Abstract
Estrogenic compounds such as 17β-estradiol (E2) and methoxychlor (MXC) induce oxidative stress damage in breast cells and mouse ovarian follicles, respectively. However, little is known about whether estrogenic compounds cause oxidative stress in the ovarian surface epithelium (OSE). Thus, this work tested the hypothesis that E2 and MXC cause oxidative stress in the OSE. To test this hypothesis, we employed an improved mouse tissue culture assay in which OSE cells were treated with hydrogen peroxide (H2O2; positive control), MXC, or E2 ± the anti-oxidant vitamin E, or progesterone. The cells then were subjected to a novel direct immunofluorescent assay in which cells in the microtiter plate were reacted with antibodies that detect oxidative damage to DNA (8-hydroxy-2′-deoxyguanosine). The signal was identified with a tyramide Alexa Fluor fluorescent probe and quantified by microfluorimetry. Correction for cellularity was carried out for each well with a fluorescent DNA dye system (CyQuant) at a different wavelength. After 24 h, the mean Alexa Fluor CyQuant ratio was 11.3 ± 0.9 for controls, 132 ± 15 for H2O2 treated positive control cells (p ≤ 0.01 from control), 105 ± 6.6 for E2 treated cells (p ≤ 0.01 from control), and 64 ± 5.1 for MXC-treated cells (p ≤ 0.01 from control). After 72 h, the mean ratio was 121 ± 10.6 for controls, 391 ± 23 for H2O2 treated cells (p ≤ 0.01 from control), 200 ± 15 for E2 treated cells (p ≤ 0.03), and 228 ± 21 for MXC-treated cells (p ≤ 0.01). Further, vitamin E, but not progesterone, protected OSE cells from E2- and MXC-induced oxidative damage. This study demonstrates the feasibility of direct immunofluorescent quantitation of DNA adducts in cell cultures without DNA extraction. Moreover, these data indicate that E2 and MXC produce oxidative DNA damage in the OSE, and that this damage is prevented by the anti-oxidant vitamin E.
Keywords: ovarian surface epithelium, oxidative stress damage, DNA adducts, estrogen
An imbalance between free radical pro-oxidants and anti-oxidants has important implications for both physiological and pathological processes in the reproductive tract (Agarwal et al., 2005). For example, superoxide radicals, possibly of neutrophilic origin, are generated during ovulation and during regression of the corpus luteum in the ovary. These free radicals have important physiologic roles in regulating the ovarian follicular cycle, possibly through inhibition of steroid production (Behrman et al., 2001). Nonphysiologic effects of free radicals include premature ovarian follicular atresia via apoptosis. Apoptosis is induced by many ovarian toxicants such as the xenoestrogen pesticide methoxychlor (MXC), and there is evidence that oxidative stress is central to this process (Gupta et al., 2006a). Moreover, clinical observations have linked increased levels of reactive oxygen species to decreased female fertility (Agarwal et al., 2006).
In distinction to the studies demonstrating that free radicals mediate apoptosis and senescence, and hence limit cell growth, is the growing body of evidence that intermediates of oxygen reduction induce DNA damage and are putative sources of neoplastic transformation. Valko et al. (2004) detail the sequence of DNA damage and formation of mutagenic 8-hydroxy-2′-deoxyguanosine (8-OH-dG), leading to GC-TA transversions and potential carcinogenesis. Further, Valko et al. (2006) note that oxidative DNA damage has been identified in various neoplasms, and suggest such damage may be some of the earliest genetic abnormality associated with malignancy. The ovarian surface epithelium (OSE) is of particular interest as a major source of ovarian cancer. Hence, the presence of 8-OH-dG as a marker of DNA mutation may serve as a sentinel event for subsequent ovarian neoplastic transformation. Because experimental evidence for ovarian carcinogenesis by exogenous agents is limited, the ability to detect abnormal DNA in the isolated OSE could prove of particular value in identifying agents with carcinogenic potential.
