Summary
Cortical motor areas are thought to contribute “higher order processing” but what that processing might include is unknown. Previous studies of the smooth pursuit-related discharge of supplementary eye field (SEF) neurons did not distinguish activity associated with the preparation for pursuit from discharge related to processing or memory of the target motion signals. Using a new, memory-based task, which was designed to separate these components, we show that the SEF contains signals coding retinal image-slip-velocity, memory and assessment of visual motion-direction, the decision of whether or not to pursue, and the preparation for pursuit eye movements. Bilateral muscimol injection into SEF resulted in directional errors in smooth pursuit, errors of whether or not to pursue, and impairment of initial correct eye movements. These results suggest an important role for the SEF in memory and assessment of visual motion-direction and the programming of appropriate pursuit eye movements.
Keywords: Frontal cortex, Supplementary eye fields, Smooth pursuit, Visual motion, Memory, Decision-making, Movement preparation, Monkey
Introduction
Motor-related, cortical areas have long been thought to contribute more to movements than simple commands. These “higher-order” processes have been suggested to include memory, prediction, timing, abstraction or targets, etc. but specific demonstrations of how these processes are manifest during specific movements has been lacking generally. Smooth pursuit eye movements are a well-defined, model motor system for the study of some of these higher-order processes because we know a great deal about the goal of the movements and the signals and areas involved.
Smooth pursuit eye movements allow us to see well in everyday life, by assuring accurate visual information about moving objects. They do this by keeping the image stable on the fovea (i.e., the high acuity portion of the retina) in response to visual information about the velocity of the slip of objects' images on the retina. To maintain images on the foveae during movement, prediction is used to compensate for the delays involved in processing visual motion information and/or eye velocity commands. The pursuit system is quite efficient at prediction (e.g., Becker and Fuchs 1985) but the neural mechanisms of prediction are still not well understood. They may use memory of visual motion (e.g., Assad and Maunsell 1995; Bisley et al. 2004), however, it is unknown where the memory of visual motion for predictive smooth pursuit is stored (e.g., Collins and Barnes 2005).
Prediction-related neuronal discharge during smooth pursuit has been reported in the supplementary eye fields (SEF) in the dorsomedial frontal cortex (Heinen 1995; Heinen and Liu 1997; de Hemptinne et al. 2008; also Kim et al. 2005). However, in those studies, discharge related to preparation for pursuit eye movements could not be separated from discharge related to processing of target motion signals or their memory. Although the SEF contains smooth pursuit-related neurons that discharge during pursuit (Schall 1991; Heinen 1995; Heinen and Liu 1997), their role in pursuit eye movements is not well understood for the following reasons; 1) electrical microstimulation of the SEF does not induce smooth pursuit eye movements, although it facilitates smooth pursuit initiation and enhances anticipatory pursuit eye velocity (Missal and Heinen 2001, 2004). This is in contrast to the effect of electrical microstimulation on the saccadic system, which induces saccadic eye movements (e.g., Schlag and Schlag-Rey 1987). 2) Over half of pursuit-related SEF neurons do not signal eye velocity during pursuit (Fukushima et al. 2004), and 3) SEF lesions have minimal effects on pursuit eye movements (see a review by Tehovnik et al. 2000). It has been suggested that SEF is involved in the process of guiding anticipatory pursuit (see Leigh and Zee 2006 for a review).
Using a new, memory-based smooth pursuit task that was designed to permit a dissection of neuronal responses into components associated with memory of visual motion-direction, the decision-making process of whether or not to pursue moving spots, and preparation for and execution of pursuit eye movements, in the present study we show that the SEF contains various signals reflecting each of these components. Muscimol injection into the bilateral SEF resulted in impairment of correct pursuit eye movements, suggesting an important role of the SEF in appropriate execution of smooth pursuit eye movements.
Results
As illustrated schematically in Fig. 1A (see Methods for details), our task used random-dot patterns as the cue for action. After the initial fixation, cue 1 was presented for 0.5 s at 10 °/s. It consisted of a moving random-dot-pattern for which the monkeys were required to remember both its color and the direction of visual motion. After a delay (Fig. 1A, delay 1), cue 2 was presented and consisted of a stationary random-dot-pattern. If the color of cue 2 was the same as that of cue 1 (go signal), the animal was required to prepare to pursue a spot that would move in the direction instructed by cue 1. If the color of cue 2 was different from the cue 1 color (i.e., no-go signal), the animal was required not to pursue but to maintain fixation of the stationary fixation spot. After another delay (Fig. 1A, delay 2), monkeys were required to execute the correct action by selecting one of 3 spots (one fixed at the center and two moving away in opposite directions) based on the memory of cue 1 and the instructions of cue 2 (Fig. 1A, action). Thus, this oculomotor task separated behavioral periods that required memory of visual motion-direction (delay 1), decision-making (cue 2, go or no-go), and preparation and execution for pursuit (go, delay 2, action) or maintaining fixation (no-go, delay 2 and action).
Fig. 1.
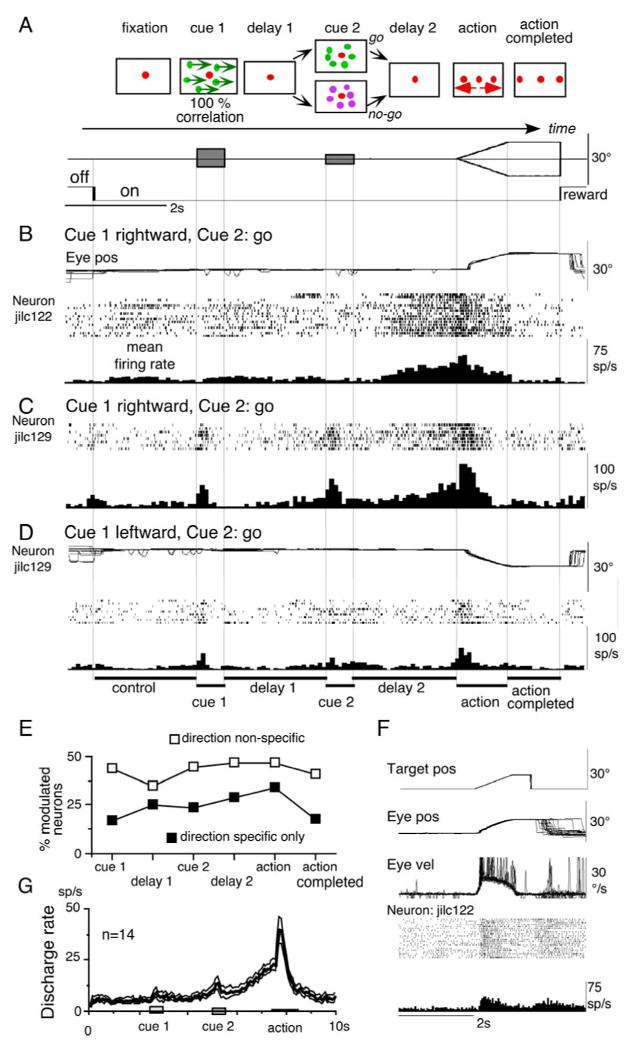
Task conditions and discharge of SEF neurons. A, task conditions. B-D, spike rasters and averaged histograms of 2 SEF neurons during go trials as indicated. Top traces in B and D are superimposed eye position (eye pos). E, percentage of modulated neurons that preferred go trials during different task periods. F, discharge of the same neuron shown in B during a simple ramp-tracking task of a single spot. Saccade velocities (eye vel) are clipped. G, mean (±SE) discharge of movement preparation neurons. See text.
Early in their training (typically after 6-8 months of training), the two monkeys performed the final action using saccades with latencies typically 260-300 ms followed by smooth-pursuit eye movements (Fukushima et al. 2008). Later (typically after a year of training), saccade latency shortened typically to 220 ms. Moreover, preceding the saccades, smooth-pursuit eye movements appeared in the correct response direction, at latencies typically of 130-150 ms (e.g., Fig. 8E, thin line, pointed by arrow).
Fig. 8.
