl-substituted PAHs comprise up to 95% of total PAHs in environmentally relevant mixtures such as crude oil (Fig. 3). In a review and evaluation of several developmental PAH toxicity models, Barron et al. (2004) proposed that alkyl-phenanthrenes were the primary PAHs driving the toxicity of Alaska North Slope Crude to herring and salmon embryos after the Exxon Valdez oil spill (Carls et al., 1999, Heintz et al., 1999, 2002). The toxicity of sediment extracts derived from oil sands and enriched with alkylated PAHs indicates that alkyl substitution is a significant contributor to embryotoxicity (Colavecchia et al., 2004; Rhodes et al., 2005).
FIG. 3.
Hydrocarbon content of Alaska North Crude Oil (adapted from Wang et al., 2002, 2003).
Alkyl-substituted phenanthrenes such as retene induce CYP1A activity and cause signs of BSD in ELS of rainbow trout, zebrafish, and medaka (Billiard et al., 1999, Brinkworth et al., 2003; Hawkins et al., 2002 Kiparissis et al., 2002). As discussed above, these data indicated that the toxicity of retene is tied to its metabolism. But why would retene metabolites be more toxic than parent? The process of metabolism likely generates ROS, but this would not explain why toxicity is enhanced when CYP1A metabolism is partially inhibited. Another explanation, already introduced above, is the generation of specific metabolites with a uniquely high toxicity. Tabash (2003) demonstrated that retene metabolites generated in vivo by retene-exposed juvenile trout, or in vitro by liver S-9 fractions from juvenile trout exposed to BNF, were primarily benzylic alcohols, i.e., metabolites with hydroxylations on the alkyl side chains. These alcohols are unique to alkyl-PAHs since hydroxylation of unsubstituted PAHs can only form phenols. It is also possible that that the presence of alkyl side chains causes the formation of specific and highly toxic phenols because hydroxylations are restricted to specific double bonds.
For other alkyl-substituted homologs of phenanthrene, both the size and substitution pattern of alkyl groups determines potency for BSD in medaka; compounds substituted in the 1,7 positions are the most toxic to embryos (Kiparissis et al., 2002). Some alkyl-PAHs, including alkylated napthalenes and anthracenes, do not cause BSD in medaka (Kiparissis et al., 2002; Turcotte et al., 2005).
Oxidative Stress
There is strong evidence of a role for oxidative stress in PAH synergistic toxicity. Upregulated antioxidant defenses appear to be acting in both the short-term and heritable adaptation to PAH toxicity in the Elizabeth River. Killifish indigenous to this site display elevated hepatic total glutathione levels (Bacanskas et al., 2004). Laboratory-raised offspring from this population appear better equipped to withstand oxidative stress, as evidenced by increased resistance to a model pro-oxidant, tert-butyl hydroperoxide, higher basal total oxy-radical scavenging capacity, glutathione concentrations, and MnSOD protein levels (Meyer et al., 2003a). Furthermore, treatment of reference site killifish embryos to the model pro-oxidant cumene hydroperoxide resulted in a similar phenotype as seen in PAH and DLC toxicity, whereas cotreatment of embryos with vitamin-E-acetate showed potential for reducing PAH synergistic toxicity (Wassenberg, 2004).
Vitamin E is thought to be maternally loaded into the yolk sac of salmonids. Exposure of larval rainbow trout to the alkyl-PAH retene caused a 20% decrease in whole body vitamin E concentrations (Bauder et al., 2005). Coexposure to retene and to 10 μg/l vitamin E restored antioxidant levels to control levels and significantly reduced the severity of BSD (Bauder et al., 2005). However, in the same study, known pro-oxidants such as paraquat, tert-butyl hydroperoxide, and carbon tertrachloride did not increase tissue concentrations of hydroperoxides or cause signs of BSD (Bauder et al., 2005). These paradoxical results could be a function of exposure conditions, chemical uptake, different modes of action between retene and the pro-oxidants, or the limits of detection of the hydroperoxide method.
In summary, it is clear that multiple mechanisms govern the adverse developmental effects of PAHs and that the toxic potency of complex mixtures is unquestionably a function of their components and how they interact. The accumulation by fish of potent CYP1A inducers (e.g., BaP) means that all other aromatic constituents accumulated from a mixture may be subject to oxygenation to metabolites that are more or less toxic than the parent compounds. Similarly, the presence of low molecular weight PAHs that inhibit CYP1A activity, or that competitively bind to the AHR (e.g., 2-aminoanthracene, Wassenberg and Di Giulio, 2004b), will modulate CYP1A activity and the metabolism and toxicity of other PAHs. Moreover, the loss of low molecular weight PAHs with weathering of crude oil may cause a progressive shift in the extent of compound interactions. Overall, due to their complex nature and multiple cellular targets, the developmental toxicity of PAHs is not easily predicted from hydrophobicity (i.e., narcotic mode of action) or the AHR activity of individual PAHs (Incardona et al., 2006). Understanding the mechanism underlying PAH effects is paramount for understanding and characterizing their risk. This includes characterizing the regulation and contribution of other AHR-mediated genes in the ELS fish model to PAH toxicity.
IMPLICATIONS FOR RISK ASSESSMENT
PAHs invariably occur in the environment as mixtures, and assessing the risks of complex mixtures such as PAHs is challenging. For example, in sediment samples from the PAH-contaminated Elizabeth River, VA site discussed earlier, BaP (AHR agonist) and FL (CYP1A inhibitor) comprised 11 and 26%, respectively, of total PAHs measured (18 individual compounds) (Vogelbein and Unger, 2003). The marked synergy between PAHs acting as AHR agonists and as CYP1A inhibitors described herein suggests that for some PAH mixtures, the common assumption of additivity may greatly underestimate these risks. This is consistent with recent arguments that widely accepted models of PAH toxicity are oversimplified and that TEQ models do not predict their interactive, developmental effects (Incardona et al., 2006). Elucidating the mechanistic basis for PAH interactions will act to reduce the degree of uncertainty in such risk assessments. Unquestionably, a detailed chemical characterization of PAH mixture constituents, beyond the standard typical 16 nonsubstituted PAHs, at each particular site would be required to predict nonadditive effects. Biological testing of selected complex mixtures would improve the confidence in risk assessments that are based primarily on analytical data.
FUNDING
Superfund Basic Research Grant (P42 ES10356); Environmental Health Sciences Training Grant (T32 ES07031) to R.T.D.; Discovery Grant from the Natural Sciences and Engineering Research Council of Canada to P.V.H.