Previously, we have shown that MXC has a proliferative effect on the OSE, which is mediated through stimulation of cell cycle regulators and inhibition of apoptosis (Symonds et al., 2005). Because MXC has been shown to induce oxidative stress in the mouse ovarian follicle (Gupta et al., 2006a, b), we hypothesized it may also do so in the OSE. Using a modification of our previously described method to isolate OSE in microtiter plates (Symonds et al., 2005, 2006), we exposed the cells to MXC and 17β-estradiol (E2) to compare their effects to the known free radical inducing agent hydrogen peroxide (H2O2) upon DNA oxidative damage, both in short-term (1 h) and long-term (24 and 72 h) timeframes. To measure abnormal DNA products, we developed an immunoassay directed against 8-OH-dG using an ultrasensitive tyramide-fluorescent system and fluorimetric quantitation. To counter cytotoxic effects of H2O2 and possible stimulatory effects of MXC and E2, we corrected results for cellularity. Further, we evaluated the ability of the known anti-oxidant, vitamin E (α-tocopherol), as well as progesterone to counteract the oxidative effect of H2O2. Finally, we assessed the effect of cotreatment of vitamin E with MXC, and with E2 on 8-OH-dG products.
MATERIALS AND METHODS
Animals.
Female FVB mice were used in all experiments. The mice were maintained at the University of Maryland Central Animal Facility, provided food and water ad libitum and euthanized between postnatal days 60 and 90. Temperature was maintained at 22° ± 1°C, and animals were subjected to 12-h light:dark cycles. The University of Maryland School of Medicine Institutional Animal Use and Care Committee approved all procedures involving animal care, euthanasia, and tissue collection.
Primary cultures of OSE cells.
Primary cells were isolated using a modification of previously reported techniques (Symonds et al., 2005, 2006). In brief, ovaries were excised from mice aseptically and individually placed in 0.5 ml of Medium 199E (BioSource, Rockville, MD) with 1 mg of type IV collagenase (Sigma-Aldrich, Inc., St Louis, MO). After 30 min of incubation at 37°C, each ovary was vortexed for 2 min, the ovaries were removed, and the suspension was vortexed again for 2 min to disaggregate cell clusters. The suspension was diluted with additional Medium 199E containing 15% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA) and an antimicrobial solution (10 μl/ml) containing 10 mg/ml streptomycin, 10 mU/ml penicillin G, and 25 μg/ml amphotericin (Sigma-Aldrich, Inc.). Aliquots containing 100–250 cells/100 μl from a single ovary were pipetted into 96-well plates coated with type I collagen (CellCoat, Greiner Bio-One, Frickenhause, Germany) with frequent mixing during aliquoting. Control and treatment wells were from the same suspension. Cultures were incubated at 37°C with 5% CO2, with a media change each third day, and cells were grown to confluence (usually for 7–14 days) before treatment. Use of the modifications, type IV collagenase and collagen coated plates, permitted plating a smaller number of cells and more rapid growth, with fewer growth failures and less stromal contamination than in our previous studies (Symonds et al., 2005, 2006).
Treatment of cultures.
In a first set of experiments, we evaluated the effect of E2 and MXC on 8-OH-dguanine products after short-term (1 h) and long-term exposure (24 and 72 h). Controls cells were exposed to vehicle (dimethyl sulfoxide) or 15mM of H2O2 (positive control). The dose of H2O2 was based on pilot studies in the laboratory which indicated that this dose gave the best antibody response. Treated cells were exposed to 0.1μM of E2 (Sigma-Aldrich, Inc.) or 3μM MXC (ChemService, West Chester, PA). Doses of E2 and MXC were selected based on our previous studies (Symonds et al., 2006). To assess the effects of vitamin E and progesterone in reversing the induction of 8-OH-dG products by H2O2, OSE cells were treated for 72 h with either 15mM of H2O2, 100mM of vitamin E (α-tocopherol, Sigma-Aldrich T3251), or 100mM of vitamin E plus 15mM of H2O2. In some experiments, cells were treated with 1μM progesterone (Sigma-Aldrich P8783) or 1μM progesterone plus 15mM of H2O2. In a final experiment to determine the effect of vitamin E on E2 and MXC, OSE cells were treated for 72 h either with 15mM of H2O2, 0.1μM of E2, 0.1μM of E2 plus 100mM of vitamin E, 3μM MXC, or 3μM MXC plus vitamin E. Doses of vitamin E and progesterone were based on available literature showing biologic potency at these levels (Rae et al., 2004; Zhang and Omaye, 2000).
Immunofluorescent assay for 8-OH-dG.