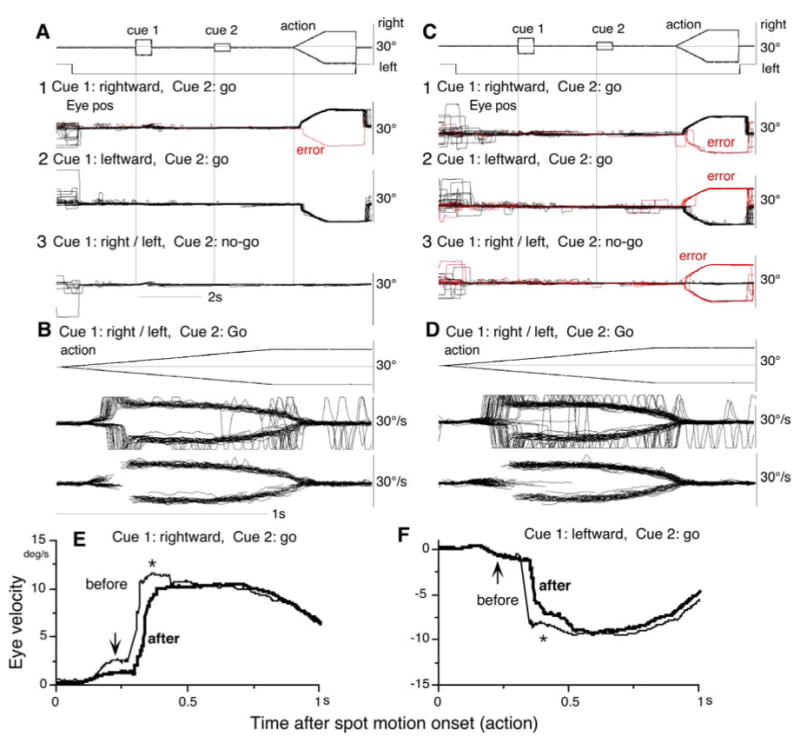
Inactivation of bilateral SEF. Eye position (A, C) and velocity (B, E, D, F) aligned at the onset of cue 1 before muscimol infusion (A, B) and after infusion (C, D). E and F compare de-saccaded and averaged eye velocity before (thin lines) and after (thick lines) infusion for rightward (E) and leftward pursuit (F) correct performance. De-saccaded portions were connected by straight lines. See text.
Discharge of task-related neurons in the SEF
We analyzed the activity of a total of 208 neurons in SEF of 2 monkeys that exhibited modulation during our task. Discharge characteristics of neurons in the 2 monkeys were similar. To assess during which period(s) of our task (Fig. 1A) SEF neurons were active, we measured mean discharge rates of individual neurons during the different periods, and compared the mean rate (±SD) for each period with the mean rate (±SD) during the initial fixation (Fig. 1A) for each neuron (e.g., Fig. 1B-D, control, see Methods). Of the 208, 158 neurons preferred go trials (see Data analysis). Figure 1B and C-D illustrate 2 example neurons recorded in the left SEF during go trials. Both showed clear discharge during the delay 2 and the action periods. Preferred directions for the discharge during delay 2 and action periods were rightward for both neurons (e.g., Fig. 1C vs D). In addition, the neuron shown in Fig. 1C exhibited a brief discharge at cue 1 and cue 2.
Figure 1E plots the percentage of modulated neurons during each period in go trials. Over half of the 158 neurons exhibited significant modulation (higher or lower than control) during every period from cue 1 to action. Modulated neurons during go trials included those that exhibited direction specific modulation (Fig. 1E, filled squares) and those that did not (direction non-specific, open squares). The great majority (>80 %) exhibited excitation as illustrated in Fig. 1 (B, C). In the following sections, we performed quantitative analyses of the excitatory responses.
Direction specific neurons active during the action period included pursuit-related neurons as identified by their discharge in a simple pursuit task (see Data analysis for details). Discharge of a representative neuron is shown in Fig. 1F where only a single spot was shown during delay 2 and action periods in Fig. 1A. This is the same neuron shown in Fig. 1B, but unlike the discharge in Fig. 1B, the discharge before the onset of pursuit eye movements was not observed during simple pursuit (Fig. 1F), suggesting that the discharge depended on a task that required movement preparation (e.g., Mann et al. 1988). Figure 1G plots mean (±SE) discharge rates of a group of SEF neurons (n=14) that exhibited directional responses during the action period on go trials in their preferred direction (duration of delay 1 and delay 2 was set for 2 s). These neurons also exhibited a directional response during delay 2 but not during delay 1, as example neurons show in Fig. 1B and C-D (see below).
Classification of direction- and instruction- specific neurons
Our monkeys were required to remember both the color and the direction of cue 1 visual motion and to associate them with the cue 2 instruction for the appropriate action (Fig. 1A). Because tested neurons responded similarly when the color of cue 1 was changed (see Methods, also Fukushima et al. 2008), the most important information during cue 1 for SEF neurons is the direction of visual motion. We searched for neurons that carried the direction- and instruction- specific information during delay 1 and delay 2. For neurons that showed such responses, we presented cue 1 visual motion either in the preferred direction or anti-preferred direction and cue 2 instruction was either go or no-go. Therefore, there are four possible combinations of neurons that showed direction- and instruction- specific responses during go-trials. We found all 4 groups of neurons in the SEF (Table 1, 1-4). Direction specific delay 1 activity was observed in 39 neurons and they were further divided into 2 groups based on whether their activity during delay 2 was directional and whether it was affected by the preparation of pursuit eye movement direction. The first group (14/39) did not have directional delay 2 activity, so it was not affected by preparation of pursuit eye movement direction. We call this group of neurons visual memory neurons (Table 1, 1) for the reasons as described below. The second group (25/39) showed directional delay 2 activity during preparation of pursuit eye movements. We call this group of neurons visual memory + movement preparation neurons (Table 1, 2). The third group (n=20, Table 1, 3) exhibited a direction specific response during delay 2 but not delay 1 (e.g., Fig. 1B, C, movement preparation neurons). Our task has also revealed a fourth group (50/208, Table 1, 4) that preferred no-go trials (no-go neurons).
Table 1.
Classification of direction- and instruction- specific SEF neurons.
| Go trials | No-go trials | |||
|---|---|---|---|---|
| Direction- instruction- specific neuron groups | delay 1 | delay 2 | delay 1 | delay 2 |
| 1. Visual memory | yes | no | yes | no |
| 2. Visual memory + movement preparation | yes | yes | yes | no |
| 3. Movement preparation | no | yes | no | no |
| 4. No-go | no | no | no | yes |
Visual memory neurons
This group of neurons exhibited direction specific discharge only during delay 1 in both go and no-go trials (n=14, Table 1, 1). About half of them (6/14) also showed directional activity during cue 1. Discharge of a representative neuron is shown in Fig. 2 (A, B). It exhibited clear discharge during cue 1 and the discharge was maintained during delay 1 when rightward (but not leftward) visual motion was presented at cue 1 during go trials and no-go trials (Fig. 2A1-2 vs B1-2). The continuation of this delay 1 discharge during the cue 2 period was not significantly affected by the cue 2 instruction that required the monkey to prepare for action (i.e., whether or not to pursue moving spots; go or no-go, Fig. 2A1 vs B1). Furthermore, the continuation of the delay 1 instruction was not significantly influenced by the monkey's preparation of pursuit direction. This is also seen when the monkey made an error (Fig. 2A1, red trace in eye pos); instead of performing rightward pursuit, the monkey performed leftward pursuit. Despite this error, discharge similar to that during correct trials was clearly observed during delay 1 (Fig. 2A1, red raster).
Fig. 2.
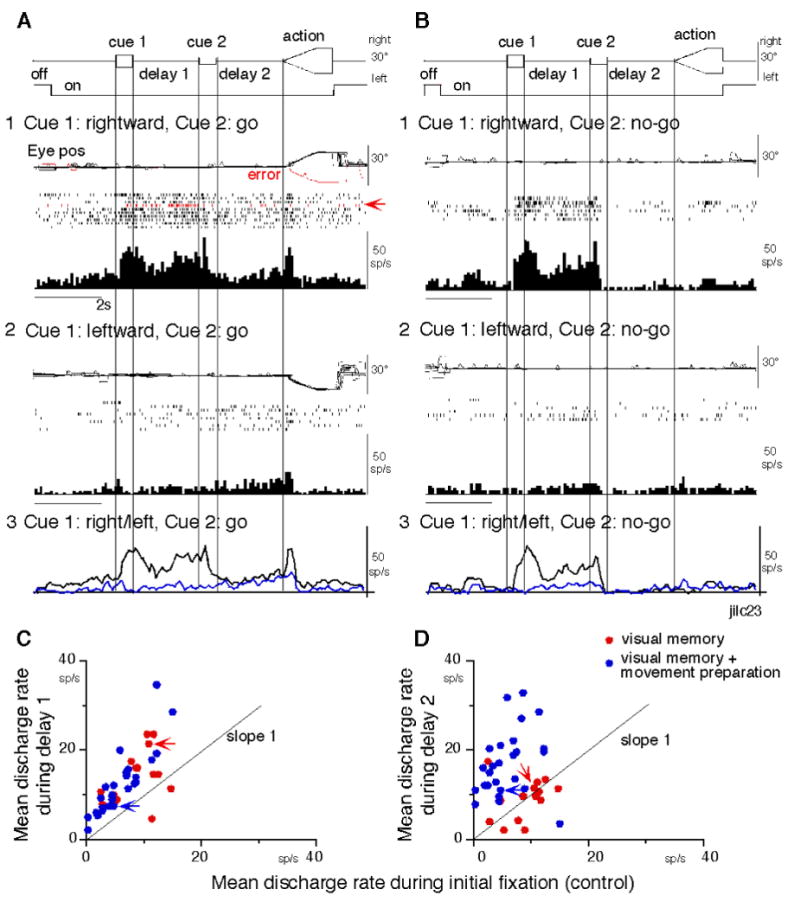
Discharge of representative visual memory neuron and comparison with visual memory + movement preparation neurons during delay periods. A1-2, rightward (A1) and leftward (A2) visual motion go trials. B1-2, rightward (B1) and leftward (B2) no-go trials. Red traces pos and spike raster in A1 highlight an error trial. A3 -B3 compare mean discharge rightward (black) / leftward (blue) for cue 1 visual motion go- no-go trials, respectively. C, mean discharge of visual memory neurons (red) and visual memory + movement preparation neurons (blue) during delay 1 against initial fixation. D, mean discharge of visual memory neurons (red) and visual memory + movement preparation neurons (blue) during delay 2 against initial fixation. In C and D, red arrows are mean rates of the neuron shown in A-B. Blue arrows are mean rates of the neuron shown in Fig. 3A.
