Abstract
Studies investigating the predictors of growth in infants born to HIV-infected women in developing countries are limited. Using data from 886 Tanzanian HIV-infected women and their infants, we examined the impact of maternal socioeconomic and immunological status, infant characteristics at birth, and HIV, diarrhea and respiratory infections on infants’ monthly length-for-age (LAZ) and length-for-weight (WLZ) z-scores during the first 2 years of life. We used restricted cubic splines to estimate average adjusted growth curves by categories of each predictor. LAZ decreased significantly during the first 2 years. WLZ increased from birth to 4 months but decreased significantly thereafter. Greater maternal schooling significantly reduced deterioration in LAZ and WLZ scores from birth to 24 months, while maternal CD4 cell counts ≥200 mm−3 at baseline were associated with reduced deterioration in LAZ scores. Infants born pre-term or with low-birth weight were significantly more stunted and wasted than their reference groups at all time points though their rate of growth faltering was slower. Infant-HIV status was strongly associated with significantly greater deterioration in LAZ and WLZ scores, beginning at about 4 months of age. Episodes of diarrhea or respiratory infections were related to significantly lower WLZ but not LAZ scores, independent of infant-HIV status. In conclusion, maternal schooling, immunological status and infant infections are important predictors of early growth in children born to HIV-positive women.
Keywords: HIV, growth, infants, morbidity, Tanzania, LAZ scores, WLZ scores
Introduction
HIV infection affects an estimated 2.3 million children worldwide, the vast majority of whom (90%) live in sub-Saharan Africa (SSA) [1]. Pediatric HIV infection is associated with linear and ponderal growth retardation [2] and, in SSA, HIV is likely to be a significant contributor to the burden of child malnutrition, morbidity and mortality [3–6].
A number of longitudinal studies have documented the impact of maternally acquired HIV infection on infant growth retardation in SSA [7–10]. However, few studies have quantified the independent contribution of other maternal and infant factors to growth in infants exposed to HIV. Furthermore, it is unclear to what extent the timing of infant HIV infection affects subsequent growth, since most studies have assessed infant-HIV status at the end of the growth period of interest.
Using data from a cohort of infants born to mothers living with HIV in Tanzania, we examined the effect of time-independent maternal and infant factors and time-dependent infant morbidities including HIV infection, diarrhea and respiratory illness on infant growth during the first 2 years.
Methods
Study design and population
From 1995 to 1997, 1078 HIV-infected pregnant women between 12 and 27 weeks gestation who attended four prenatal clinics in Dar es Salaam, Tanzania were enrolled in a randomized controlled trial (RCT) examining the effects of vitamin supplementation on pregnancy outcomes, mother-to-child transmission of HIV, and other mother and infant health and survival endpoints. Detailed descriptions of the trial design were published previously [11, 12]. Briefly, participating women were randomized in a two-by-two factorial design to 1 of 4 groups to receive, from the time of enrollment and after delivery, a daily oral dose of (i) multivitamins (B-complex, C and E), (ii) vitamin A and β-carotene, (iii) multivitamins including vitamin A and β-carotene, or (iv) placebo. All women received the standard of antenatal care prevalent at the time. At enrollment, nurses collected data from the women on age, education, socioeconomic and marital status, obstetric history and anthropometry. Study physicians performed a complete medical examination and collected blood specimens. Information on the risks and benefits of infant feeding options among HIV-infected women was provided according to World Health Organization (WHO) and Tanzanian Ministry of Health guidelines; virtually all of the women (>99%) chose to breastfeed.
Participating women were encouraged to give birth at the study clinic in Muhimbili National Hospital. At delivery, a research midwife measured the newborn's recumbent length to the nearest 0.1 cm with an infant length board and weight to the nearest 0.1 kg on calibrated balance-beam scales (model 725; Seca, Hamburg, Germany) with the use of standard techniques [13].
Infants and their mothers were followed monthly at the study clinic. At each scheduled visit, physical examinations were conducted by study physicians and trained research nurses measured the infants’ recumbent length and weight. Mothers were queried regarding the number of days, if any, during the previous month the child presented with respiratory signs or diarrhea, defined as ≥3 watery stools in the prior 24 h, and whether blood and/or mucus was present in stools. Respiratory signs included fever, cough, difficulty breathing, chest retractions and difficulties with eating, drinking or breast-feeding. Respiratory rate was measured using a stopwatch on the day of the monthly visit; rapid respiratory rate (RRR) was defined as ≥50 breaths per minute, for infants, and ≥40 breaths per minute, for children aged >1 year.