After treatment, cells were washed with phosphate buffered saline (PBS), air dried for 15 min, and fixed with 70% ethanol for 30 min. Plates were washed between each subsequent step three times with PBS and 100 μl of reagent was applied at each step. After fixation, cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) for 10 min, followed by epitope retrieval with 0.5% dodecyl sulfate (Flukia, Sigma-Aldrich) for 10 min. Blocking was performed with 1% bovine serum albumin (BSA) for 60 min, followed by overnight incubation with 1 μg/ml mouse monoclonal anti-8-hydroxy-2'-deoxyguanosine in 1% BSA (origin from Japanese Institute to Control Aging, Genox, Baltimore, MD). Molecular Probes Anti-mouse Tyramide Signal Amplification System with Alexa Fluor 594 (Molecular Probes, Eugene, OR) was utilized to quantify the bound anti-8-hydroxy-2'-deoxyguanosine. This procedure included incubation for 60 min with 1:100 horse-radish peroxidase in 1% blocking agent, followed by 10-min incubation with tyramide Alexa Fluor working solution constituted as per manufacturer's specifications. After washing, fluorescence was measured with a microtiter plate fluorimeter (CytoFluor 4000, PerSeptive Biosystems, Framingham, MA) with excitation at 590 nm and emission detection at 645 nm (50% gain), yielding a value in arbitrary fluorescent units. Incubation was carried out with CyQuant NF (Molecular Probes) reagent at 39°C for 60 min, followed by reading at excitation 485/emission 530 nm (30% gain). The Alexa Fluor value was then divided by the CyQuant value for each individual cell to give a ratio normalized for cellularity.
Statistical analysis.
Data were analyzed using SPSS statistical software (SPSS, Inc., Chicago, IL). ANOVA with Tukey's post hoc test was used for multiple comparisons between treatment groups. Data are presented as means ± standard error of the mean (n = 8 per treatment group). A p value ≤ 0.05 was considered significant.
RESULTS
The results indicate that short-term (1 h) exposure to H2O2, but not E2 or MXC, significantly increased oxidative damage in the OSE. The mean adduct ratio (fluorimetric signal for anti-8-hydroxy-2'-deoxyguanosine to fluorimetric CyQuant signal) was 23.8 ± 1.7 for controls, 54.1 ± 8.2 for H2O2-treated cells (n = 8, p ≤ 0.01 from control), 33 ± 1.4 for E2 treated cells (n = 8, p = 0.5), and 34.9 ± 2.5 for MXC-treated cells (n = 8, p = 0.29).
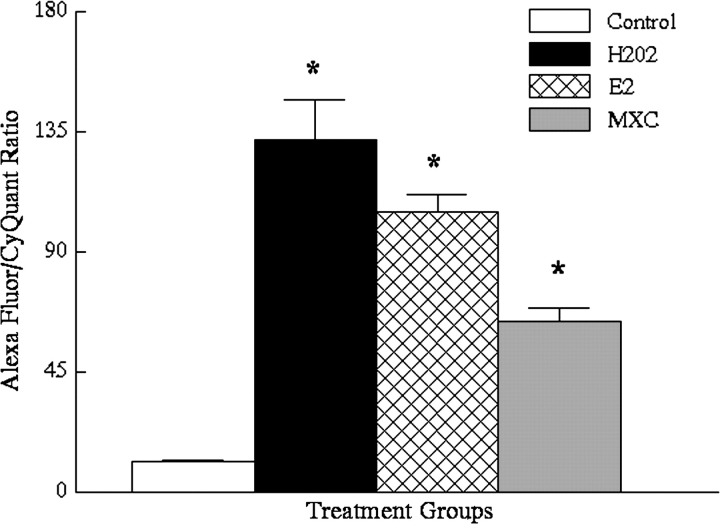
Further, the data indicate that longer-term (24 h) exposure to H2O2, E2, or MXC significantly increased oxidative damage in the OSE (Fig. 1). Specifically, the mean ratio was 11.3 ± 0.9 for vehicle controls, 132 ± 15 for H2O2 treated cells (n = 8, p ≤ 0.01 from control), 105 ± 6.6 for E2 treated cells (n = 8, p ≤ 0.01 from vehicle controls), and 64 ± 5.1 for MXC-treated cells (n = 8, p ≤ 0.01 from vehicle controls) (Fig. 1).