The activity during delay 2 of go trials was not significantly affected by pursuit eye movement direction (Fig. 2A1 vs A2). This is also illustrated in Fig. 2A3, which shows similar mean discharge rates during delay 2 when the cue 1 instruction was rightward (black) or leftward (blue). However, this delay 2 activity was significantly higher than the delay 2 activity during no-go trials (Fig. 2B1-3, P< 0.05). These results suggest that the delay 1 activity of this neuron reflected memory of the visual motion-direction presented by cue 1 but that the delay 2 activity was unaffected by the preparation of pursuit eye movement direction, although it may have reflected “go” signals, the direction of which was not specified yet (i.e., direction non-specific, Fig. 1E, Table 1, see below).
Visual memory + movement preparation neurons
This group of neurons exhibited congruent directionality during delay 1 and during delay 2 in go-trials (n=25, Table 1, 2). Figure 3A shows activity of a representative neuron. It exhibited clear discharge during the late period of the delay 1 when leftward (but not rightward) visual motion was presented at cue 1 during go trials and no-go trials (Fig. 3A1 vs A2, A3 vs A4), and this delay 1 activity was basically similar during go- and no-go trials (Fig. 3A1 vs A3), like the discharge of visual memory neurons (Fig. 2A1 vs B1). In addition, when the cue 2 instructed “go” to prepare to pursue in the congruent direction (Fig. 3A1), this neuron exhibited robust discharge during the late period of delay 2 following a pause after cue 2 onset. This delay 2 activity suggests that it was related to preparation for leftward pursuit eye movements, because the discharge was not observed in association with rightward pursuit (Fig. 3A2 vs A1) or during no-go trials (A3-4 vs A1, Table 1).
Fig. 3.
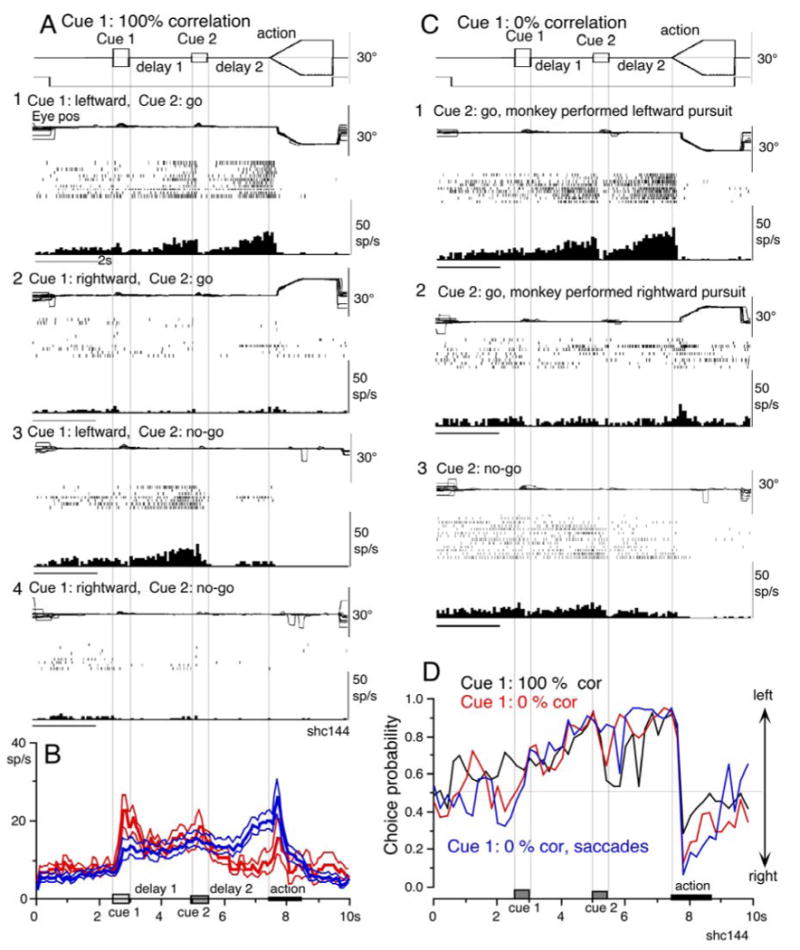
Representative visual memory + movement preparation neuron and time course of discharge modulation. A and C, cue 1 motion was 100 % and 0 % correlation, respectively. A1-2, go trials when cue 1 was leftward (A1) and rightward (A2) visual motion. A3-4, no-go trials, leftward (A3) and rightward (A4) as cue 1. B, time course of mean (±SE) discharge modulation of visual memory neurons (red, n=13) and visual memory + movement preparation neurons (blue, n=22) during go trials in their preferred directions. C1 and 2, go trials sorted into leftward (A1) and rightward pursuit (A2). C3, no-go trials. D, choice probability (CP) time course for go trials sorted on whether the monkey pursued towards left or right with 100 % (black) and 0 % correlation (red). Blue trace, CP time course for go trials for saccades when cue 1 was presented with 0 % correlation.
To compare mean discharge rate of visual memory neurons and visual memory +movement preparation neurons during delay periods, Fig. 2C and D plot mean discharge rate of individual neurons during delay 1 and delay 2 against mean rate during initial fixation. There was no significant difference in the distribution of the two groups during delay 1 (Fig. 2C, P>0.1), but discharge of most neurons is clearly greater than that during fixation (unity slope line). Likewise, the two groups showed a significant difference in distribution during delay 2 (Fig. 2D, P<0.001). We also calculated the average mean ratio of delay 2 discharge rate divided by average control fixation rate for all tested neurons of each group. The ratios for visual memory neurons and visual memory + movement preparation neurons were 1.1 and 2.9, respectively, indicating that the mean ratio was nearly 3 times larger for the latter neurons (Fig. 2D).
Figure 3B plots time course of mean (±SE) discharge rates of visual memory neurons (red, n=13) and visual memory + movement preparation neurons (blue, n=22) during go trials in their preferred directions when the duration of delay 1 and delay 2 was set at 2 s. The initial response to cue 1 was larger for visual memory neurons (Fig. 3B, red), but the 2 groups of neurons maintained similar discharge rates during the delay 1 and also during cue 2. This suggests that the delay 1 activity of the 2 groups of SEF neurons depended on the direction of visual motion presented by cue 1, but the activity was minimally affected by preparation for pursuit eye movement direction. During delay 2, the discharge of the 2 groups of neurons clearly diverged (Fig. 3B, red vs blue), suggesting that this divergence reflected preparation for pursuit eye movement direction instructed by the cue 2 for visual memory + movement preparation neurons (Fig. 3B, blue).
Correlation of delay 1 and delay 2 activity during go trials
Because the delay 1 activity of visual memory neurons (Fig. 3B, red) did not seem to reflect preparation for pursuit eye movement direction as stated above (Fig. 2A1-3), the congruent directionality in preferred directions in the two delay periods of visual memory + movement preparation neurons suggests the possibility that the delay 1 information about the direction of visual motion may have been used for further processing in the preparation of pursuit eye movement directions. To examine this possibility, we let the monkeys choose pursuit directions themselves and examined how visual memory + movement preparation neurons discharged during delay 1 and delay 2. To encourage the animals to make the direction choice themselves, we used the paradigm devised by Newsome and Pare (1988, 0 % correlation) that moved each dot randomly in different directions at cue 1 (see Methods). If the color of cue 2 was the same as cue 1, it instructed “go” and the monkey followed one of the 2 moving spots either towards the preferred or towards the opposite (i.e., anti-preferred) direction. If the color of cue 2 was different from that of cue 1, it instructed no-go.
During 0 % correlation, cue 1 does not provide the necessary information about the direction of visual motion (Newsome and Pare 1988). Our monkeys pursued one of the 2 moving spots randomly with nearly equal probability during the action period of these go trials. For example, in 216 go trials with 0 % correlation, the monkey performed rightward and leftward pursuit in 51 % (110/216) and 49 % (106/216) of the trials, respectively. We sorted eye and cell trials based on the monkeys' choice of either the preferred direction of delay 2 activity or the anti-preferred direction of the neuron (tested by 100 % correlation). Because visual memory + movement preparation neurons had discharge related to preparation for pursuit (see above), we predicted that delay 2 activity during 0 % correlation should be correlated with the pursuit preferred direction. Our question was whether delay 1 activity was correlated with delay 2 activity.