References
- Andreasen EA, Hahn ME, Heideman W, Peterson RE, Tanguay RL. The zebrafish (Danio rerio) aryl hydrocarbon receptor type 1 is a novel vertebrate receptor. Mol. Pharmacol. 2002a;62:234–249. doi: 10.1124/mol.62.2.234. [DOI] [PubMed] [Google Scholar]
- Andreasen EA, Spitsbergen JM, Tanguay RL, Stegeman JJ, Heideman W, Peterson RE. Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: Effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Toxicol. Sci. 2002b;68:403–419. doi: 10.1093/toxsci/68.2.403. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Peterson RE, Heideman W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006;94:175–182. doi: 10.1093/toxsci/kfl093. [DOI] [PubMed] [Google Scholar]
- Arzuaga X, Calcaño W, Elskus A. The DNA de-methylating agent 5-azacytidine does not restore CYP1A induction in PCB resistant Newark Bay killifish (Fundulus heteroclitus) Mar. Environ. Res. 2004;58:517–520. doi: 10.1016/j.marenvres.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Arzuaga X, Elskus A. Evidence for resistance to benzo[a]pyrene and 3,4,3’4’-tetrachlorobiphenyl in a chronically polluted Fundulus heteroclitus population. Mar. Environ. Res. 2002;54:247–251. doi: 10.1016/s0141-1136(02)00184-8. [DOI] [PubMed] [Google Scholar]
- ATSDR. CERCLA Priority List of Hazardous Substances. Washington, DC: Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services; 1997. [Google Scholar]
- ATSDR. CERCLA Priority List of Hazardous Substances. Washington, DC: Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services; 2005. [Google Scholar]
- Bacanskas LR, Whitaker J, Di Giulio RT. Oxidative stress in two populations of killifish (Fundulus heteroclitus) with differing contaminant exposure histories. Mar. Environ. Res. 2004;58:597–601. doi: 10.1016/j.marenvres.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Barron MG, Carls MG, Heintz R, Rice SD. Evaluation of fish early life stage toxicity models of chronic embryonic exposures to complex polycyclic aromatic hydrocarbon mixtures. Toxicol. Sci. 2004;78:60–67. doi: 10.1093/toxsci/kfh051. [DOI] [PubMed] [Google Scholar]
- Barron MG, Carls MG, Short JW, Rice SD. Photoenhanced toxicity of aqueous phase and chemically dispersed weathered Alaska North Slope crude oil to Pacific herring eggs and larvae. Environ. Toxicol. Chem. 2003;22:650–660. [PubMed] [Google Scholar]
- Basu N, Billiard S, Fragoso N, Omoike A, Tabash S, Brown S, Hodson P. Ethoxyresorufin-O-deethylase induction in trout exposed to mixtures of polycyclic aromatic hydrocarbons. Environ. Toxicol. Chem. 2001;20:1244–1251. [PubMed] [Google Scholar]
- Bauder MB, Palace VP, Hodson PV. Is oxidative stress the mechanism of blue sac disease in retene-exposed trout larvae? Environ. Toxicol. Chem. 2005;24:694–702. doi: 10.1897/04-23r.1. [DOI] [PubMed] [Google Scholar]
- Belair CD, Peterson RE, Heideman W. Disruption of erythropoiesis by dioxin in the zebrafish. Dev. Dyn. 2001;222:581–594. doi: 10.1002/dvdy.1213. [DOI] [PubMed] [Google Scholar]
- Bello SM, Franks DG, Stegeman JJ, Hahn ME. Acquired resistance to Ah receptor agonists in a population of Atlantic killifish (Fundulus heteroclitus) inhabiting a marine superfund site: In vivo and in vitro studies on the inducibility of xenobiotic metabolizing enzymes. Toxicol. Sci. 2001;60:77–91. doi: 10.1093/toxsci/60.1.77. [DOI] [PubMed] [Google Scholar]
- Bello SM, Heideman W, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits regression of the common cardinal vein in developing zebrafish. Toxicol. Sci. 2004;78:258–266. doi: 10.1093/toxsci/kfh065. [DOI] [PubMed] [Google Scholar]
- Billiard SM. Kingston, Ontario, Canada: Department of Biology, Queen's University; 2002. Toxicological significance of CYPIA induction in teleosts exposed to polycyclic aromatic hydrocarbons (PAH). Ph.D. Dissertation. [Google Scholar]
- Billiard SM, Hahn ME, Franks DG, Peterson RE, Bols NC, Hodson PV. Binding of polycyclic aromatic hydrocarbons (PAHs) to teleost aryl hydrocarbon receptors (AHRs) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002;133:55–68. doi: 10.1016/s1096-4959(02)00105-7. [DOI] [PubMed] [Google Scholar]
- Billiard SM, Querbach K, Hodson PV. Toxicity of retene to early life stages of two freshwater fish species. Environ. Toxicol. Chem. 1999;18:2070–2077. [Google Scholar]
- Billiard SM, Timme-Laragy AR, Wassenberg DM, Cockman C, Di Giulio RT. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol. Sci. 2006;92:526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- Bols NC, Schirmer K, Joyce EM, Dixon DG, Greenberg BM, Whyte JJ. Ability of polycyclic aromatic hydrocarbons to induce 7-ethoxyresorufin-O-deethylase activity in a trout liver cell line. Ecotoxicol. Environ. Saf. 1999;44:118–128. doi: 10.1006/eesa.1999.1808. [DOI] [PubMed] [Google Scholar]
- Brammell BF, Price DJ, Birge WJ, Elskus AA. Apparent lack of CYP1A response to high PCB body burdens in fish from a chronically contaminated PCB site. Mar. Environ. Res. 2004;58:251–255. doi: 10.1016/j.marenvres.2004.03.067. [DOI] [PubMed] [Google Scholar]
- Brinkworth LC, Hodson PV, Tabash S, Lee P. CYP1A induction and blue sac disease in early developmental stages of rainbow trout (Oncorhynchus mykiss) exposed to retene. J. Toxicol. Environ. Health A. 2003;66:627–646. doi: 10.1080/15287390309353771. [DOI] [PubMed] [Google Scholar]
- Brown SB, Fisk AT, Brown M, Villella M, Muir DCG, Evans RE, Lockhart WL, Metner DA, Cooley HM. Dietary accumulation and biochemical responses of juvenile rainbow trout (Oncorhynchus mykiss) to 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) Aquat. Toxicol. 2002;59:139–152. doi: 10.1016/s0166-445x(01)00246-6. [DOI] [PubMed] [Google Scholar]
- Brunnberg S, Andersson P, Lindstam M, Paulson I, Poellinger L, Hanberg A. The constitutively active Ah receptor (CA-Ahr) mouse as a potential model for dioxin exposure-effects in vital organs. Toxicology. 2006;224:191–201. doi: 10.1016/j.tox.2006.04.045. [DOI] [PubMed] [Google Scholar]
- Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N. Engl. J. Med. 2003;348:2110–2124. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- Cantrell SM, Joy-Schlezinger J, Stegeman JJ, Tillitt DE, Hannink M. Correlation of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced apoptotic cell death in the embryonic vasculature with embryotoxicity. Toxicol. Appl. Pharmacol. 1998;148:24–34. doi: 10.1006/taap.1997.8309. [DOI] [PubMed] [Google Scholar]
- Cantrell SM, Lutz LH, Tillitt DE, Hannink M. Embryotoxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): The embryonic vasculature is a physiological target for TCDD-induced DNA damage and apoptotic cell death in medaka (Orizias latipes) Toxicol. Appl. Pharmacol. 1996;141:23–34. doi: 10.1006/taap.1996.0256. [DOI] [PubMed] [Google Scholar]
- Carls MG, Rice SD, Hose JE. Sensitivity of fish embryos to weathered crude oil: Part I. Low-level exposure during incubation causes malformations, genetic damage, and mortality in larval pacific herring (Clupea pallasi) Environ. Toxicol. Chem. 1999;18:481–493. [Google Scholar]
- Carney SA, Chen J, Burns CG, Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol. Pharmacol. 2006a;70:549–561. doi: 10.1124/mol.106.025304. [DOI] [PubMed] [Google Scholar]
- Carney SA, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol. Pharmacol. 2004;66:512–521. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Carney SA, Prasch AL, Heideman W, Peterson RE. Understanding dioxin developmental toxicity using the zebrafish model. Birth Defects Res. A Clin. Mol. Teratol. 2006b;76:7–18. doi: 10.1002/bdra.20216. [DOI] [PubMed] [Google Scholar]
- Chen CM, Cooper KR. Developmental toxicity and EROD induction in the Japanese medaka (Oryzias latipes) treated with dioxin congeners. Bull. Environ. Contam. Toxicol. 1999;63:423–429. doi: 10.1007/s001289900997. [DOI] [PubMed] [Google Scholar]
- Choi H, Jedrychowski W, Spengler J, Camann DE, Whyatt RM, Rauh V, Tsai W-Y, Perera FP. International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environ. Health Perspect. 2006;114:1744–1750. doi: 10.1289/ehp.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colavecchia MV, Backus SM, Hodson PV, Parrott JL. Toxicity of oil sands to early life stages of fathead minnows (Pimephales promelas) Environ. Toxicol. Chem. 2004;23:1709–1718. doi: 10.1897/03-412. [DOI] [PubMed] [Google Scholar]
- Colavecchia MV, Hodson PV, Parrott JL. CYP1A induction and blue sac disease in early life stages of white suckers (Catostomus commersoni) exposed to oil sands. J. Toxicol. Environ. Health A. 2006;69:967–994. doi: 10.1080/15287390500362154. [DOI] [PubMed] [Google Scholar]
- Courtenay SC, Grunwald CM, Kreamer G-L, Fairchild WL, Arsenault JT, Ikonomou M, Wirgin II. A comparison of the dose and time response of CYP1A1 mRNA induction in chemically treated Atlantic tomcod from two populations. Aquat. Toxicol. 1999;47:43–69. [Google Scholar]
- Di Toro DM, McGrath JA, Hansen DJ. Technical basis for narcotic chemicals and polycyclic aromatic hydrocarbon criteria. I. Water and tissue. Environ. Toxicol. Chem. 2000;19:1951–1970. [Google Scholar]
- Dong W, Teraoka H, Tsujimoto Y, Stegeman JJ, Hiraga T. Role of aryl hydrocarbon receptor in mesencephalic circulation failure and apoptosis in zebrafish embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2004;77:109–116. doi: 10.1093/toxsci/kfh023. [DOI] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Yamazaki K, Tsukiyama S, Imani S, Imagawa T, Stegeman JJ, Peterson RE, Hiraga T. 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: Local circulation failure in the dorsal midbrain is associated with increased apoptosis. Toxicol. Sci. 2002;69:191–201. doi: 10.1093/toxsci/69.1.191. [DOI] [PubMed] [Google Scholar]
- Douben PET. In: PAHs: An Ecotoxicological Perspective. Douben PET, editor. West Sussex, UK: John Wiley and Sons; 2003. p. 392. [Google Scholar]
- Dragin N, Dalton TP, Miller ML, Shertzer HG, Nebert DW. For dioxin-induced birth defects, mouse or human CYP1A2 in maternal liver protects whereas mouse CYP1A1 and CYP1B1 are inconsequential. J. Biol. Chem. 2006;281:18591–18600. doi: 10.1074/jbc.M601159200. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Zacharia LC, Barchiesi F, Imthurn B, Jackson EK. CYP450- and COMT-derived estradiol metabolites inhibit activity of human coronary artery SMCs. Hypertension. 2003;41:807–813. doi: 10.1161/01.HYP.0000048862.28501.72. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK, Gillespie DG, Rosselli M, Barchiesi F, Krust A, Keller H, Zacharia LC, Imthurn B. Cytochromes 1A1/1B1- and catechol-O-methyltransferase-derived metabolites mediate estradiol-induced antimitogenesis in human cardiac fibroblast. J. Clin. Endocrinol. Metab. 2005;90:247–255. doi: 10.1210/jc.2003-032154. [DOI] [PubMed] [Google Scholar]
- Elonen GE, Spehar RL, Holcombe GW, Johnson RD, Fernandez JD, Erickson RJ, Tietge JE, Cook PM. Comparitive toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin to seven freshwater fish species during early life-stage development. Environ. Toxicol. Chem. 1998;17:472–483. [Google Scholar]
- Elskus AA. Toxicant resistance in wildlife: Fish populations. In: Robertson LW, Hansen LG, editors. PCB: Recent Advances in Environmental Toxicology and Health Effects. Lexington, KY: University Press of Kentucky; 2001. pp. 273–276. [Google Scholar]
- Elskus AA, Monosson E, McElroy AE, Stegeman JJ, Woltering DS. Altered CYP1A expression in Fundulus heteroclitus adults and larvae: A sign of pollutant resistance? Aquat. Toxicol. 1999;45:99–113. [Google Scholar]
- Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol. Appl. Pharmacol. 1996;140:173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- Feuston MH, Hamilton CE, Schreiner CA, Mackerer CR. Developmental toxicity of dermally applied crude oils in rats. Journal of Toxicology and Environmental Health. 1997;52:79–93. doi: 10.1080/00984109708984054. [DOI] [PubMed] [Google Scholar]