FIG. 1.
Results of DNA adduct assay (expressed as ratio of arbitrary fluorescent units for anti-8-hydroxy-2'-deoxyguanosine/ CyQuant) for 24-h treated OSE cells. Control = vehicle-treated; H2O2-treated (n = 8, p ≤ 0.01); E2-treated (n = 8, p ≤ 0.01); and MXC-treated cells (n = 8, p ≤ 0.01). Asterisks indicate significant differences from vehicle-treated controls by ANOVA.
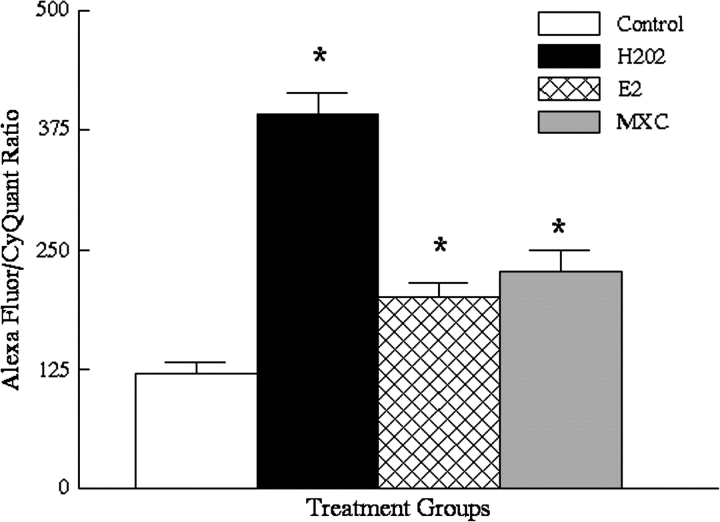
Similarly, the data indicate that 72-h exposure to H2O2, E2, or MXC significantly increased oxidative damage in the OSE. The mean ratio was 121 ± 10.6 for vehicle controls, 391 ± 23 for H2O2 treated cells (n = 8, p ≤ 0.01 from vehicle controls), 200 ± 15 for E2-treated cells (n = 8, p ≤ 0.03 from vehicle controls), and 228 ± 21 for MXC-treated cells (n = 8, p ≤ 0.01 from vehicle controls) (Fig. 2).
FIG. 2.
Results of DNA adduct assay for 72-h treated OSE cells. Abbreviations and symbol as in Figure 1 (H2O2, n = 8, p ≤ 0.01; E2, n = 8, p ≤ 0.03; MXC, n = 8, p ≤ 0.01).
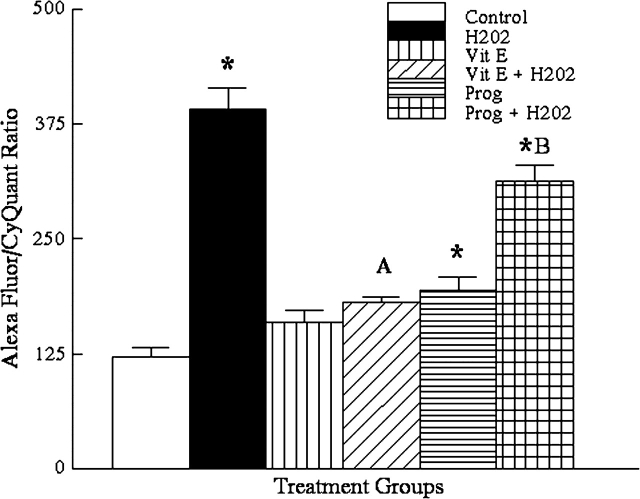
Because H2O2 caused oxidative damage in the OSE, we further evaluated whether vitamin E or progesterone could protect OSE cells from H2O2 induced oxidative damage (Fig. 3). The mean values were 120 ± 11 for vehicle control cells, 391 ± 23 for H2O2 treated cells (n = 8, p ≤ 0.01 compared with vehicle controls), 159 ± 14 for vitamin E (n = 8, p = 0.42 compared with vehicle controls), 181 ± 5.5 for vitamin E plus H2O2 treated cells (n = 8, p = 0.88 compared with vitamin E treatment alone), 194 ± 15 for progesterone treated cells (n = 8, p ≤ 0.01 compared with vehicle controls), and 314 ± 17 for progesterone plus H2O2 treated cells (n = 8, p ≤ 0.01 compared with progesterone alone). Thus, vitamin E, but not progesterone, prevented the production of DNA adducts by H2O2 (Fig. 3).