Morbidity classifications
Incident episodes of respiratory illness were classified in 3 ways: ‘cough plus fever’; ‘cough plus other’ defined as cough in combination with difficult breathing, chest retractions, or refusal to eat, drink or breast-feed; or ‘cough plus RRR’ defined as cough with RRR on the day of clinic visit. Incident episodes of diarrhea were defined as ‘acute’ which included all periods with ≥1 day but <14 days of diarrhea or ‘persistent’ defined as lasting ≥14 days. Acute diarrhea was further classified as ‘dysentery’ if mucus and/or blood were present or as ‘watery’ if mucus and blood were absent.
Details on the assessment of infant-HIV status are published elsewhere [14, 15]. Briefly, blood samples were collected from the infants at birth, 6 weeks, and every 3 months thereafter. HIV infection was defined as a positive result from a polymerase chain reaction test at any age or a positive enzyme-linked immunosorbent assay result, confirmed by a western blot test, in children aged ≥18 months. The time of transmission was estimated as the midpoint between the last negative and the first positive samples.
At 6 months of age and every 6 months thereafter, all children received an oral dose of vitamin A (200 000 IU, or 100 000 IU if <1 year of age) per the Tanzanian standard of pediatric care. Anti-retroviral medications were unavailable in this setting at the time of the study. The study protocol was approved by the Research and Publications Committee of Muhimbili University College of Health Sciences, the Ethical Committee of the National AIDS Control Program of the Tanzanian Ministry of Health and the Human Subjects Committee of the Harvard School of Public Health.
Statistical analyses
Length-for-age (LAZ) and weight-for-length (WLZ) z-scores were calculated from the WHO/National Center for Health Statistics reference using EPI INFO 6.0 software (Centers for Disease Control and Prevention, Atlanta, GA). To examine associations between baseline maternal or child covariates and growth indicators, we estimated average LAZ and WLZ curves for each category of predictors using mixed-effects models (PROC MIXED; SAS Institute Inc., Cary, NC) with restricted cubic splines [16]; knots were placed at ages 1, 2, 3, 6, 9, 12, 15, 18 and 22 months. Models included the predictors of interest, linear and spline terms for infant age in months, and interaction terms between the predictors and the age variables. Random effects for the intercept and the linear term for age (slope) were included to account for the within-person correlation of measurements in the estimation of the variance [17]. We estimated differences in z-scores from 0 to 24 months between categories of each predictor from the spline models. Models were adjusted for those characteristics that were significantly associated with growth outcomes in univariate analyses (p < 0.05) or were considered mechanistically relevant, including the vitamin-regimen assignment of the mother. Confidence intervals (CIs) around the estimates of association were constructed using robust estimates of the variance [18].
We analyzed associations between infant diarrhea or respiratory morbidity and LAZ or WLZ, using generalized estimating equations in which we specified a normal distribution with the identity link and an exchangeable correlation structure. Exposure status in the models (incident episode of morbidity) was updated prior to the monthly anthropometric measurements. The effect of HIV infection as a time-varying exposure was examined by building adjusted LAZ or WLZ curves from spline models, where the HIV status of each infant was updated each month prior to the anthropometry assessment. Estimates of association were obtained at 6, 12 and 24 months as the adjusted differences in the first derivatives of the HIV-specific growth curves at those time points. We tested for interactions between HIV status and diarrhea or respiratory morbidities with the use of the likelihood ratio test. All analyses were conducted using Statistical Analysis Software (SAS) version 9.
Results
Of the 1078 women enrolled in the RCT of vitamin supplementation, we excluded 3 who were nonpregnant, 6 who died before delivery and 42 who had an unknown date of delivery and/or pregnancy outcome. For the analyses of growth endpoints, we included 886 singletons born alive who had at least one set of anthropometric measurements taken at or after delivery and at ≤24 months of age. Baseline characteristics in this subset did not differ significantly from the original group of participants. The mothers of the infants included in this study were on average ± SD 24.8 ± 4.8 years of age and enrolled at 20.3 ± 3.1 weeks of gestation. Approximately 20% were above Stage 1 of the WHO classification for HIV disease and 12% had CD4 counts <200 cells mm−3 at the time of enrollment (Table 1). The incidence of preterm birth (<37 weeks) was 24%, whereas 11% of the infants weighed <2500 g at birth. Infants contributed a total 13 434 months to follow-up, with a median 21.0 months per infant (interquartile range [IQR] = 4.3, 23.7). Two-hundred and eight infants died prior to 24 months of age. HIV status was available on 852 infants of whom 29% had become HIV infected by 24 months.
Table 1.