Figure 3C plots sorted trials during 0 % correlation for leftward pursuit (C1), rightward pursuit (C2) and no-go trials (C3) of the same neuron shown in Fig. 3A. As expected, when the monkey made leftward pursuit (i.e., in the preferred direction of this neuron tested by 100 % correlation), discharge modulation during the late period of delay 2 was much stronger compared to the trials where the monkey made rightward pursuit (Fig. 3C1 vs C2). This suggests that the delay 2 activity did indeed reflect preparation for pursuit eye movements. In addition, the stronger discharge during the delay 1 in the same trials (Fig. 3C1 vs C2) suggests that this discharge was also related to the monkey's choice and preparation for the subsequent pursuit eye movement direction independent of the cue 1 stimulus itself, which was non-directional during 0 % correlation.
To evaluate these results further, we calculated choice probability (CP, Britten et al. 1996) and its time course based on whether the monkey pursued in the preferred direction of the neuron (tested by 100 % correlation) or anti-preferred direction during go trials (see Data analysis). The results are plotted in Fig. 3D (red) for the same neuron when cue 1 was presented as 0 % correlation and are compared with the CP time course when cue 1 was presented as 100 % correlation (Fig. 3D, black). The 2 curves were basically similar. After cue 1, the CP increased and reached greater than 0.8 during the late period of the delay 1. Following a brief decrease at cue 2, the CP again increased and was maintained between 0.8-0.9 during delay 2 until the action period (Fig. 3D).
For 10 visual memory + movement preparation neurons, we calculated the CP during go trials when the cue 1 was presented as 0 % correlation. Mean CP values during the delay 1 and 2 periods were 0.70 and 0.77, respectively. Figure 4 plots mean (±SE) CP time course curves of the 10 neurons during 100 % (A) and 0 % (B) correlation conditions. The two curves were basically similar. After cue 1, mean CP increased above 0.6 (0.7-0.8, Fig. 4A, B). After cue 2, the CP further increased during 0 % correlation (B) and remained near 0.8 during delay 2 until the action period. There was a brief dip in mean CP at cue 2, especially in the 0 % correlation condition (Fig. 4B).
Fig. 4.
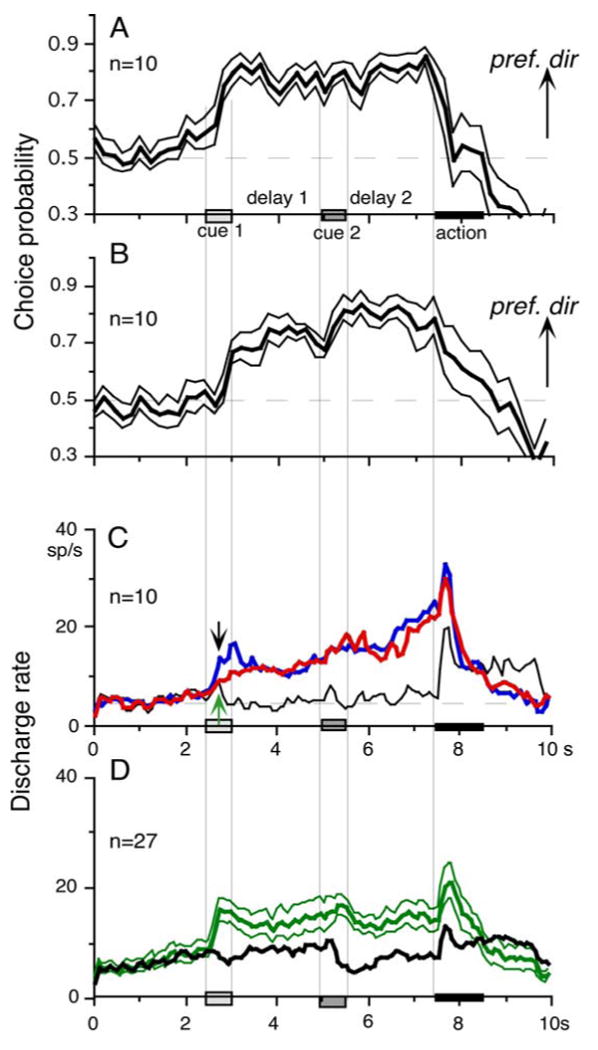
Time course of choice probability (CP) for go trials, mean (±SE) discharge modulation. A, B plot mean (±SE) CP of 10 visual memory + movement preparation during go trials sorted on pursuit in the preferred directions during delay 2 when cue 1 was 100 % (A) and 0 % correlation (B). C, blue and red traces compare mean discharge rates of the 10 neurons in preferred directions during go trials when cue 1 was 100 % (blue) and 0 % correlation (red). Black trace is mean discharge rate of the same neurons during go trials in anti-preferred directions when cue 1 was 0 % correlation. Dashed horizontal, gray lines in A and B are CP 0.5 values. Dashed line in C is mean discharge rate during control fixation period. In D, we selected SEF neurons that exhibited directional response to cue 1 visual motion and compared their mean discharge rates when the monkeys performed the pursuit instructed by cue 1 (i.e., preferred directions for cue 1) or anti-preferred directions.
Figure 4C (red and blue) plots mean discharge rates of these 10 neurons during go trial pursuit in their preferred directions when cue 1 was presented as 0 % and 100 % correlation, respectively. For comparison, Fig. 4C (black) plots mean discharge rates of the same neurons during pursuit in their anti-preferred directions when the cue 1 was presented with 0 % correlation. The initial response to cue 1 was largest during 100 % correlation (Fig. 4C, blue, indicated by downward arrow). The two curves during 0 % correlation (Fig. 4C, red and black) exhibited a slight increase in discharge rate after presentation of cue 1 but diverged clearly 240 ms later (green arrow). The time course of discharge modulation of the 2 curves for pursuit in the preferred directions during 100 % and 0 % correlation (Fig. 4C, red and blue) was basically similar, and they were clearly different from the mean discharge rates of the same neurons during pursuit in the anti-preferred directions (Fig. 4C, black). These results indicate that the delay 1 activity of visual memory + movement preparation neurons (e.g., Fig. 3A) covaried with both the delay 2 activity and monkeys' choice for final pursuit eye movement direction, suggesting that the congruent directionality during delay 1 and 2 reflected motion-direction assessment and preparation for subsequent pursuit eye movement direction, respectively (see Discussion).
Smooth pursuit vs saccade
The congruent directionality of delay 1 and 2 discharge of visual memory + movement preparation neurons was also observed when moving two spots stepwise during the action period so that the monkeys made saccades instead of smooth pursuit (Fig. 1A, see Methods). The results were similar in the 10 neurons tested. Figure 5A illustrates the discharge of the same neuron (Fig. 3A) during leftward (Fig. 5A1) and rightward saccades (Fig. 5A2) when cue 1 was presented with 100 % correlation. Clearly, the activity during delay 1 and 2 was higher when the monkey made leftward saccades (Fig. 5A1) than rightward (A2). Delay 1 activity was also observed when cue 1 motion was leftward during go- (Fig. 5A1 vs A2) and no-go trials (A3 vs A4). For comparison, Fig. 5C shows discharge of the same neuron during a visually guided saccade task with a single spot. Unlike the discharge in Fig. 5A1 and A2, no consistent pre-saccadic activity was observed during visually guided saccades (Fig. 5C).
Fig. 5.
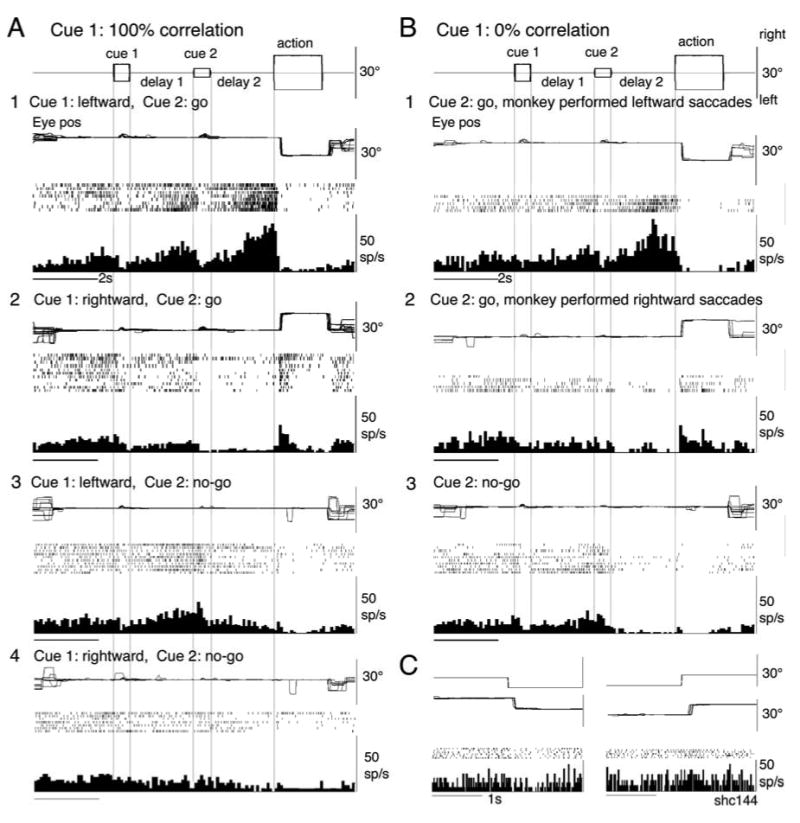
Discharge of representative visual memory + movement preparation neuron during saccade task. A and B, cue 1 was 100 % and 0 % correlation, respectively. A1 and 2, go trials for saccades when cue 1 was leftward (A1) and rightward (A2) visual motion (100 % correlation). A3 and A4, no-go trials as indicated. B1 and B2, go trials for saccades, cue 1 was 0 % correlation. Traces were grouped into leftward saccades (B1) and rightward saccades (B2). B3, no-go trials. C, discharge during a visually guided saccade task with a single spot.