- Förlin L, Celander M. Studies of the inducibility of P450 1A in perch from the PCB-contaminated Lake Jarnsjon in Sweden. Mar. Environ. Res. 1995;39:85–88. [Google Scholar]
- Franklin MR. Inhibition of mixed-function oxidations by substrates forming reduced cytochrome P450 metabolic-intermediate complexes. Pharmacol. Therapeutics A. 1977;2:227–245. [Google Scholar]
- Goldstone HM, Stegeman JJ. Molecular mechanisms of 2,3,7,8-tetrachlorodibenzo-p-dioxin cardiovascular embryotoxicity. Drug Metab. Rev. 2006;38:261–289. doi: 10.1080/03602530600570099. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Fernandez-Salguero P. The aryl hydrocarbon receptor: Studies using the AHR-null mice. Drug Metab. Dispos. 1998;26:1194–1198. [PubMed] [Google Scholar]
- Guiney PD, Smolowitz RM, Peterson RE, Stegeman JJ. Correlation of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction of cytochrome P4501A in vascular endothelium with toxicity in early life stages of lake trout. Toxicol. Appl. Pharmacol. 1997;143:256–273. doi: 10.1006/taap.1996.8051. [DOI] [PubMed] [Google Scholar]
- Guiney PD, Walker MK, Spitsbergen JM, Peterson RE. Hemodynamic dysfunction and cytochrome P4501A mRNA expression induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin during embryonic stages of lake trout development. Toxicol. Appl. Pharmacol. 2000;168:1–14. doi: 10.1006/taap.2000.8999. [DOI] [PubMed] [Google Scholar]
- Hahn ME, Karchner SI, Shapiro MA, Perera SA. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13743–13748. doi: 10.1073/pnas.94.25.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn ME. Mechanisms of innate and acquired resistance to dioxin-like compounds. Rev. Toxicol. 1998;2:395–443. [Google Scholar]
- Hahn ME. Dioxin toxicology and the aryl hydrocarbon receptor: Insights from fish and other non-traditional models. Mar. Biotechnol. 2001;3:S224–S238. doi: 10.1007/s10126-001-0045-y. [DOI] [PubMed] [Google Scholar]
- Hahn ME. Aryl hydrocarbon receptors: Diversity and evolution. Chem. Biol. Interact. 2002;141:131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Handley HM. Woods Hole, MA: Woods Hole Oceanographic Institution (WHOI)/Massachusetts Institute of Technology (MIT) Joint Graduate Program in Oceanography; 2003. Zebrafish cardiovascular cDNA microarrays: Expression profiling and gene discovery in embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Ph. D. Dissertation. [Google Scholar]
- Handley-Goldstone HM, Grow MW, Stegeman JJ. Cardiovascular gene expression profiles of dioxin exposure in zebrafish embryos. Toxicol. Sci. 2005;85:683–693. doi: 10.1093/toxsci/kfi116. [DOI] [PubMed] [Google Scholar]
- Hawkins SA, Billiard SM, Tabash SP, Brown RS, Hodson PV. Altering cytochrome P4501A activity affects polycyclic aromatic hydrocarbon metabolism and toxicity in rainbow trout (Oncorhynchus mykiss) Environ. Toxicol. Chem. 2002;21:1845–1853. [PubMed] [Google Scholar]
- Heid SE, Walker MK, Swanson HI. Correlation of cardiotoxicity mediated by halogenated aromatic hydrocarbons to aryl hydrocarbon receptor activation. Toxicol. Sci. 2001;61:187–196. doi: 10.1093/toxsci/61.1.187. [DOI] [PubMed] [Google Scholar]
- Heintz RA, Rice SD, Wertheimer AC, Bradshaw RF, Thrower FP, Joyce JE, Short JW. Delayed effects on growth and marine survival of pink salmon Oncorhynchus gorbuscha after exposure to crude oil during embryonic development. Mar. Ecol. Prog. Ser. 2002;208:205–216. [Google Scholar]
- Heintz RA, Short JW, Rice SD. Sensitivity of fish embryos to weathered crude oil: Part II. Increased mortality of pink salmon (Oncorhynchus gorbuscha) embryos incubating downstream from weathered Exxon Valdez crude oil. Environ. Toxicol. Chem. 1999;18:494–503. [Google Scholar]
- Helder T. Effects of 2,3,7,8-tetrachlorodibenzo-dioxin (TCDD) on early life stages of rainbow trout (Salmo gairdneri, Richardson) Toxicology. 1981;19:101–112. doi: 10.1016/0300-483x(81)90092-5. [DOI] [PubMed] [Google Scholar]
- Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio) Toxicol. Appl. Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Bello SM, Prasch AL, Peterson RE, Heideman W. Water permeability and TCDD-induced edema in zebrafish early-life stages. Toxicol. Sci. 2004;78:78–87. doi: 10.1093/toxsci/kfh056. [DOI] [PubMed] [Google Scholar]
- Hodson PV, Qureshi K, Noble CA, Akhtar P, Brown RS. Inhibition of CYP1A enzymes by alpha-naphthoflavone causes both synergism and antagonism of retene toxicity to rainbow trout (Oncorhynchus mykiss) Aquat. Toxicol. 2007;81:275–285. doi: 10.1016/j.aquatox.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Gay ML. Embryotoxic effects of benzo[a]pyrene, chrysene, and 7,12-dimethylbenz[a]anthracene in petroleum hydrocarbon mixtures in mallard ducks. J. Toxicol. Environ. Health. 1981;7:775–787. doi: 10.1080/15287398109530019. [DOI] [PubMed] [Google Scholar]
- Hornung MW, Cook PM, Fitzsimmons PN, Kuehl DW, Nicols JW. Tissue distribution and metabolism of benzo[a]pyrene in embryonic and larval medaka (Oryzias latipes) Toxicol. Sci. 2007;100:393–405. doi: 10.1093/toxsci/kfm231. [DOI] [PubMed] [Google Scholar]
- Hornung MW, Spitsbergen JM, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters cardiovascular and craniofacial development and function in sac fry of rainbow trout (Oncorhynchus mykiss) Toxicol. Sci. 1999;47:40–51. doi: 10.1093/toxsci/47.1.40. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Carls MG, Teraoka H, Sloan CA, Collier TK, Scholz NL. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ. Health Perspect. 2005;113:1755–1762. doi: 10.1289/ehp.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol. Appl. Pharmacol. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Ivnitski I, Elmaoued R, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) inhibition of coronary development is preceded by a decrease in myocyte proliferation and an increase in cardiac apoptosis. Teratology. 2001;64:201–212. doi: 10.1002/tera.1065. [DOI] [PubMed] [Google Scholar]