Attained LAZ scores of 886 infants born to HIV-infected Tanzanian mothers by maternal and infant characteristics
| N (%) | LAZ scores |
||||
|---|---|---|---|---|---|
| 0 montha | 24 monthsa | Change 0–24 monthsa | Adjusted difference in change 0–24 months [95% CI]b | ||
| Mother's age (years) | |||||
| <20 | 105 (12.0) | − 0.6 ± 0.1 | −2.1 ± 0.2 | −1.5 ± 0.1 | Reference |
| 20–24 | 359 (40.5) | −0.4 ± 0.1 | −2.0 ± 0.1 | −1.5 ± 0.1 | 0.0 [−0.4, 0.5] |
| 25–29 | 275 (31.0) | −0.4 ± 0.1 | −1.9 ± 0.1 | −1.5 ± 0.1 | 0.1 [−0.4, 0.5] |
| ≥30 | 147 (16.6) | −0.5 ± 0.1 | −1.8 ± 0.1 | −1.3 ± 0.1 | 0.3 [−0.2, 0.7]c |
| Mother's level of schooling (years) | |||||
| None | 70 (7.9) | −0.4 ± 0.1 | −2.3 ± 0.2 | −1.9 ± 0.2 | Reference |
| <5 | 44 (5.0) | −0.7 ± 0.2 | −2.3 ± 0.2 | −1.5 ± 0.3 | 0.4 [−0.3, 1.1] |
| 5–9 | 679 (76.6) | −0.4 ± 0.0 | −2.0 ± 0.1 | −1.5 ± 0.1 | 0.3 [−0.1, 0.8] |
| >9 | 93 (10.5) | −0.4 ± 0.1 | −1.5 ± 0.2 | −1.1 ± 0.2 | 0.9 [0.3, 1.4]d |
| Mother is primiparous | |||||
| No | 581 (67.0) | −0.4 ± 0.0 | −1.9 ± 0.1 | −1.5 ± 0.1 | Reference |
| Yes | 286 (33.0) | −0.4 ± 0.1 | −2.0 ± 0.1 | −1.6 ± 0.1 | −0.1 [−0.3, 0.2] |
| Mother's height (cm) | |||||
| ≥150 | 798 (90.1) | −0.4 ± 0.0 | −1.9 ± 0.1 | −1.4 ± 0.1 | Reference |
| <150 | 88 (9.9) | −0.9 ± 0.1 | −2.5 ± 0.2 | −1.6 ± 0.2 | −0.3 [−0.6, 0.1] |
| Mother's CD4 counts at first prenatal visit (cells mm −3) | |||||
| <200 | 103 (12.4) | −0.6 ± 0.1 | −2.5 ± 0.2 | −2.0 ± 0.2 | Reference |
| 200 – 500 | 483 (57.9) | −0.4 ± 0.1 | −1.9 ± 0.1 | −1.5 ± 0.1 | 0.5 [0.1, 1.0] |
| >500 | 248 (29.7) | −0.4 ± 0.1 | −1.9 ± 0.1 | −1.5 ± 0.1 | 0.5 [0.0, 1.0]e |
| Infant sex | |||||
| Male | 450 (50.9) | −0.4 ± 0.1 | −1.6 ± 0.2 | −1.3 ± 0.2 | Reference |
| Female | 434 (49.1) | −0.4 ± 0.1 | −1.9 ± 0.1 | −1.4 ± 0.1 | −0.2 [−0.4, 0.0] |
| Gestational age at birth (week) | |||||
| ≥37 | 674 (76.1) | −0.3 ± 0.0 | −1.9 ± 0.1 | −1.6 ± 0.1 | Reference |
| <37 | 212 (23.9) | −1.1 ± 0.1 | −2.1 ± 0.1 | −1.1 ± 0.1 | 0.6 [0.3, 0.9] |
| Infant birth weight (g) | |||||
| ≥2500 | 732 (88.9) | −0.3 ± 0.0 | −1.9 ± 0.1 | −1.6 ± 0.1 | Reference |
| <2500 | 91 (11.1) | −2.1 ± 0.2 | −2.6 ± 0.2 | −0.5 ± 0.2 | 1.1 [0.6, 1.5] |
aUnadjusted mean values and change ± SE were estimated from restricted cubic splines regression models.
bFrom restricted cubic splines regression models adjusted for education, maternal age, primiparity, height, and CD4 cell counts of the mother at baseline, and infant sex, age, low-birth weight, and HIV status; 95% CI were calculated using robust variance estimates.
cp for trend = 0.01.
dp for trend = 0.22.
ep for trend = 0.19.
Mean infant LAZ scores decreased by 1.38 units (95% CI = 1.26, 1.51) from birth to 24 months. Infants born to mothers with >9 years of schooling, older mothers or mothers with CD4 counts ≥200 cells mm−3 exhibited significantly less deterioration in LAZ scores compared to their respective reference groups (all p < 0.01; Table 1). Infants born low-birth weight or preterm had significantly lower LAZ scores for the entirety of the follow-up, but the decreases in LAZ scores over time were significantly less than those observed in their reference counterparts.