When cue 1 was presented with 0 % correlation, both delay 1 and delay 2 activities were much higher when the monkey made leftward saccades than rightward (Fig. 5B1 vs B2). The CP time course for saccade trials for the preferred direction was similar to the time course for smooth pursuit (Fig. 3D, blue). No-go trials during 0 % correlation at cue 1 resulted in mixed trials; some included high discharge rates and others low discharge rates (Fig. 5B3, also Fig. 3C3, see Discussion). These results indicate that SEF activity reflects motion direction assessment and movement preparation and is common for smooth pursuit and saccades.
Movement preparation neurons
This group of neurons exhibited a direction specific response during delay 2 but not delay 1 during go-trials (Table 1). They discharged before the onset of pursuit eye movements in the task that required preparation for pursuit eye movements (e.g., Fig. 1B vs F). About half of them (9/20) also exhibited directional discharge to cue 2 when it instructed the monkeys to prepare for subsequent pursuit eye movements in the preferred direction (Fig. 1C vs D). The CP time course was examined using 0 % correlation in 6 of these neurons. All of them exhibited higher CP values (>0.8) during delay 2 for their preferred directions.
No-go neurons
Discharge of a representative neuron is shown in Fig. 6. During go trials, it exhibited discharge during the action period, regardless of the pursuit direction (Fig. 6A1). When the cue 2 instruction was “no-go” (Fig. 6A2), it exhibited a stronger discharge at cue 2 and during delay 2 compared to the direction non-specific discharge during go trials. Furthermore, when the monkey made an error during the action period by pursuing a leftward moving spot during a no-go trial (Fig. 6A2, red trace), this neuron nearly stopped discharging at cue 2 and during delay 2. These results suggest that discharge of this neuron reflected the monkey's decision to maintain fixation and not to pursue.
Fig. 6.
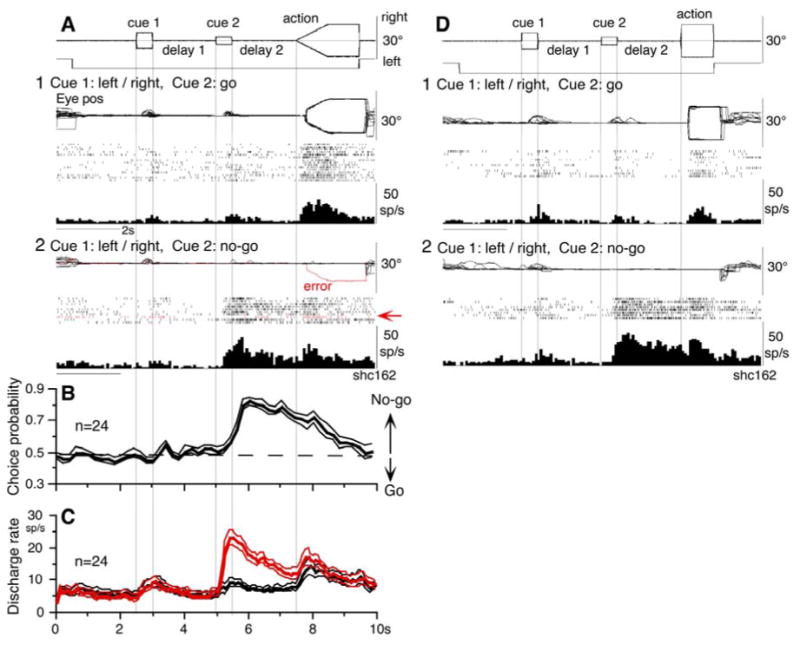
No-go neurons. A and D, representative neuron during smooth pursuit (A) and saccade tasks (D). A1, go trials when cue 1 was rightward and leftward visual motions. A2, no-go trials. Red traces (arrows) highlight an error trial. B, CP time course for 24 no-go neurons during no-go and go- trials. C, time course of mean (±SE) discharge of the 24 neurons during no-go (red) and go-(black) trials. D1 and D2, go trials and no-go trials for saccades, respectively.
The CP was computed during delay 2 with respect to the monkeys' choice based on whether they maintained fixation (i.e., no-go) or if they pursued a moving spot, regardless of its directions (Fig. 6A1 vs A2). Neurons that exhibited CP above 0.7 during delay 2 when they performed no-go were classified as no-go neurons (n=50, see Data analysis). Mean (±SE) CP during delay 2 were 0.87 (±0.07). Figure 6B plots mean (±SE) CP time course curves of 24 neurons when the duration of both delay 1 and delay 2 was set at 2s. The CP increased after cue 2. Figure 6C compares mean (±SE) discharge rates of the same neurons during no-go trials (red) and go trials (black). The difference in discharge modulation during cue 2 and delay 2 is clear.
No-go related discharge was also observed when the 2 spots were moved stepwise during the action period (Fig. 1A) so that the monkeys performed saccades. Figure 6D illustrates discharge of the same neuron (Fig. 6A) during rightward and leftward saccades (Fig. 6D1) and no-go trials (Fig. 6D2). Clearly, when the cue 2 instructed no-go, discharge was stronger during delay 2 (Fig. 6D2).
Preferred vs anti-preferred directions and location of responsive neurons
To examine how visual motion during cue 1 affected the activity of the overall population of SEF neurons during go trials, we sorted all SEF neurons that exhibited directional responses to cue 1 at 100 % correlation, and compared their mean discharge rates when the monkeys performed pursuit eye movements in the preferred direction with mean rates in the anti-preferred direction. Figure 4D plots mean rates of a total of 27 neurons for preferred directions (green, mean ±SE) and anti-preferred directions (black) when the duration of delay 1 and delay 2 was set at 2 s. These included 5 visual memory neurons, 14 visual memory + movement preparation neurons, 4 movement preparation neurons, and 4 others that did not exhibit directional responses during delay 1 and 2. SEF neurons that exhibited directional responses to cue 1 visual motion (Fig. 4D, green vs black) maintained clearly higher discharge rates during both delay 1 and delay 2. Their response to the identical cue 2 stimulus for the “go” instruction was clearly larger for the preferred direction compared to the anti-preferred direction (Fig. 4D), indicating directional modulation of cue 2 responses (e.g., Fig. 1C vs D).
Figure 7 summarizes schematically the locations of the electrode penetrations (see Methods) where we recorded the 4 groups of neurons (Table 1) on a surface view of the dorsomedial frontal cortex of monkey J (see Methods). These neurons were intermingled in the SEF region (Fig. 7, key). Locations of the electrode penetrations in monkey S were similar, although no-go neurons were recorded more caudally (~ 6 mm) as well.
Fig. 7.
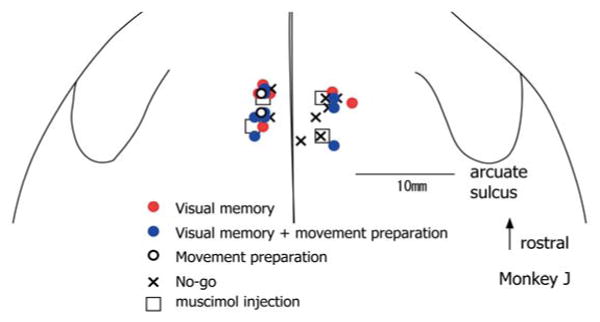
Recording locations of 4 groups (key) of neurons in the dorsomedial frontal cortex in monkey J. Muscimol injection sites are shown by open squares.