- Ivnitski-Steele ID, Friggens M, Chavez M, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) inhibition of coronary vasculogenesis is mediated, in part, by reduced responsiveness to endogenous angiogenic stimuli, including vascular endothelial growth factor A (VEGF-A) Birth Defects Res. A Clin. Mol. Teratol. 2005;73:440–446. doi: 10.1002/bdra.20137. [DOI] [PubMed] [Google Scholar]
- Ivnitski-Steele ID, Sanchez A, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin reduces mycocardial hypoxia and vascular endothelial growth factor expression during chick embryo development. Birth Defects Res. A Clin. Mol. Teratol. 2004;70:51–58. doi: 10.1002/bdra.10151. [DOI] [PubMed] [Google Scholar]
- Jin B, Park DW, Nam K-W, Oh GT, Lee Y-S, Ryu D-Y. CpG methylation in the mouse CYP1A2 promoter. Toxicol. Lett. 2004;152:11–18. doi: 10.1016/j.toxlet.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Jönsson ME, Orrego R, Woodin BR, Goldstone JV, Stegeman JJ. Basal and 3,3’,4,4’,5-pentachlorobiphenyl-induced expression of cytochrome P450 1A, 1B and 1C genes in zebrafish. Toxicol. Appl. Pharmacol. 2007a;221:29–41. doi: 10.1016/j.taap.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson ME, Jenny MJ, Woodin BR, Hahn ME, Stegeman JJ. Role of AHR2 in the expression of novel cytochrome P450 1 family genes, cell cycle genes, and morphological defects in developing zebra fish exposed to 3,3’,4,4’,5-pentachlorobiphenyl or 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2007b;100:180–193. doi: 10.1093/toxsci/kfm207. [DOI] [PubMed] [Google Scholar]
- Karchner SI, Franks DG, Hahn ME. AHR1B, a new functional aryl hydrocarbon receptor in zebrafish: Tandem arrangement of ahr1b and ahr2 genes. Biochem. J. 2005;392:153–161. doi: 10.1042/BJ20050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchner SI, Franks DG, Kennedy SW, Hahn ME. The molecular basis for differential dioxin sensitivity in birds: Role of the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6252–6257. doi: 10.1073/pnas.0509950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchner SI, Franks DG, Powell WH, Hahn ME. Regulatory interactions among three members of the vertebrate aryl hydrocarbon receptor family: AHR repressor, AHR1, and AHR2. J. Biol. Chem. 2002;277:6949–6959. doi: 10.1074/jbc.M110779200. [DOI] [PubMed] [Google Scholar]
- Karchner SI, Powell WH, Hahn ME. Identification and functional characterization of two highly divergent aryl hydrocarbon receptors (AHR1 and AHR2) in the teleost Fundulus heteroclitus. Evidence for a novel subfamily of ligand-binding basic helix loop helix-Per-ARNT-Sim (bHLH-PAS) factors. J. Biol. Chem. 1999;274:33814–33824. doi: 10.1074/jbc.274.47.33814. [DOI] [PubMed] [Google Scholar]
- Kiparissis Y, Blazeski S, Hodson PV, Cai X, Snieckus V. Embryotoxicity of Alkyl-Substituted PAHs. 2002. Canadian Network of Toxicology Centres (CNTC), 2002 Annual Symposium. [Google Scholar]
- Korashy HM, El-Kadi AO. The role of the aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab. Rev. 2006;38:411–450. doi: 10.1080/03602530600632063. [DOI] [PubMed] [Google Scholar]
- Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, Bentz M, Southard J, Bradfield CA. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer JS, Zheng J. The sources, transport, and fate of PAHs in the marine environment. In: Douben PET, editor. PAHs: An Ecotoxicological Perspective. West Sussex, UK: John Wiley and Sons; 2003. pp. 9–33. [Google Scholar]
- Lin TM, Ko K, Moore RW, Buchanan DL, Cooke PS, Peterson RE. Role of the aryl hydrocarbon receptor in the development of control and 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed male mice. J. Toxicol. Environ. Health A. 2001;64:327–342. doi: 10.1080/152873901316981312. [DOI] [PubMed] [Google Scholar]
- Lund AK, Goens MB, Kanagy NL, Walker MK. Cardiac hypertrophy in aryl hydrocarbon receptor null mice is correlated with elevated angiotensin II, endothelin-1, and mean arterial blood pressure. Toxicol. Appl. Pharmacol. 2003;193:177–187. doi: 10.1016/j.taap.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Ma Q, Lu AYH. CYP1A induction and human risk assessment: An evolving tale of in vitro and in vivo studies. Drug Metab. Dispos. 2007;35:1009–1016. doi: 10.1124/dmd.107.015826. [DOI] [PubMed] [Google Scholar]
- Marty GD, Heintz RA, Hinton DE. Histology and teratology of pink salmon larvae near the time of emergence from gravel substrate in the laboratory. Can. J. Zool. 1997;75:978–988. [Google Scholar]
- McCarty LS, Mackay D. Enhancing ecotoxicological modeling and assessment: Body residues and modes of toxic action. Environ. Sci. Technol. 1993;27:1718–1728. [Google Scholar]
- McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor is activated by modified low-density lipoprotein. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1412–1417. doi: 10.1073/pnas.0607296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador J. Rationale and procedures for using the tissue-residue approach for toxicity assessment and determination of tissue, water, and sediment quality guidelines for aquatic organisms. Hum. Ecol Risk Assess. 2006;12:1018–1073. [Google Scholar]
- Merchant M, Morrison V, Santostefano M, Safe S. Mechanism of action of aryl hydrocarbon receptor antagonists: Inhibition of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced CYP1A1 gene expression. Arch. Biochem. Biophys. 1992;298:389–394. doi: 10.1016/0003-9861(92)90426-w. [DOI] [PubMed] [Google Scholar]