WLZ scores increased significantly from birth to 4 months (1.15 Z [1.03, 1.27]), but decreased significantly from 4 months to 24 months (−1.06 Z [−1.12, −0.95]). The overall change in WLZ from birth to 24 months was 0.09 Z [−0.05, 0.23]. A statistically significant trend was observed between maternal schooling and WLZ score change (Table 2). Primiparity was associated with positive changes in WLZ scores, while older maternal age was associated with greater deterioration in infant WLZ scores. As with LAZ, WLZ scores were lower among infants born preterm or low-birth weight; however, scores deteriorated to a lesser extent among these infants over the 24 months compared to their reference groups.
Table 2.
Attained WLZ scores of 886 infants born to HIV-infected Tanzanian mothers by maternal and infant characteristics
| N (%) | WLZ scores |
||||
|---|---|---|---|---|---|
| 0 montha | 24 monthsa | Change 0–24 monthsa | Adjusted difference in change 0–24 months [95% CI]b | ||
| Mother's age (years) | |||||
| <20 | 105 (12.0) | −1.2 ± 0.1 | −0.8 ± 0.2 | 0.4 ± 0.2 | Reference |
| 20–24 | 359 (40.5) | −0.7 ± 0.1 | −0.8 ± 0.1 | −0.1 ± 0.1 | −0.5 [−1.0, 0.0] |
| 25–29 | 275 (31.0) | −0.6 ± 0.1 | −0.6 ± 0.1 | 0.0 ± 0.1 | −0.4 [−0.9, 0.1] |
| ≥30 | 147 (16.6) | −0.6 ± 0.1 | −0.9 ± 0.1 | −0.2 ± 0.2 | −0.7 [−1.2, −0.1]c |
| Mother's level of schooling (years) | |||||
| None | 70 (7.9) | −1.3 ± 0.1 | −1.1 ± 0.3 | 0.2 ± 0.3 | Reference |
| <5 | 44 (5.0) | −0.8 ± 0.2 | −1.5 ± 0.4 | −0.6 ± 0.5 | −0.9 [−1.8, −0.1] |
| 5–9 | 679 (76.6) | −0.7 ± 0.1 | −0.7 ± 0.2 | −0.1 ± 0.2 | −0.3 [−0.9, 0.3] |
| >9 | 93 (10.5) | −0.7 ± 0.1 | −0.3 ± 0.2 | 0.4 ± 0.3 | 0.2 [−0.3, 0.7]d |
| Mother is primiparous | |||||
| No | 581 (67.0) | −0.6 ± 0.1 | −0.7 ± 0.1 | −0.1 ± 0.1 | Reference |
| Yes | 286 (33.0) | −1.0 ± 0.1 | −0.7 ± 0.1 | 0.2 ± 0.1 | 0.4 [0.1, 0.7] |
| Mother's height (cm) | |||||
| ≥150 | 798 (90.1) | −0.7 ± 0.0 | −0.7 ± 0.1 | −0.0 ± 0.1 | Reference |
| <150 | 88 (9.9) | −1.0 ± 0.2 | −0.9 ± 0.1 | 0.1 ± 0.2 | 0.1 [−0.2, 0.5] |
| Mother's CD4 counts at first prenatal visit (cells mm−3) | |||||
| <200 | 103 (12.4) | −0.8 ± 0.1 | −1.0 ± 0.2 | −0.1 ± 0.2 | Reference |
| 200–500 | 483 (57.9) | −0.7 ± 0.1 | −0.7 ± 0.1 | 0.0 ± 0.1 | −0.1 [−0.5, 0.4] |
| >500 | 248 (29.7) | −0.7 ± 0.1 | −0.7 ± 0.1 | −0.0 ± 0.1 | −0.1 [−0.6, 0.4]e |
| Infant sex | |||||
| Male | 450 (50.9) | −0.5 ± 0.1 | −0.8 ± 0.2 | −0.3 ± 0.2 | Reference |
| Female | 434 (49.1) | −0.6 ± 0.1 | −0.8 ± 0.1 | −0.1 ± 0.1 | 0.2 [−0.1, 0.5] |
| Gestational age at birth (week) | |||||
| ≥37 | 674 (76.1) | −0.6 ± 0.1 | −0.7 ± 0.1 | −0.1 ± 0.1 | Reference |
| <37 | 212 (23.9) | −1.1 ± 0.1 | −0.8 ± 0.1 | 0.3 ± 0.2 | 0.4 [0.0, 0.7] |
| Infant birthweight (g) | |||||
| ≥2500 | 732 (88.9) | −0.6 ± 0.0 | −0.7 ± 0.1 | 0.0 ± 0.1 | Reference |
| <2500 | 91 (11.1) | −2.7 ± 0.2 | −1.2 ± 0.2 | 1.5 ± 0.3 | 1.6 [0.9, 2.2] |
aUnadjusted mean values and change ± SE were estimated from restricted cubic splines models.