FIG. 3.
Results of DNA adduct assay for 72-h treated OSE cells showing the effect of vitamin E and progesterone on H2O2 induced oxidative stress DNA adducts (Vit E = vitamin E; prog = progesterone; other abbreviations as in Fig. 1). Asterisks indicate significant elevations from control (H2O2, n = 8, p ≤ 0.01; prog, n = 8, p ≤ 0.01). The letter A indicates no significant difference between vitamin E alone and vitamin E + H2O2 (n = 8, p = 0.88). The letter B indicates a significant difference between progesterone alone and progesterone + H2O2 (n = 8, p ≤ 0.01).
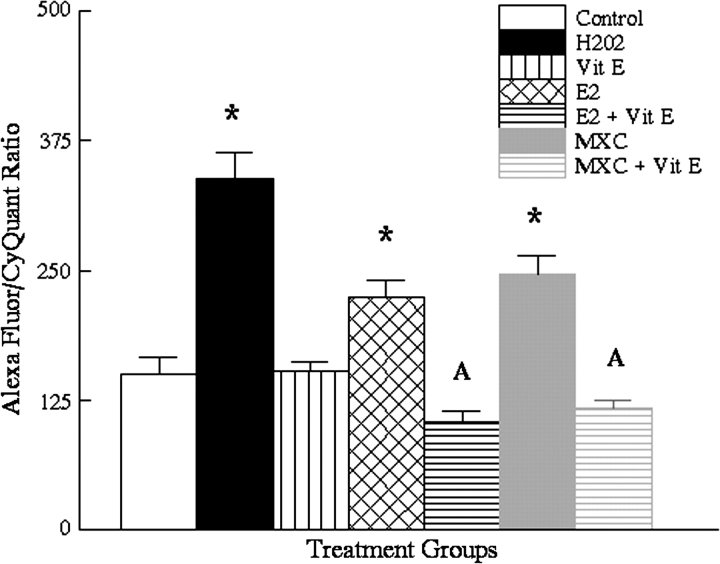
In a second set of experiments, we evaluated the ability of vitamin E to counter the oxidative damage induced by E2 and MXC (Fig. 4). The mean values were 150 ± 17 for controls, 338 ± 25 for H2O2 treated cells (n = 8, p ≤ 0.01 compared with vehicle controls), 153 ± 9 for vitamin E (n = 8, p = 1.00 compared with vehicle controls), 224 ± 16 for E2 alone (n = 8, p ≤ 0.02, compared with vehicle controls), 105 ± 10 for E2 and vitamin E treated cells (n = 8, p = 0.30 compared with vitamin E alone), 247 ± 17 for MXC (n = 8, p ≤ 0.01 compared with vehicle controls), and 118 ± 7 (for MXC + vitamin E–treated cells (n = 8, p = 0.65 compared with vitamin E alone). Thus, treatment with vitamin E abolished the increased levels of 8-OH-dG adducts produced by E2 and MXC (Fig. 4).
FIG. 4.
Results of DNA adduct assay for 72-h treated OSE cells showing the effect of vitamin E on E2 and MXC-induced oxidative stress DNA adducts. Asterisks indicate a significant difference from vehicle-treated controls. The letter A indicates no significant difference between vitamin E alone and vitamin E + E2 (n = 8, p = 0.30) or between vitamin E and vitamin E + MXC (n = 8, p = 0.65).