- Merchant M, Safe S. In vitro inhibition of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced activity by α-naphthoflavone and 6-methyl-1,3,8-trichlorodibenzofuran using an aryl hydrocarbon (Ah)-responsive construct. Biochem. Pharmacol. 1995;50:663–668. doi: 10.1016/0006-2952(95)00180-8. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Di Giulio RT. Heritable adaptation and associated fitness costs in killifish (Fundulus heteroclitus) inhabiting a polluted estuary. Ecol. Appl. 2003;13:490–503. [Google Scholar]
- Meyer JN, Nacci DE, Di Giulio RT. Cytochrome P4501A (CYP1A) in killifish (Fundulus heteroclitus): Heritability of altered expression and relationship to survival in contaminated sediments. Toxicol. Sci. 2002;68:69–81. doi: 10.1093/toxsci/68.1.69. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Smith JD, Winston GW, Di Giulio RT. Antioxidant defenses in killifish (Fundulus heteroclitus) exposed to contaminated sediments and model prooxidants: Short-term and heritable responses. Aquat. Toxicol. 2003a;65:377–395. doi: 10.1016/j.aquatox.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Wassenberg DM, Karchener SI, Hahm ME, Di Giulio RT. Expression and inducibility of aryl hydrocarbon receptor pathway genes in wild-caught killifish (Fundulus heteroclitus) with different contaminant histories. Environ. Toxicol. Chem. 2003b;22:2337–2343. doi: 10.1897/02-495. [DOI] [PubMed] [Google Scholar]
- Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, et al. Loss of teratogenic response to 2,3,7,8-tetrachloro-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- Monosson E, Stegeman JJ. Cytochrome P450E (P4501A) induction and inhibition in winter flounder by 3,3’,4,4’-tetrachlorobiphenyl: Comparison of response in fish from Georges Bank and Narragansett Bay. Environ. Toxicol. Chem. 1991;10:765–774. [Google Scholar]
- Mulero-Navarro S, Carvajal-Gonzalez JM, Herranz M, Ballestar E, Fraga MF, Ropero S, Esteller M, Fernandez-Salguero PM. The dioxin receptor is silenced by promoter hypermethylation in human acute lymphoblastic leukemia through inhibition of Sp1 binding. Carcinogenesis. 2006;27:1099–1104. doi: 10.1093/carcin/bgi344. [DOI] [PubMed] [Google Scholar]
- Murray M, Reidy GF. Selectivity in the inhibition of mammalian cytochromes P-450 by chemical agents. Pharmacol. Rev. 1990;42:85–101. [PubMed] [Google Scholar]
- Nacci D, Coiro L, Champlin D, Jayaraman S, McKinney R, Gleason TR, Munns WR, Jr, Specker JL, Cooper KR. Adaptations of wild populations of the estuarine fish Fundulus heteroclitus to persistent environmental contaminants. Mar. Biol. 1999;134:9–17. [Google Scholar]
- Nacci DE, Champlin D, Coiro L, McKinney R, Jayaraman S. Predicting the occurrence of genetic adaptation to dioxinlike compounds in populations of the estuarine fish Fundulus heteroclitus. Environ. Toxicol. Chem. 2002;21:1525–1532. [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nebert DW. The Ah locus: Genetic differences in toxicity, cancer, mutation, and birth defects. Crit. Rev. Toxicol. 1989;20:153–174. doi: 10.3109/10408448909017908. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat. Rev. Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- Niimi AJ, Palazzo V. Biological half-lives of eight polycyclic aromatic hydrocarbons (PAHs) in rainbow trout (Salmo gairdneri) Water Res. 1986;20:503–507. [Google Scholar]
- Ontario Ministry of the Environment. Scientific Criteria Document for Standard Development No. 4-84. Polychloinated Dibenzo-p-Dioxins (PCDDs) and Polychlorinated Dibenzofurans (PCDFs) Ontario: OMOE; 1985. [Google Scholar]
- Ownby DR, Newman MC, Mulvey M, Vogelbein WK, Unger MA, Arzayus LF. Fish (Fundulus heteroclitus) populations with different exposure histories differ in tolerance of creosote-contaminated sediments. Environ. Toxicol. Chem. 2002;21:1897–1902. [PubMed] [Google Scholar]
- Parrott JL, Hodson PV, Servos MR, Huestis SL, Dixon DG. Relative potencies of polychlorinated dibenzo-p-dioxins and dibenzofurans for inducing mixed function oxygenase activity in rainbow trout. Env. Toxicol. Chem. 1995;14:1041–1050. [Google Scholar]
- Payne JF, Fancey LL, Rahimtula AD, Porter EL. Review and perspective on the use of mixed-function oxygenase enzymes in biological monitoring. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1987;86:233–245. doi: 10.1016/0742-8413(87)90074-0. [DOI] [PubMed] [Google Scholar]
- Peters JM, Narotsky MG, Elizondo G, Fernandez-Salguero PM, Gonzalez FJ, Abbott BD. Amelioration of TCDD-induced teratogenesis in aryl hydrocarbon receptor (AhR)-null mice. Toxicol. Sci. 1999;47:86–92. doi: 10.1093/toxsci/47.1.86. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: Cross-species comparisons. Crit. Rev. Toxicol. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Piskorska-Pliszczynska J, Keys B, Safe S, Newman MS. The cytosolic receptor binding affinities and AHH induction potencies of 29 polynuclear aromatic hydrocarbons. Toxicol. Lett. 1986;34:67–74. doi: 10.1016/0378-4274(86)90146-3. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Andreasen EA, Peterson RE, Heideman W. Interactions between 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and hypoxia signaling pathways in zebrafish: Hypoxia decreases responses to TCDD in zebrafish embryos. Toxicol. Sci. 2004;78:68–77. doi: 10.1093/toxsci/kfh053. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Tanguay RL, Mehta V, Heideman W, Peterson RE. Identification of zebrafish ARNT1 homologs: 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in the developing zebrafish requires ARNT1. Mol. Pharmacol. 2006;69:776–787. doi: 10.1124/mol.105.016873. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Prince R, Cooper KR. Comparisons of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on chemically impacted and nonimpacted subpopulations of Fundulus heteroclitus: II. Metabolic considerations. Environ. Toxicol. Chem. 1995;14:589–595. [Google Scholar]