bFrom restricted cubic splines regression models adjusted for education, maternal age, primiparity, height, and CD4 cell counts of the mother at baseline, and infant sex, age, low-birth weight and HIV status; 95% CI were calculated using robust variance estimates.
c p for trend <0.001.
dp for trend = 0.04.
ep for trend = 0.44.
Infants who became HIV positive during follow-up exhibited significant deterioration in both LAZ and WLZ scores beginning at ∼4 months postpartum (Fig. 1). The adjusted mean differences in attained LAZ scores between those infants who were HIV positive and those who were HIV negative at 6, 12 and 24 months were −0.23 ([−0.39, −0.09] p = 0.003), −0.38 ([−0.52, −0.24] p < 0.001) and −0.63 ([−0.84, −0.41] p < 0.001), respectively. The corresponding adjusted mean differences in WLZ scores were −0.38 ([−0.56, −0.20] p < 0.001), −0.35 ([−0.55, −0.16] p < 0.001) and −0.69 ([−0.95, −0.42] p < 0.001).
Fig. 1.
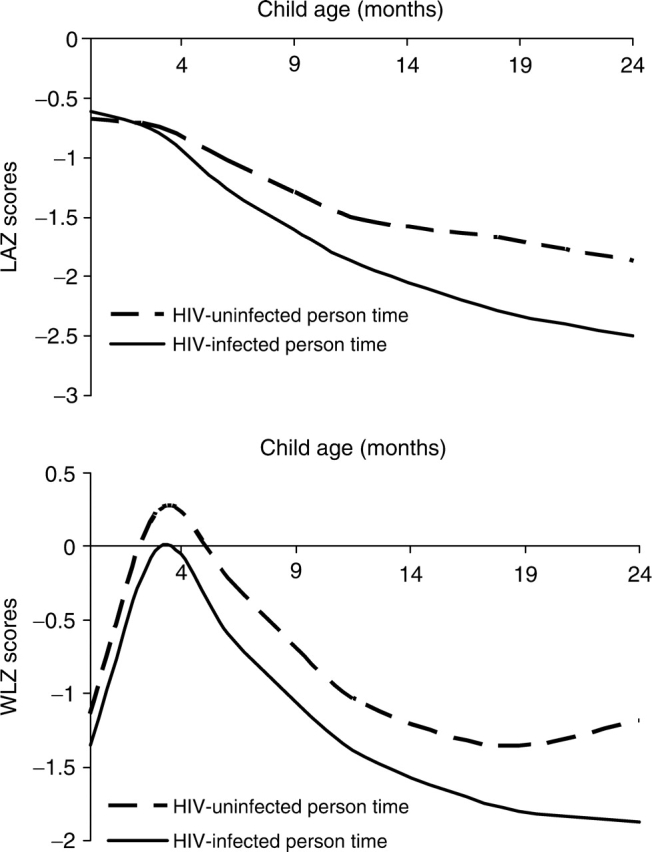
Infant growth by HIV status in the first 2 years of life. Adjusted LAZ and WLZ scores by child HIV-uninfected and infected person-time for the first 2 years of life were obtained from restricted cubic splines regression models adjusted for education, age, primiparity, height and CD4+ cell counts of the mother at baseline, infant sex, and birth weight. The adjusted mean differences in attained LAZ scores between those infants who were HIV positive and those who were HIV negative at 6, 12 and 24 months were −0.23 ([−0.39, −0.09] p = 0.003), −0.38 ([−0.52, −0.24] p < 0.001) and −0.63 ([−0.84, −0.41] p < 0.001), respectively. The corresponding adjusted mean differences in WLZ scores were −0.38 ([−0.56, −0.20] p < 0.001), −0.35 ([−0.55, −0.16] p < 0.001) and −0.69 ([−0.95, −0.42] p < 0.001).
Diarrhea or respiratory morbidities in the preceding month were not associated with subsequent differences in LAZ scores (Table 3). Infants who experienced acute diarrhea, watery diarrhea, dysentery, cough plus fever or cough plus other had WLZ scores that were ∼0.2 units lower than those of infants who did not experience these episodes (p < 0.05). Interactions between HIV status and diarrhea or respiratory illness were not statistically significant (p for interactions all >0.05).