Chemical inactivation of SEF
To further examine whether SEF could be involved in visual motion-memory and the decision-making process of whether or not to pursue moving spots, we injected muscimol into the SEF bilaterally at the locations where we recorded responsive neurons (Fig. 7, open squares; see Methods). Results were consistent in the 2 monkeys. Representative results are shown in Fig. 8 before (A, B) and after (C, D) infusion for either rightward or leftward cue 1 motion. Before muscimol infusion (Fig. 8A, B), our monkeys performed the task well with few errors (red traces) in both go- and no-go trials (Fig. 8A1-3). After muscimol infusion, however, direction errors often appeared during go trials (Fig. 8C1-2, red traces) and even go/no-go errors appeared (Fig. 8C3, red traces) as well. There was no directional preference for errors during go- and no-go trials. Injections were repeated on 7 different days with 2-3 days between each. Mean (±SD) error rates before infusion were 8.8±3.3 (range 4.4-12.9) %. After infusion, mean (±SD) error rates significantly increased to 21.1±4.9 % (range 14.3-30.0, P<0.05).
Figure 8E and F compare the latency of correct pursuit eye movements after the onset of the action signal. There was an initial pursuit component before the catch-up saccades (Fig. 8B, D), and the rightward component was larger compared to leftward before muscimol injection in both monkeys (e.g., Fig. 8E, downward arrow). After muscimol injection, this component decreased (Fig. 8E). Latencies of catch-up saccades were also delayed after muscimol infusion. This delay was observed during leftward pursuit even though the initial pursuit component before catch-up saccades was not affected (Fig. 8F, upward arrow). After the catch-up saccade, pursuit eye velocity (i.e., post-saccadic pursuit eye velocity) decreased in both rightward and leftward directions (Fig. 8E, F, *, P<0.05). However, there was no significant difference in the maintenance of pursuit eye velocity before and after muscimol infusion during the period 0.5-0.7 s after the onset of spot motion (P>0.5, Fig. 8E, F). These results indicate that chemical inactivation of SEF not only increased directional errors and go/no-go errors, but also impaired the initial eye velocity of correct pursuit.
Discussion
The SEF is reported to play an important role in complex behaviors such as learning-related activity (Chen and Wise 1995; Nakamura et al. 1998), planning of saccades (Olson et al. 2000), sequential saccades (Isoda and Tanji 2002; Lu et al. 2002), decision-making processes (Coe et al. 2002), and antisaccades (Schlag-Rey et al. 1997). Although the SEF also contains pursuit-related neurons that discharge during smooth pursuit (Schall 1991; Heinen 1995; Heinen and Liu 1997; Fukushima et al. 2004), their role in pursuit eye movements was not clear, especially because SEF lesions do not impair simple pursuit using a single spot (see a review by Tehovnik et al. 2000; also Fukushima et al. 2003), so it had been suggested that SEF is involved in guiding anticipatory pursuit (see Introduction, also Leigh and Zee 2006 for a review).
Using a new, memory-based smooth pursuit task, the present study has demonstrated that SEF contains signals that code assessment of visual motion-direction and hold that assessment in working memory, the decision of whether or not to pursue moving spots, and the preparation of the direction of the ensuing pursuit eye movement. Chemical inactivation of SEF, bilaterally, resulted in direction errors in the execution of smooth pursuit, delay in latencies of corrective saccades, decrease of initial (but not of maintenance phase of) pursuit eye velocities, and errors of whether or not to pursue moving spots. These results indicate that the SEF discharge assesses visual motion direction, remembers it, and uses it to program appropriate smooth pursuit eye movements.
SEF and memory for visual motion direction
Our results indicate that neuronal discharge reflecting working memory for visual motion direction is found in the SEF because, in our task, signals encoding the memory of visual motion direction were required in order to produce the appropriate pursuit eye movement commands. It has been reported that potential sites for visual motion-memory are the middle temporal cortical area (MT) (Newsome and Pare 1988; Britten et al. 1992; Bisley et al. 2004) and medial superior temporal area (MST) (Cerebrini and Newsome 1994, 1995; also Kawawaki et al. 2006). For a direct comparison with SEF neuronal activity, it would be necessary to examine neuronal activity in task conditions that isolate such activity. Although Britten et al. (1996) have reported that individual MT neurons weakly but significantly predict the monkey's directional decisions, Seidmann et al. (1998) have shown that delay period activity signaling the remembered direction of motion is not observed in MT neurons. Although some activity has also been reported in MST neurons, it is minimal (Bisley et al. 2004). Preliminary study in our laboratory tested the activity of 100 MST neurons that exhibited a visual motion response during cue 1. However, none of them exhibited maintained cue 1 discharge during delay 1 (Shichinohe et al. unpub. obs.). These observations suggest that, during delay 1 in our task, MT and MST probably do not provide signals for the motion-direction memory.
The dorsolateral prefrontal cortex also contains neurons that respond to visual motion (Kim and Shadlen 1999; Zaksas and Pasternak 2006). This region has been linked to temporal storage of sensory signals for working memory (Goldman-Rakic 1995). Kim and Shadlen (1999) have demonstrated that visual motion responses can be maintained during a delay period in prefrontal cortex neurons. However, in their studies, discharge related to the memory of visual motion could not be separated from discharge related to movement preparation (also Zaksas and Pasternak 2006).
Qualitatively similar signals reflecting the direction of visual motion were also found in the frontal eye fields (FEF) and SEF (Kim and Shadlen 1999; Fukushima et al. 2002, 2008). In preliminary studies, we recorded 160 neurons in the caudal FEF of the same monkeys that exhibited modulation using the same task, and found a significantly lower percentage of direction-specific neurons in FEF than SEF during delay 1 (Shichinohe et al. unpub. obs.). Unilateral muscimol infusion into FEF did not induce directional errors in our task (Fukushima et al. 2008). These results, taken together, suggest the uniqueness of SEF in maintaining memory of visual motion direction.
We do not know how SEF visual memory signals are generated. The dorsolateral prefrontal cortex may participate in the generation as suggested earlier (Kim and Shadlen 1999; Zaksas and Pasternak 2006). SEF contained many neurons that exhibited direction non-specific discharge during delay 1 (Fig. 1E). Discharge characteristics of these neurons were not homogeneous. Some of them exhibited gradually increasing activity during delay 1 until cue 2 as though their discharge had reflected anticipation of cue 2 (e.g., Chen and Wise 1995). There were also neurons whose discharge was similar to that shown in Fig. 2A1 except for the lack of directionality. They may have signaled visual motion, the direction of which was not specified. SEF discharge reflecting visual memory must have been generated as a result of the monkeys' learning to associate cue 1 with the cue 2 instruction (e.g., Mann et al. 1988). Direction non-specific neurons may have participated in a process of this learning. It is also possible that their activity during delay 1 signaled the context of cue 1 to associate it with the cue 2 no-go instruction. Analysis of their discharge is the subject of a paper in preparation.
SEF and motion-direction assessment and preparation for pursuit eye movements
Our results indicate that visual memory + movement preparation neurons code directionality during delay 1, hold visual motion-memory, and during delay 2 use if for preparation for subsequent pursuit eye movement directions (Figs. 3-4). Using 0 % correlation at cue 1 (Newsome and Pare 1988), we forced the monkeys to choose the pursuit direction themselves. It has been shown that the sensitivity to visual motion of most neurons in MT and MST is very similar to the psychophysical sensitivity of the monkeys (Britten et al. 1992; Cerebrini and Newsome 1994). Lack of motion-direction information during 0 % correlation at cue 1 and the lack of a delay 1 response in MST neurons during 100 % correlation in our task (Shichinohe et al. unpub obs; also Seidmann et al. 1998 for MT neurons, see above) suggests that MT and MST neurons cannot provide the signals coding visual motion direction during delay 1 that were used for the monkeys' choice of final pursuit direction (e.g., Fig. 3C; also Britten et al. 1992; Cerebrini and Newsome 1994).
In contrast, our results from the CP analysis of visual motion + movement preparation neurons show that the delay 1 activity during 0 % correlation at cue 1 covaried with the monkeys' choice for pursuit direction and that the CP time courses during 0 % and 100 % correlation were very similar (Figs. 3C-D, 4B). During 0 % correlation, the monkeys seemed to search for a visual motion direction cue, but cue 1 itself did not provide the information (Newsome and Pare 1988). We think that the delay 1 activity of visual memory + movement preparation neurons during 0 % correlation reflected the monkeys' motion-direction assessment. Our results showing that the CP found in the SEF with 0% correlated motion is much higher than that found in MT (e.g., Purushothaman and Bradley, 2005) are consistent with our interpretation. Clear divergence of mean discharge rates for preferred and anti-preferred directions (Fig. 4C, red and black, indicated by green arrow) may suggest that at 240 ms after presentation of 0 % correlation at cue 1, the monkeys reached an assessment of motion-direction (cf., Kiani et al. 2008). It is unlikely that the delay 1 activity of our neurons contained signals for preparation for action, because the activity was rarely affected by the cue 2 instruction to go or no-go (Figs. 2A1 vs B1, 3A1 vs A3) or by preparation of the pursuit eye movement direction when the monkey made a pursuit direction error (Fig. 2A1, red). Notice that no-go trials during 0 % correlation at cue 1 resulted in mixed trials; some included high discharge rates and others low discharge rates (rasters in Figs. 3C3, 5B3). It is possible that trials with high and low discharge rates reflected the monkeys' attempt to find motion-direction and their assessment about its direction until the no-go instruction was given by cue 2. This conclusion is supported by the observation (Shichinohe et al. unpub. obs.) that, in 3 of 4 visual motion neurons tested, the CP time course showed values >0.8 during delay 1 for pursuit in the preferred direction.