- Ramesh A, Walker SA, Hood DB, Guillén MD, Schneider K, Weyand EH. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int. J. Toxicol. 2004;23:301–333. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- Rhodes S, Farwell A, Hewitt LM, MacKinnon M, Dixon DG. The effects of dimethylated and alkylated polycyclic aromatic hydrocarbons on the embryonic development of the Japanese medaka. Ecotoxicol. Environ. Saf. 2005;60:247–258. doi: 10.1016/j.ecoenv.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Rifkind AB. CYP1A in TCDD toxicity and in physiology-with particular reference to CYP dependent arachidonic acid metabolism and other endogenous substrates. Drug Metab. Rev. 2006;38:291–335. doi: 10.1080/03602530600570107. [DOI] [PubMed] [Google Scholar]
- Roark SA, Nacci D, Coiro L, Champlin D, Guttman SI. Population genetic structure of a nonmigratory estuarine fish (Fundulus heteroclitus) across a strong gradient of polychlorinated biphenyl contamination. Environ. Toxicol. Chem. 2005;24:717–725. doi: 10.1897/03-687.1. [DOI] [PubMed] [Google Scholar]
- Roy NK, Courtenay SC, Chambers RC, Wirgin II. Characterization of the aryl hydrocarbon receptor repressor and a comparison of its expression in Atlantic tomcod from resistant and sensitive populations. Environ. Toxicol. Chem. 2006;25:560–571. doi: 10.1897/05-347r.1. [DOI] [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs), dibenzp-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: Environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs) Crit. Rev. Toxicol. 1990;21:51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- Safe S. Development of bioassays and approaches for the risk assessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds. Environ. Health Perspect. 1993;101:S317–S325. doi: 10.1289/ehp.93101s3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal MK, Li Y-L. Deleterious effects of polynuclear aromatic hydrocarbon on blood vascular system of the rat fetus. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2007;80:367–373. doi: 10.1002/bdrb.20122. [DOI] [PubMed] [Google Scholar]
- Schnekenburger M, Talaska G, Puga A. Chromium cross-links histone deacetylase 1-DNA methyltransferase 1 complexes to chromatin, inhibiting histone-remodeling marks critical for transcriptional activation. Mol. Cell Biol. 2007;20:7089–7101. doi: 10.1128/MCB.00838-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsbergen JM, Walker MK, Olsen JR, Peterson RE. Pathologic alterations in early life stages of lake trout Salvalinus namaycush exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Aquat. Toxicol. 1991;19:41–72. [Google Scholar]
- Sundberg H, Ishaq R, Tjärnlund V, Åkerman G, Grunder K, Bandh C, Broman D, Balk L. Contribution of commonly analyzed polycylic aromatic hydrocarbons (PAHs) to potential toxicity in early life stages of rainbow trout (Oncorhynchus mykiss) Can. J. Fish. Aquat. Sci. 2006;63:1320–1333. [Google Scholar]
- Tabash SP. Kingston, Ontario, Canada: Department of Chemistry, Queen's University; 2003. An investigation of the metabolism of alkyl substituted polycyclic aromatic hydrocarbons by cytochrome P450 enzymes. Ph. D. Dissertation. [Google Scholar]
- Takahashi Y, Suzuki C, Kamataki T. Silencing of CYP1A1 expression in rabbits by DNA methylation. Biochem. Biophys. Res. Commun. 1998;247:383–386. doi: 10.1006/bbrc.1998.8791. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem. Biophys. Res. Commun. 2003;304:223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- Testa B, Jenner P. Inhibitors of cytochrome P-450s and their mechanism of action. Drug Metab. Rev. 1981;12:1–117. doi: 10.3109/03602538109011082. [DOI] [PubMed] [Google Scholar]
- Thackaberry EA, Jiang Z, Johnson CD, Ramos KS, Walker MK. Toxicogenomic profile of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the murine fetal heart: Modulation of cell cycle and extracellular matrix genes. Toxicol. Sci. 2005a;88:231–241. doi: 10.1093/toxsci/kfi301. [DOI] [PubMed] [Google Scholar]
- Thackaberry EA, Nunez BA, Ivnitski-Steele ID, Friggins M, Walker MK. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on murine heart development: Alteration in fetal and postnatal cardiac growth, and postnatal cardiac chronotropy. Toxicol. Sci. 2005b;88:242–249. doi: 10.1093/toxsci/kfi302. [DOI] [PubMed] [Google Scholar]
- Timme-Laragy AR, Cockman CJ, Matson CW, Di Giulio RT. Synergistic induction of AHR regulated genes in developmental toxicity from co-exposure to two model PAHs in zebrafish. Aquat. Toxicol. 2007;85:241–250. doi: 10.1016/j.aquatox.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Meyer JN, Waterland R, Di Giulio RT. Analysis of CpG methylation in the killifish CYP1A promoter. Comp. Biochem. Physiol. C. 2005;141:406–411. doi: 10.1016/j.cbpc.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Tokizane T, Shiina H, Igawa M, Enokida H, Urakami S, Kawakami T, Ogishima T, Okino ST, Li LC, Tanaka Y, et al. Cytochrome P450 1B1 is overexpressed and regulated by hypomethylation in prostate cancer. Clin. Cancer Res. 2005;11:5793–5801. doi: 10.1158/1078-0432.CCR-04-2545. [DOI] [PubMed] [Google Scholar]
- Toomey BH, Bello S, Hahn ME, Cantrell S, Wright P, Tillitt DE, Di Giulio RT. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces apoptotic cell death and cytochrome P4501A expression in developing Fundulus heteroclitus embryos. Aquat. Toxicol. 2001;53:127–138. doi: 10.1016/s0166-445x(00)00161-2. [DOI] [PubMed] [Google Scholar]
- Turcotte D, Abudulai N, Witherly K, Hodson P, Brown R. Abstract. Baltimore, MD: SETAC 26th Annual Meeting in North America; 2005. Identification of metabolites from alkyl-anthracene compounds in rainbow trout (Oncorhynchus mykiss) [Google Scholar]