Table 3.
Adjusted difference in LAZ and WLZ scores of Tanzanian children born to HIV-infected mothers by the incidence of infant morbidity episodes in the month preceding anthropometric assessmenta
| Episodesb | N child-months | LAZ scores ± SD | Adjusted difference in LAZ scores [95% CI] | WLZ scores ± SD | Adjusted difference in WLZ scores [95% CI] |
|---|---|---|---|---|---|
| Acute diarrhea | |||||
| No | 8082 | − 1.2 ± 1.2 | 0.0 | −0.2 ± 1.2 | Reference |
| Yes | 999 | −1.4 ± 1.2 | 0.0 [−0.1, 0.0] | −0.5 ± 1.2 | −0.2 [− 0.3, −0.2] |
| Persistent diarrhea | |||||
| No | 9036 | −1.2 ± 1.2 | 0.0 | −0.3 ± 1.2 | Reference |
| Yes | 45 | −1.2 ± 1.3 | 0.0 [−0.3, 0.2] | −0.5 ± 1.3 | −0.2 [−0.5, 0.1] |
| Watery diarrhea | |||||
| No | 8082 | −1.2 ± 1.2 | 0.0 | −0.2 ± 1.2 | Reference |
| Yes | 999 | −1.4 ± 1.1 | −0.1 [−0.2, 0.0] | −0.6 ± 1.2 | −0.2 [−0.3, −0.1] |
| Dysentery | |||||
| No | 8508 | −1.2 ± 1.2 | 0.0 | −0.2 ± 1.2 | Reference |
| Yes | 573 | −1.3 ± 1.1 | 0.0 [−0.1, 0.0] | −0.5 ± 1.2 | −0.2 [−0.3, −0.2] |
| Cough + RRR | |||||
| No | 8979 | −1.2 ± 1.2 | 0.0 | −0.3 ± 1.2 | Reference |
| Yes | 102 | −1.3 ± 1.1 | 0.1 [−0.1, 0.2] | −0.5 ± 1.3 | −0.2 [−0.3, 0.0] |
| Cough + fever | |||||
| No | 8021 | −1.2 ± 1.2 | 0.0 | −0.2 ± 1.2 | Reference |
| Yes | 1060 | −1.4 ± 1.2 | 0.0 [−0.1, 0.0] | −0.6 ± 1.2 | −0.2 [−0.3, −0.1] |
| Cough + otherc | |||||
| No | 8650 | −1.2 ± 1.1 | 0.0 | −0.2 ± 1.2 | Reference |
| Yes | 431 | −1.5 ± 1.2 | 0.0 [−0.7, 0.7] | −0.7 ± 1.3 | −0.2 [−0.3, −0.2] |
aAdjusted differences and robust SE were calculated from generalized estimating equations with LAZ or WLZ scores as the outcomes and covariates that included each episode plus maternal education, age, height, CD4 counts at baseline, parity, and infant low-birth weight, sex, age, and HIV status as a time-varying covariate.
bThe occurrence of at least one episode was assessed during the month prior to each anthropometric measurement.
cCough + other was defined as cough with at least one of the following events: difficulty breathing, chest retractions and refusal to eat, drink, or breastfeed.
Discussion
The objective of our study was to examine maternal and infant predictors of growth among infants born to mothers living with HIV. Infant-HIV status was independently associated with deficits in LAZ and WLZ scores, while infant morbidities such as diarrhea or respiratory illnesses independently reduced subsequent WLZ but not LAZ scores. Infants whose mothers had greater schooling, were primiparous, or had higher CD4 counts at baseline exhibited significantly less growth faltering, independent of infant HIV infection. Low-birth weight and preterm babies, despite slower deterioration in LAZ and WLZ scores, were significantly more stunted and wasted than their normal weight or normal term counterparts for the duration of the follow-up.
A recent review by Arpadi [2] estimated that ∼50% of HIV-infected children experience abnormal growth patterns. A limitation of previous studies in SSA has been the use of a time-independent measure of HIV status as predictor of growth. It is possible that infants classified as HIV positive in these studies contributed disease-free time to growth estimates, potentially leading to an underestimation of the effect of HIV on growth faltering. In our study, infant-HIV status was assessed at birth, 6 weeks postpartum and every 3 months thereafter enabling us to update exposure to HIV prior to the anthropometric assessments. We noted that infant HIV infection was associated with significant reductions in both WLZ and LAZ scores beginning at ∼4 months postpartum. This finding supports the active and early follow-up of infants born to HIV-infected mothers so that a timely diagnosis and provision of antiretroviral therapy (ART) and nutrition support can be implemented if needed, to ameliorate HIV-associated growth faltering.