DISCUSSION
The altered nucleoside, 8-OH-dG, is a major product of oxidative damage to DNA and represents a marker for such damage (Machella et al., 2005). We have developed a quantitative immunoassay to measure this in cultured mouse OSE without DNA extraction based on tyramide-fluorescent localization of monoclonal antibodies to 8-OH-dG and adjustment for cellularity. Our results confirm previous work of Gupta et al. (2006a) that MXC induces oxidative stress damage to the ovary, and extends those findings to an additional tissue compartment, the OSE. Short-term (1 h) incubation with MXC did not significantly affect levels of 8-OH-dG in the OSE. However, after longer-term exposure (24 and 72 h), MXC produced a sixfold greater signal than untreated controls at 24 h exposure, and a twofold elevation at 72 h. Our previous studies on the effect of MXC on the OSE demonstrated proliferative effects, diminished apoptosis, and genomic expression of cell cycle regulators, Bcl-2, Bax, and cytochrome P450 (CYP450) enzymes, which occurred within a time frame of 14 days of treatment (Symonds et al., 2005, 2006). Therefore, these current results suggest oxidative stress may be one of the earliest cellular alterations induced by MXC. The mechanism by which the response to MXC-induced oxidative stress relates to our previous demonstration of MXC-induced OSE proliferation and decreased apoptosis is at present unclear. However, the results appear to be consistent with the study of Young et al. (2004) demonstrating increased resistance to oxidative stress-induced apoptosis in human OSE which was oncogenically transformed with H-RasV12. In the latter study, upregulation of anti-oxidant proteins in immortalized cells was the most prominent of 2200 proteins studied, suggesting that the growth characteristics of the OSE may be linked to its response to oxidative stress.
Our data indicating that E2 treatment is as effective as MXC in inducing oxidative damage were unexpected because estrogen is generally considered an anti-oxidant in many tissues. For example, Lund et al. (1999) found that E2 protected isolated ewe follicles from H2O2 stress-induced apoptosis, and Miller et al. (2006) found that E2 mitigated MXC-induced atresia. However, the effects of estrogen on oxidative stress appear complicated and may be dependent on the specific target cells. Patel and Bhat (2004) demonstrated that E2 in comparison to a weak estrogen (17-alpha-ethinylestradiol) induced elevated levels of an oxidative stress marker, 8-iso-PGF(2alpha), in estrogen receptor alpha (ER-α) positive hamster kidney tumor (H301) cells. Likewise, Mobley and Brueggemeier (2004) demonstrated that E2 treatment of ERα positive MCF-7 breast cancer cells, but not ER-negative MDA-MB-231 cancer cells, resulted in a diminished ability to metabolize peroxide, making the cells more sensitive to oxidative damage. Thus, E2 appears to have a paradoxical anti-oxidant effect in some tissues (such as the follicle) and an oxidant effect on others (such as the OSE- and ER-positive breast cancer cells).
The reasons that E2 acts as an anti-oxidant in the ovarian follicle and an oxidant in the OSE are unknown. It is possible that metabolic differences in ovarian follicles and the OSE lead to differences in the oxidant properties of E2. In the OSE, E2 could be converted into pro-oxidant and carcinogenic metabolites. Specifically, catechol-estrogens that are produced by cytochrome P450-mediated hydroxylation of estrogens (Hiraku et al., 2001; Tsuchiya et al., 2005) in the position adjacent to the phenolic hydroxyl group and further oxidized to ortho-quinones have been implicated in these detrimental effects. From ortho-quinones, via a redox cycle, superoxide radical anions are generated which are ultimately responsible for oxidative damage (Bolton, 2002; Bolton et al., 2000; Cavalieri et al., 1997; Zhu and Conney, 1998). This hypothesis is supported by data reported by Patel and Bhat (2004), which demonstrate abolition of sensitivity to oxidative damage through inhibition of CYP450 enzymes. Previously, we demonstrated that the mouse OSE does have the appropriate CYP450 enzymes, specifically CYP 1B1, to produce catechol metabolites of E2 (Symonds et al., 2006), which could account for the oxidative metabolism of E2 in the OSE. Although it would seem that granulosa cells have the ability to upregulate CYP 1B1 under certain circumstances (Vidal et al., 2006), it is unclear that this metabolic pathway is significant for E2 in the follicle. Moreover, E2 may have the ability to recruit compensatory anti-oxidant enzymes more efficiently in some tissues than others. Alternatively, it is possible that the pro-oxidative properties of E2 relate to differences in the distribution of ER in the ovary. The ovarian follicle predominately contains ER-β expressed in the granulosa cells (Fitzpatrick et al., 1999) and the OSE predominately contains ER-α (Pelletier et al., 2000). It is possible that E2 binding to ER-β induces anti-oxidant pathways, whereas E2 binding to ER-α induces oxidant pathways.