The importance of motion-direction information in the SEF is supported by the results that muscimol infusion into bilateral SEF resulted in errors in pursuit directions (Fig. 8C). Note that direction errors and even go/no-go errors increased after muscimol infusion from the control mean error rate of 8.8 (±3.3SD) % to significantly higher error rate of 21.1 (±4.9SD) %. It should be noted that the increase in error rates was not due to loss of alertness or general attention, because there was no significant difference in the maintenance of pursuit eye movements (Fig. 8E, F). However, it should also be noted that the mean error rate of 21 % after muscimol infusion indicates that the monkeys still could perform the task (Fig. 8E and F, thin vs thick traces). These results suggest that, although the SEF is important for working memory of visual motion direction in our task, it is not the sole area for this function. Other brain areas, most probably the prefrontal cortex, may also participate in this function (c.f., Kim and Shadlen 1999; Zaksas and Pasternak 2006).
SEF and decision for go or no-go
The present results demonstrate the existence of no-go neurons for smooth pursuit in the SEF (Fig. 6). No-go neurons were reported earlier in a saccadic and pursuit go/no-go tasks in the SEF (Mann et al. 1988; Kim et al. 2005). The existence of no-go neurons along with impairment in performing no-go trials after muscimol infusion in the present study (Fig. 8) suggests that SEF is necessary for the decision-making process of whether or not to pursue moving spots during our task. Our results also show that no-go SEF signals were common for both saccadic and smooth pursuit eye movements in our task conditions (Fig. 6A vs D). Notice that the direction specific activity reflecting visual motion-memory and preparation for action was also common in smooth pursuit and saccadic systems (Fig. 5A, B), suggesting that, in SEF, these signals, although they are context dependent (e.g., Figs. 1B vs 1F, 5A1 vs 5C; Mann et al. 1988), are not separated for the two eye movement systems (Krauzlis 2005).
Our results showed that no-go neurons discharged during the action period in go trials (Fig. 6). It is unlikely that their discharge reflected a visual response to spot motion, because they did not discharge at cue 1. Their discharge may partly contribute to performance monitoring (see Discussion of Emeric et al. 2008).
Role of SEF in visual motion-memory and preparation for pursuit eye movements
Using a new memory-based, smooth-pursuit task, the present results show that the SEF contains signals reflecting retinal image-slip-velocity, memory and assessment of visual motion-direction, the decision whether to pursue, and the preparation for and execution of pursuit eye movements (Table 1). The signals and the congruent discharge during delay 1 and 2 (Fig. 4A-C) seem to reflect stages of processing that take place within the SEF. The final stages in this conversion are direction-specific eye movement signals to pursue a chosen spot during the action period. Such signals are commonly found in the FEF pursuit area (see Leigh and Zee 2006 for a review). Also, the first signal (i.e., directional visual motion signals induced by cue 1) and signals reflecting preparation for pursuit eye movements during delay 2 are commonly found in the FEF (Fukushima et al. 2008; also Kim and Shadlen 1999). These results suggest that both SEF and FEF are involved in appropriate execution of smooth pursuit eye movements and that they may have different roles (Mann et al. 1988; Fukushima et al. 2006). The present study has revealed an important role of the SEF in memory of visual motion direction, a decision-making process for aborting pursuit of moving spots, and preparation of appropriate pursuit eye movements.
Methods
Two monkeys (Macaca fuscata, 5-6 years old) were used. All procedures were performed in strict compliance with the guidelines for the Care and Use of Animals of National Institutes of Health. Our specific procedures were approved by the Animal Care and Use Committee of Hokkaido University School of Medicine. Our methods for animal preparation, training, recording and data analysis are described elsewhere in detail (e.g., Fukushima et al., 2000, 2008), and are summarized here briefly. Each monkey was sedated with ketamine hydrochloride (5 mg/kg, i.m.), then anesthetized with sodium pentobarbital (25 mg/kg, i.p.). Additional anesthesia (0.5-1.0% halothane mixed with 50% nitrous oxide and 50% oxygen) was administrated as necessary. Under aseptic conditions, head-holders were affixed to the skull. Vertical and horizontal components of eye movements were recorded using a scleral search coil (Fuchs and Robinson 1966).
Monkeys were seated in a primate chair in darkness with the head firmly restrained, facing a 22-inch computer display (Mitsubishi, RDF 221S, 120 Hz) placed 65cm away from the eyes. Visual objects (spot and random-dot-patterns, see below) were presented in the central 10° by 10° of the visual field. The tasks are schematically illustrated in Fig. 1A. A red fixation spot appeared in the center and the monkeys were required to fixate it (Fig. 1A, fixation). At cue 1, a random dot pattern was presented (each 0.5° spot, presented across 40 % of the 10° × 10° area, ~150 dots) and was moved along one of 8 directions separated by 45° at 10°/s for 0.5 s (Fig. 1A, cue 1): horizontal (right or left), vertical (up or down) or 4 diagonal directions. Each dot in the pattern moved in the same direction (i.e., 100 % correlation, Newsome and Pare 1988). In successive trials, the direction of the moving pattern (e.g., right or left) was random but the frequency of its occurrence was equal. The monkeys were required to remember the color of the pattern and the direction of movement. After a delay (Fig. 1A, delay 1 of 1-4s, typically 2s), a stationary pattern was presented as the 2nd cue (Fig. 1A, cue 2) (each 0.5° spot, presented across 40 % of the 10° × 10° area, ~150 dots). If the color of cue 2 was the same as cue 1, it instructed the monkeys to prepare to pursue a spot that would move in the direction instructed by cue 1 (i.e., go). If the color of the cue 2 was different, it instructed the monkeys not to pursue (i.e., no-go) but to maintain fixation of a stationary spot. After the 2nd delay, which lasted a fixed period of 1-4 s in a block of trials (Fig. 1A, delay 2, typically 2s), the monkeys were required to perform the pursuit eye movement by selecting the correct spot (Fig. 1A, action). For this, the fixation spot remained stationary, but spawned 2 identical spots; one moved in the direction instructed by cue 1 and the other moved in the opposite direction at 10°/s. The monkeys were required to respond correctly, either to pursue the correct spot or not to pursue (i.e., no-go) by maintaining fixation of the spot that remained stationary. The frequency of occurrence of the fixation condition was set at 24 % of the trials, and in the remaining 76 % of the trials, the monkeys were required to pursue one of the 2 moving spots. To examine whether responses were unique to the smooth pursuit task, we also moved the 2 spots stepwise during the action period to induce saccades.
Reward circuits compared position signals of the fixation spot during cue 1, cue 2 and two delay periods (Fig. 1A) and the correct target spot during the action period (Fig. 1A) with the monkeys' eye position signals. If the monkeys' gaze was within the error window of ± 2°, apple juice was automatically delivered to the animal at the end of each trial (Fig. 1A, reward). If the monkeys' gaze was outside the error window, the trial was aborted and was started again. The monkeys were also trained to perform the task with different cue 1 and cue 2 colors. Typically, the monkeys were trained to perform this task over several months to a year. At the start of recordings, the error rate was typically less than 10 %. A SEF chamber was installed aimed at anterior 21-25 and lateral 1-5 stereotaxic coordinates on both sides of the dorsomedial frontal cortex.
Extracellular recordings were made in 2 monkeys. To locate the SEF, we first applied microstimulation (100 μA, 20-30 cathodal pulses, 0.2 ms duration, 333 Hz) in the dorsomedial frontal cortex, while the monkeys fixated a stationary spot or performed smooth pursuit. Low threshold areas (~50 μA) for evoking saccades were located (Schlag and Schlag-Rey 1987; Missal and Heinen 2001, 2004), and we then started searching for responsive neurons using our task (Fig. 1A). Once task-related neurons were isolated, we determined their preferred directions by moving cue 1 in different directions using 100 % correlation (Newsome and Pare 1988). For cue 1, we also moved each dot randomly using 0 % correlation in a block of trials (Newsome and Pare 1988). If the color of cue 2 was the same as cue 1, it instructed “go” and the monkey followed one of the 2 moving spots. If the color of cue 2 was different from that of cue 1, it instructed no-go. Thus, the monkey was forced to remember and match the cue color in order to respond correctly. The 0 % correlation was used to force the monkeys to choose the pursuit direction to examine how SEF neurons discharged during delay 1 and delay 2. For comparison, pursuit and saccade tasks were also tested using a single spot.
Both monkeys were also used for recordings in the caudal part of the frontal eye fields in the arcuate sulcus (Fukushima et al. 2008). The stereotaxic coordinates of the caudal portion of the arcuate sulcus were measured during the initial surgery under visual observation and the correct area was confirmed by electrical stimulation that evoked saccades. As illustrated in Fig. 7, the locations of electrode penetrations in the SEF where we recorded the 4 groups of neurons (Table 1) were estimated with respect to the caudal portion of the arcuate sulcus and the remaining portions of the arcuate sulcus were estimated from the anatomy of other monkeys' brains who were of similar age and body weight.