It has been hypothesized that the effects of respiratory and diarrhea morbidity on growth faltering may be more severe or more common in HIV-infected children [5, 10]. In our study, the effects of diarrhea and respiratory morbidity on growth outcomes were independent of the infant's-HIV status, and interactions between diarrhea or respiratory infections and HIV were not significant. Our statistical power, however, may have been limited to detect small underlying differences. The lack of association with linear growth could be attributed in part to the relatively short latency period between assessment of the infections and assessment of length. A previous study in this setting suggested that the negative effect of watery diarrhea on linear growth in infants was cumulative over extended periods [10].
Low-birth weight infants and infants born preterm each had significantly lower LAZ and WLZ scores for the duration of follow-up independent of HIV status. Unexpectedly, growth deterioration was slower in these infants when compared to their normal weight or normal term counterparts. It is plausible that infants born preterm or low-birth weight experienced incomplete catch-up growth. We also cannot eliminate the possibility that increased mortality among the most malnourished of these infants may have reduced their contribution to later anthropometric measures. It is unlikely that their less pronounced growth faltering resulted from more intensive follow-up because all participating infants were examined monthly.
Infants born to mothers with higher schooling or baseline CD4 counts ≥200 cells mm−3 exhibited less growth faltering compared to their reference counterparts. Maternal schooling is consistently a predictor of improved child growth in developing countries [19, 20]. Among HIV-infected mothers, those with greater schooling may possess greater knowledge regarding self-care, adherence to medical advice and health care seeking, each of which may serve to protect the health of the mother and subsequently the child. Indeed, greater schooling among HIV-infected women was associated with improved maternal health measures in Tanzania [21] and Zambia [22]. It has been hypothesized that poor maternal health during pregnancy may adversely program the infant immune system [23–25] thus increasing susceptibility to infectious disease. Furthermore mothers with low-CD4 counts may be too weak or ill to provide adequate care to their children after delivery [5, 26].
Study limitations
Our study examined data collected longitudinally during a randomized controlled trial; thus, it is limited in that it is exploratory in nature and did not include a comparison sample of infants born to HIV-uninfected mothers. Furthermore, it could be argued that in light of recent anti-retroviral therapy rollouts our findings may not be relevant to the current situation. However, by the end of 2006, <15% of eligible children infected with HIV in Africa were receiving ART [27]. We could not examine the impact of infant feeding practices on growth due in part to low variability in the exposure during the first 2 years and because we lacked adequate data on exclusive versus mixed breast feeding during the first 6 months postpartum. Lastly, although intensive-growth monitoring was conducted, potential measurement error of anthropometric variables, especially length, may have reduced the statistical power to detect significant associations.
In conclusion, maternal schooling and immunological status and infant HIV infection and other morbidities influence growth in infants born to HIV-infected mothers independent of infant HIV infection.
Acknowledgements
This study was funded by National Institutes of Health (NICHD R01 32257 to WWF and training grant T32DK07703 to the Department of Nutrition, Harvard School of Public Health for AW).
References
- 1.Geneva: UNAIDS and WHO; 2006. AIDS Epidemic Update: special report on HIV/AIDS (December 2006) [Google Scholar]
- 2.Arpadi SM. Durban, South Africa: World Health Organization; 2005. Growth failure in HIV-infected children. Consultation on Nutrition and HIV/AIDS in Africa: Evidence, lessons and recommendations for action. Department of Nutrition for Health and Development. [Google Scholar]
- 3.Bakaki P, Kayita J, Moura Machado JE, et al. Epidemiologic and clinical features of HIV-infected and HIV-uninfected Ugandan children younger than 18 months. J Acquir Immune Defic Syndr. 2001;28:35–42. doi: 10.1097/00042560-200109010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Taha TE, Dallabetta GA, Canner JK, et al. The effect of human immunodeficiency virus infection on birthweight, and infant and child mortality in urban Malawi. Int J Epidemiol. 1995;24:1022–9. doi: 10.1093/ije/24.5.1022. [DOI] [PubMed] [Google Scholar]