We also found it surprising that progesterone induced DNA adducts under the conditions of this study and had no protective effect, because previous studies had suggested that repair of OSE DNA damage is enhanced by exogenous progesterone administration (Murdoch, 1998). It is possible that different routes of administration (direct in vitro exposure in the present study) with different metabolic consequences account for these dissimilar results.
Potentially mutagenic 8-oxo-dguanine adducts have been previously identified in hen (Murdoch et al., 2005), sheep, and human OSE, particularly in cells surrounding ovulation sites (Murdoch and Martinchick, 2004). It is possible that cytokines released from inflammatory cells that accompany ovulation produce oxidative stress. Based on the results of this study, it is also possible that exposure to follicular fluid during ovulation may be contributory because follicular fluid contains levels of estrogen at least 100 times greater than serum levels (Lindgren et al., 2002). Because frequency and duration of ovulation are recognized risk factors for ovarian carcinogenesis (Holschneider and Berek, 2000), oxidative induced DNA mutation at the time of ovulation could explain this association. Mutated OSE DNA could be especially prone to neoplastic transformation in epithelial inclusions (germinal inclusion cysts) formed by sequestration of surface epithelium during healing phase of the stigma. Such cells persist for long periods without apoptotic removal. As reported in this study, abnormal DNA adducts induced by MXC and E2 are subject to prevention in vitro by the anti-oxidant vitamin E. This is in accord with the findings of Murdoch and Martinchick (2004), which indicate a protective effect of vitamin E against oxoguanine adducts in ewes. In addition, it provides an experimental basis for some epidemiological studies suggesting a protective effect of anti-oxidant intake against ovarian cancer (Fleischauer et al., 2001).
FUNDING
Colgate-Palmolive Foundation grant; and National Institutes of Health (ES012893).
Acknowledgments
We thank Ms Janice Babus for laboratory support and Dr R. M. Santella of Columbia University for very helpful information and suggestions during development of the assay procedure.
References
- Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005;14:3–28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Curr. Opin. Obstet. Gynecol. 2006;18:325–332. doi: 10.1097/01.gco.0000193003.58158.4e. [DOI] [PubMed] [Google Scholar]
- Behrman HR, Kodaman PH, Preston SL, Gao S. Oxidative stress and the ovary. J. Soc. Gynecol. Investig. 2001;8:S40–S42. doi: 10.1016/s1071-5576(00)00106-4. [DOI] [PubMed] [Google Scholar]
- Bolton JL. Quinoids, quinoid radicals, and phenoxyl radicals formed from estrogens and antiestrogens. Toxicology. 2002;177:55–65. doi: 10.1016/s0300-483x(02)00195-6. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem. Res. Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, et al. Molecular origin of cancer: Catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc. Natl. Acad. Sci. U. S. A. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick SL, Funkhouser JM, Sindoni DM, Stevis PE, Deecher DC, Bapat AR, Merchenthaler I, Frail DE. Expression of estrogen receptor-beta protein in rodent ovary. Endocrinology. 1999;140:2581–2591. doi: 10.1210/endo.140.6.6928. [DOI] [PubMed] [Google Scholar]
- Fleischauer AT, Olson SH, Mignone L, Simonsen N, Caputo TA, Harlap S. Dietary antioxidants, supplements, and risk of epithelial ovarian cancer. Nutr. Cancer. 2001;40:92–98. doi: 10.1207/S15327914NC402_3. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol. Sci. 2006b;93:382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Schuh RA, Fiskum G, Flaws JA. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol. Appl. Pharmacol. 2006a;216:436–445. doi: 10.1016/j.taap.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Hiraku Y, Yamashita N, Nishiguchi M, Kawanishi S. Catechol estrogens induce oxidative DNA damage and estradiol enhances cell proliferation. Int. J. Cancer. 2001;92:333–337. doi: 10.1002/ijc.1193. [DOI] [PubMed] [Google Scholar]