- Uno S, Dalton TP, Sinclair PR, Gorman N, Wang B, Smith AG, Miller ML, Shertzer HG, Nebert DW. Oral exposure to benzo[a]pyrene in the mouse: Detoxification by inducible cytochrome P450 is more important than metabolic activation. Mol. Pharmacol. 2004;65:1225–1237. doi: 10.1124/mol.65.5.1225. [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton P, Shertzer HG, Genter MB, Warshawsky D, Talaska G, Nebert DW. Benzo[a]pyrene-induced toxicity: Paradoxical protection in Cyp1a1(-/-) knockout mice having increased hepatic BaP-DNA adduct levels. Biochem. Biophys. Res. Commun. 2001;289:1049–1056. doi: 10.1006/bbrc.2001.6110. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum L, Bosveld AT, Brunström B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ. Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ. Trends in hydrophobic organic contaminants in urban and reference lake sediments across the United States, 1970–2001. Environ. Sci. Technol. 2005;39:5567–5574. doi: 10.1021/es0503175. [DOI] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ, Furlong ET. Urban sprawl leaves its PAH signature. Environ. Sci. Technol. 2000;34:4064–4070. [Google Scholar]
- Vogelbein WK, Unger M. The Elizabeth River Monitoring Program 2001–2002: Association Between Mummichog Liver Histopathology and Sediment Chemical Contamination. Richmond, Virginia: Virginia Department of Environmental Quality; 2003. [Google Scholar]
- Walisser JA, Bunger MK, Glover E, Bradfield CA. Gestational exposure of Ahr and Arnt hypomorphs to dioxin rescues vascular development. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16677–16682. doi: 10.1073/pnas.0404379101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MK, Catron TF. Characterization of cardiotoxicity induced by 2,3,7, 8-tetrachlorodibenzo-p-dioxin and related chemicals during early chick embryo development. Toxicol. Appl. Pharmacol. 2000;167:210–221. doi: 10.1006/taap.2000.8992. [DOI] [PubMed] [Google Scholar]
- Walker MK, Cook PM, Butterworth BC, Zabel EW, Peterson RE. Potency of a complex mixture of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners compared to 2,3,7,8-tetrachlorodibenzo-p-dioxin in causing fish early life stage mortality. Fund. Appl. Toxicol. 1996;30:178–186. doi: 10.1006/faat.1996.0054. [DOI] [PubMed] [Google Scholar]
- Walker MK, Peterson RE. Potencies of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners, relative to 2,3,7,8-tetrachlorodibenzo-p-dioxin, for producing early life stage mortality in rainbow trout (Oncorhynchus mykiss) Aquat. Toxicol. 1991;21:219–237. [Google Scholar]
- Wang L, Scheffler BE, Willett KL. CYP1C1 messenger RNA expression is inducible by benzo[a]pyrene in Fundulus heteroclitus embryos and adults. Toxicol. Sci. 2006;93:331–340. doi: 10.1093/toxsci/kfl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hollebone B, Fingas M, Fieldhouse B, Sigouin L, Landriault M. Characteristics of Spilled Oils, Fuels, and Petroleum Products: 1. Composition and Properties of Selected Oils. US EPA; 2003. [Google Scholar]
- Wang Z, Hollebone B, Fingas M, Fieldhouse B, Sigouin L, Landriault M, Smith P. Final report to US EPA. Ottawa, Canada: Environment Canada; 2002. Development Composition Database for Selected Multicomponent Oils; p. 300. [Google Scholar]
- Wannemacher R, Rebstock A, Kulzer E, Schrenk D, Bock KW. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproduction and oogenesis in zebrafish (Branchydanio rerio) Chemosphere. 1992;24:1361–1368. [Google Scholar]
- Wassenberg DM. Durham, NC: Duke University; 2004. Interactive Effects of Polycyclic Aromatic Hydrocarbons on Cytochrome P4501A Activity and Embryonic Development in the Killifish Fundulus heteroclitus. Ph. D. Dissertation. [Google Scholar]
- Wassenberg DM, Di Giulio RT. Teratogenesis in Fundulus heteroclitus embryos exposed to a creosote-contaminated sediment extract and CYP1A inhibitors. Mar. Environ. Res. 2004a;58:163–168. doi: 10.1016/j.marenvres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic embryotoxicity of polycyclic aromatic hydrocarbon aryl hydrocarbon receptor agonists with cytochrome P4501A inhibitors in Fundulus heteroclitus. Environ. Health Perspect. 2004b;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenberg DM, Nerlinger AL, Battle LP, Di Giulio RT. Effects of the polycyclic aromatic hydrocarbon heterocycles, carbazole and dibenzothiophene, on in vivo and in vitro CYP1A activity and polycyclic aromatic hydrocarbon-derived embryonic deformities. Environ. Toxicol. Chem. 2005;24:2526–2532. doi: 10.1897/04-440r1.1. [DOI] [PubMed] [Google Scholar]
- Weinstein BM. What guides early embryonic blood vessel formation? Dev. Dyn. 1999;215:2–11. doi: 10.1002/(SICI)1097-0177(199905)215:1<2::AID-DVDY2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Whyte JJ, Jung RE, Schmitt CJ, Tillitt DE. Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit. Rev. Toxicol. 2000;30:347–570. doi: 10.1080/10408440091159239. [DOI] [PubMed] [Google Scholar]
- Willett KL, Randerath K, Zhou G-D, Safe SH. Inhibition of CYP1A1-dependent activity by the polynuclear aromatic hydrocarbon (PAH) fluoranthene. Biochem. Pharmacol. 1998;55:831–839. doi: 10.1016/s0006-2952(97)00561-3. [DOI] [PubMed] [Google Scholar]
- Willett KL, Wassenberg D, Lienesch L, Reichert W, Di Giulio RT. In vivo and in vitro inhibition of CYP1A-dependent activity in Fundulus heteroclitus by the polynuclear aromatic hydrocarbon fluoranthene. Toxicol. Appl. Pharmacol. 2001;177:264–271. doi: 10.1006/taap.2001.9296. [DOI] [PubMed] [Google Scholar]
- Wirgin I, Waldman JR. Altered gene expression and genetic damage in North American fish populations. Mutat. Res. 1998;399:193–219. doi: 10.1016/s0027-5107(97)00256-x. [DOI] [PubMed] [Google Scholar]
- Wirgin I, Waldman JR. Resistance to contaminants in North American fish populations. Mutat. Res. 2004;552:73–100. doi: 10.1016/j.mrfmmm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Zabel EW, Walker MK, Hornung MW, Clayton MK, Peterson RE. Interactions of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners for producing rainbow trout early life stage mortality. Toxicol. Appl. Pharmacol. 1995;134:204–213. doi: 10.1006/taap.1995.1185. [DOI] [PubMed] [Google Scholar]