- 5.Thea DM, St. Louis ME, Atido U, et al. A prospective study of diarrhea and HIV-1 infection among 429 Zairian infants. N Engl J Med. 1993;329:1696–702. doi: 10.1056/NEJM199312023292304. [DOI] [PubMed] [Google Scholar]
- 6.Villamor E, Misegades L, Fataki MR, et al. Child mortality in relation to HIV infection, nutritional status, and socio-economic background. Int J Epidemiol. 2005;34:61–8. doi: 10.1093/ije/dyh378. [DOI] [PubMed] [Google Scholar]
- 7.Bailey RC, Kamenga MC, Nsuami MJ, et al. Growth of children according to maternal and child HIV, immunological and disease characteristics: a prospective cohort study in Kinshasa, Democratic Republic of Congo. Int J Epidemiol. 1999;28:532–40. doi: 10.1093/ije/28.3.532. [DOI] [PubMed] [Google Scholar]
- 8.Bobat R, Coovadia H, Moodley D, et al. Growth in early childhood in a cohort of children born to HIV-1-infected women from Durban, South Africa. Ann Trop Paediatr. 2001;21:203–10. doi: 10.1080/02724930120077772. [DOI] [PubMed] [Google Scholar]
- 9.Lepage P, Msellati P, Hitimana DG, et al. Growth of human immunodeficiency type 1-infected and uninfected children: a prospective cohort study in Kigali, Rwanda, 1988 to 1993. Pediatr Infect Dis J. 1996;15:479–85. doi: 10.1097/00006454-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Villamor E, Fataki MR, Bosch RJ, et al. Human immunodeficiency virus infection, diarrheal disease and sociodemographic predictors of child growth. Acta Paediatr. 2004;93:372–9. [PubMed] [Google Scholar]
- 11.Fawzi WW, Msamanga GI, Spiegelman D, et al. Rationale and design of the Tanzania vitamin and HIV infection trial. Control Clin Trials. 1999;20:75–90. doi: 10.1016/s0197-2456(98)00045-2. [DOI] [PubMed] [Google Scholar]
- 12.Villamor E, Saathoff E, Bosch RJ, et al. Vitamin supplementation of HIV-infected women improves postnatal child growth. Am J Clin Nutr. 2005;81:880–8. doi: 10.1093/ajcn/81.4.880. [DOI] [PubMed] [Google Scholar]
- 13.Lohman TG, Roche AF, Martorell RE. Anthropometric standardization reference manual. Champaigne, IL: Human Kinetics Books; 1988. [Google Scholar]
- 14.Fawzi WW, Msamanga G, Hunter D, et al. Randomized trial of vitamin supplements in relation to vertical transmission of HIV-1 in Tanzania. J Acquir Immune Defic Syndrom. 2000;23:246–54. doi: 10.1097/00126334-200003010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Fawzi WW, Msamanga GI, Hunter D, et al. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS. 2002;16:1935–44. doi: 10.1097/00002030-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 16.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–6. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 17.Diggle PJ, Heagarty P, Liang K, et al. Analysis of longitudinal data, 2nd edn. Oxford: Oxford University Press; 2002. [Google Scholar]
- 18.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–30. [Google Scholar]
- 19.Boyle MH, Racine Y, Georgiades K, et al. The influence of economic development level, household wealth and maternal education on child health in the developing world. Social Sci Med. 2006;63:2242–54. doi: 10.1016/j.socscimed.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Cochrane SH, Leslie J, O’Hara DJ. Parental education and child health: intracountry evidence. Health Policy Educ. 1982;2:213–50. doi: 10.1016/0165-2281(82)90011-x. [DOI] [PubMed] [Google Scholar]
- 21.Villamor E, Msamanga G, Spiegelman D, et al. HIV status and sociodemographic correlates of maternal body size and wasting during pregnancy. Eur J Clin Nutr. 2002;56:415–24. doi: 10.1038/sj.ejcn.1601328. [DOI] [PubMed] [Google Scholar]
- 22.Albrecht S, Semrau K, Kasonde P, et al. Predictors of nonadherence to single-dose nevirapine therapy for the prevention of mother-to-child HIV transmission. J Acquir Immune Defic Syndr. 2006;41:114–8. doi: 10.1097/01.qai.0000179425.27036.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chougnet C, Kovacs A, Baker R, et al. Influence of human immunodeficiency virus-infected maternal environment on development of infant interleukin-12 production. J Infect Dis. 2000;181:1590–7. doi: 10.1086/315458. [DOI] [PubMed] [Google Scholar]
- 24.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–71. [PubMed] [Google Scholar]
- 25.Nielsen SD, Jeppesen DL, Kolte L, et al. Impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased thymic output and low CD4 counts. Blood. 2001;98:398–404. doi: 10.1182/blood.v98.2.398. [DOI] [PubMed] [Google Scholar]
- 26.Chisenga M, Kasonka L, Makasa M, et al. Factors affecting the duration of exclusive breastfeeding among HIV-infected and -uninfected women in Lusaka, Zambia. J Hum Lact. 2005;21:266–75. doi: 10.1177/0890334405279251. [DOI] [PubMed] [Google Scholar]
- 27.Crowley S. Washington D.C: WHO; 2007. Health Care for Children Affected by HIV. IATT on Children and HIV and AIDS, April 2007. [Google Scholar]