- Holschneider CH, Berek JS. Ovarian cancer: Epidemiology, biology, and prognostic factors. Semin. Surg. Oncol. 2000;19:3–10. doi: 10.1002/1098-2388(200007/08)19:1<3::aid-ssu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Lindgren PR, Bäckström T, Cajander S, Damber MG, Mählck CG, Zhu D, Olofsson JI. The pattern of estradiol and progesterone differs in serum and tissue of benign and malignant ovarian tumors. Int. J. Oncol. 2002;21:583–589. [PubMed] [Google Scholar]
- Lund SA, Murdoch J, Van Kirk EA, Murdoch WJ. Mitogenic and antioxidant mechanisms of estradiol action in preovulatory ovine follicles: Relevance to luteal function. Biol. Reprod. 1999;61:388–392. doi: 10.1095/biolreprod61.2.388. [DOI] [PubMed] [Google Scholar]
- Machella N, Regoli F, Santella RM. Immunofluorescent detection of 8-oxo-dG and PAH bulky adducts in fish liver and mussel digestive gland. Aquat. Toxicol. 2005;71:335–343. doi: 10.1016/j.aquatox.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol. Sci. 2006;93:180–188. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- Mobley JA, Brueggemeier RW. Estrogen receptor-mediated regulation of oxidative stress and DNA damage in breast cancer. Carcinogenesis. 2004;25:3–9. doi: 10.1093/carcin/bgg175. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ. Perturbation of sheep ovarian surface epithelial cells by ovulation: Evidence for roles of progesterone and poly(ADP-ribose) polymerase in the restoration of DNA integrity. J. Endocrinol. 1998;156:503–508. doi: 10.1677/joe.0.1560503. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Martinchick JF. Oxidative damage to DNA of ovarian surface epithelial cells affected by ovulation: Carcinogenic implication and chemoprevention. Exp. Biol. Med. 2004;229:546–552. doi: 10.1177/153537020422900613. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Van Kirk EA, Alexander BM. DNA damages in ovarian surface epithelial cells of ovulatory hens. Exp. Biol. Med. 2005;230:429–433. doi: 10.1177/15353702-0323006-11. [DOI] [PubMed] [Google Scholar]
- Patel MM, Bhat HK. Differential oxidant potential of carcinogenic and weakly carcinogenic estrogens: Involvement of metabolic activation and cytochrome P450. J. Biochem. Mol. Toxicol. 2004;18:37–42. doi: 10.1002/jbt.20005. [DOI] [PubMed] [Google Scholar]
- Pelletier G, Labrie C, Labrie F. Localization of oestrogen receptor alpha, oestrogen receptor beta and androgen receptors in the rat reproductive organs. J. Endocrinol. 2000;165:359–370. doi: 10.1677/joe.0.1650359. [DOI] [PubMed] [Google Scholar]
- Rae MT, Niven D, Critchley HO, Harlow CR, Hillier SG. Antiinflammatory steroid action in human ovarian surface epithelial cells. J. Clin. Endocrinol. Metab. 2004;89:4538–4544. doi: 10.1210/jc.2003-032225. [DOI] [PubMed] [Google Scholar]
- Symonds DA, Miller KP, Tomic D, Flaws JA. Effect of methoxychlor and estradiol on cytochrome p450 enzymes in the mouse ovarian surface epithelium. Toxicol. Sci. 2006;89:510–514. doi: 10.1093/toxsci/kfj044. [DOI] [PubMed] [Google Scholar]
- Symonds DA, Tomic D, Miller KP, Flaws JA. Methoxychlor induces proliferation of the mouse ovarian surface epithelium. Toxicol. Sci. 2005;83:355–362. doi: 10.1093/toxsci/kfi024. [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogen and its regulation in human. Cancer Lett. 2005;227:115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Vidal JD, VandeVoort CA, Marcus CB, Lazarewicz NR, Conley AJ. In vitro exposure to environmental tobacco smoke induces CYP1B1 expression in human luteinized granulosa cells. Reprod. Toxicol. 2006;22:731–737. doi: 10.1016/j.reprotox.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Young TW, Mei FC, Yang G, Thompson-Lanza JA, Liu J, Cheng X. Activation of antioxidant pathways in ras-mediated oncogenic transformation of human surface ovarian epithelial cells revealed by functional proteomics and mass spectrometry. Cancer Res. 2004;64:4577–4584. doi: 10.1158/0008-5472.CAN-04-0222. [DOI] [PubMed] [Google Scholar]
- Zhang P, Omaye ST. Beta-carotene and protein oxidation: Effects of ascorbic acid and alpha-tocopherol. Toxicology. 2000;146:37–47. doi: 10.1016/s0300-483x(00)00160-8. [DOI] [PubMed] [Google Scholar]
- Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]