To inactivate the SEF, we used a micro-recording needle (Crist Instrument) that was attached to a Hamilton syringe, and 1.0 μl of GABA agonist muscimol dissolved in physiological saline (10 μg/1 μl) was infused into the identified sites: 2 sites 2 mm apart rostro-caudally in the right and left SEF (Fig. 7, open squares). The effects of muscimol injection on monkeys' performance (Fig. 1A) were examined by changing the colors of cue 1 and cue 2. For this, we prepared 5 sets of different-colored dots and each set was presented randomly before and after infusion. This was to force the monkeys to remember the cue color in order to respond correctly thus testing their working memory in a demanding task situation.
Data analysis
To analyze the discharge of each neuron, traces were aligned on the onset of cue 1. Eye position, target position, and neuronal discharge were sorted by correct responses to the direction instructed by cue 1 and cue 2. Trials for go and no-go were sorted separately. Mean discharge rates of individual neurons during each period (Fig. 1A) were measured and compared as the mean (±SD) rate of each period versus the mean discharge rate (±SD) during the initial fixation (Fig. 1A-D). We defined significant differences as those having a P-value <0.05 using Students' t test with the significance level corrected for multiple comparisons using the Bonferroni correction. A total of 240 neurons were tested in the SEF region. Of these, 32 neurons (13 %) exhibited gradually increasing activity during the control (fixation) period as though these neurons reflected anticipation of the occurrence of cue 1 (e.g., Chen and Wise 1995). Because they responded before any cue, we were unable to estimate control discharge rate accurately, so we did not include these neurons. Further analysis was done on 208 neurons.
The monkeys occasionally made small eye movements during the delay periods (e.g., Fig. 2). Some were blinks. These eye movements did not contribute to the observed neuronal responses.
Direction specific neurons during the action period included pursuit-related neurons in a pursuit task (e.g., Fig. 1F). We tested simple ramp pursuit and/or sinusoidal pursuit in a total of 38 neurons that exhibited modulation during the action period during go trials (Fig. 1A). These included 16 direction specific neurons and 22 direction non-specific neurons (Fig. 1E). A majority of the former (12/16=75%) but only a minority of the latter (3/22=14%) exhibited modulation during a simple pursuit task like Fig.1F: typically weaker discharge modulation during movements (but rarely before the onset of eye movements) compared to the modulation in our memory-based pursuit task (e.g., Fig. 1B). Neurons that exhibited directional modulation both during delay 1 and delay 2 included pursuit-related neurons identified by sinusoidal pursuit (7/9 neurons tested). However, neurons that showed directional modulation only during delay 1 did not show directional response during pursuit (action) and we did not test these neurons using a simple pursuit task.
Responses during delay 1 and 2 periods were evaluated by choice probability (CP, Britten et al. 1996: Zaksas and Pasternak 2006). The evolution of the CP over the time course of each trial was calculated with respect to the monkeys' choice based on whether they pursued in the preferred direction of the neuron or anti-preferred direction. CP values were computed using a sliding window of 200 ms duration incremented in steps of 100 ms from the initial fixation to the end of action period (e.g., Fig. 4A), typically for 10 s.
The evolution of the CP was also calculated for neurons that discharged during delay 2 during no-go trials with respect to the monkeys' performance based on whether they maintained fixation or if they pursued a spot regardless of direction (Fig. 6). Neurons were classified as no-go neurons if they exhibited CP above 0.7 during delay 2 when the monkeys performed no-go. Because these neurons preferred the no-go trials, they were not included in the percentage of modulated neurons during go trials summarized in Fig. 1E.
To analyze the effects of muscimol injection, 80-100 trials were aligned with the onset of cue 1 before and after injection. Error trials were counted. Eye position and velocity traces were examined for correct performance. De-saccaded eye velocity for correct responses was averaged to compare mean velocity.
Although the two monkeys are still being used for other experiments, we are certain that recordings were from the SEF region because the discharge characteristics, the recording locations estimated relative to the arcuate sulcus (Fig. 7), and the stereotaxic coordinates and electrical stimulation for saccadic eye movements were similar to our previous studies in which recording locations were confirmed histologically.
Acknowledgments
This research was supported by Grant-in-Aid for Scientific Research on Priority Areas (System study on higher-order brain functions) (17022001) and (C)(20500351) from the MEXT of Japan, and National Institutes of Health Grants EY-06558 (NEI) and RR-00166.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assad JA, Maunsell JHR. Neuronal correlates of inferred motion in primate posterior parietal cortex. Nature. 1995;373:518–521. doi: 10.1038/373518a0. [DOI] [PubMed] [Google Scholar]
- Becker W, Fuchs AF. Prediction in the oculomotor system: smooth pursuit during transient disappearance of a visual target. Exp Brain Res. 1985;57:562–575. doi: 10.1007/BF00237843. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Zalsas D, Droll JA, Pasternak T. Activity of neurons in cortical area MT during a memory for motion task. J Neurophysiol. 2004;91:286–300. doi: 10.1152/jn.00870.2003. [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- Cerebrini S, Newsome WT. Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. J Neurosci. 1994;14:4109–4124. doi: 10.1523/JNEUROSCI.14-07-04109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerebrini S, Newsome WT. Microstimulation of extrastriate area MST influences performance on a direction discrimination task. J Neurophysiol. 1995;73:437–448. doi: 10.1152/jn.1995.73.2.437. [DOI] [PubMed] [Google Scholar]
- Chen LL, Wise SP. Neuronal activity in the supplementary eye field during acquisition of conditional oculomotor associations. J Neurophysiol. 1995;73:1101–1121. doi: 10.1152/jn.1995.73.3.1101. [DOI] [PubMed] [Google Scholar]
- Coe B, Tomihara K, Matsuzawa M, Hikosaka O. Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J Neurosci. 2002;22:5081–5090. doi: 10.1523/JNEUROSCI.22-12-05081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CJ, Barnes GR. Scaling of smooth anticipatory eye velocity in response to sequences of discrete target movements in humans. Exp Brain Res. 2005;167:404–413. doi: 10.1007/s00221-005-0044-8. [DOI] [PubMed] [Google Scholar]
- de Hemptinne C, Lefevre P, Missal M. Neuronal basis of directional expectation and anticipatory pursuit. J Neurosci. 2008;28:4298–4310. doi: 10.1523/JNEUROSCI.5678-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex. J Neurophysiol. 2008;99:759–772. doi: 10.1152/jn.00896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Akao T, Takeichi N, Kaneko CRS, Fukushima K. Involvement of the frontal oculomotor areas in developmental compensation for the directional asymmetry in smooth pursuit eye movements in young primates. Ann N Y Acad Sci. 2003;1004:451–456. [Google Scholar]
- Fukushima J, Akao T, Takeichi N, Kurkin S, Kaneko CRS, Fukushima K. Pursuit-related neurons in the supplementary eye fields: discharge during pursuit and passive whole body rotation. J Neurophysiol. 2004;91:2809–2825. doi: 10.1152/jn.01128.2003. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Akao T, Kurkin S, Kaneko CRS, Fukushima K. The vestibular-related frontal cortex and its role in smooth-pursuit eye movements and vestibular-pursuit interactions. J Vestibular Res. 2006;16:1–22. [PMC free article] [PubMed] [Google Scholar]
- Fukushima K, Sato T, Fukushima J, Shinmei Y, Kaneko CRS. Activity of smooth pursuit-related neurons in the monkey periarcuate cortex during pursuit and passive whole body rotation. J Neurophysiol. 2000;83:563–587. doi: 10.1152/jn.2000.83.1.563. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Yamanobe T, Shinmei Y, Fukushima J. Predictive responses of peri-arcuate pursuit neurons to visual target motion. Exp Brain Res. 2002;145:104–120. doi: 10.1007/s00221-002-1088-7. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Akao T, Shichinohe N, Nitta T, Kurkin S, Fukushima J. Predictive signals in the pursuit area of the monkey frontal eye fields. Prog Brain Res. 2008;171:433–440. doi: 10.1016/S0079-6123(08)00664-X. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Heinen SJ. Single neuron activity in the dorsomedial frontal cortex during smooth pursuit eye movements. Exp Brain Res. 1995;104:357–361. doi: 10.1007/BF00242022. [DOI] [PubMed] [Google Scholar]
- Heinen SJ, Liu M. Single-neuron activity in the dorsomedial frontal cortex during smooth-pursuit eye movements to predictable target motion. Visual Neurosci. 1997;14:853–865. doi: 10.1017/s0952523800011597. [DOI] [PubMed] [Google Scholar]
- Isoda M, Tanji J. Cellular activity in the supplementary eye field during sequential performance of multiple saccades. J Neurophysiol. 2002;88:3541–3545. doi: 10.1152/jn.00299.2002. [DOI] [PubMed] [Google Scholar]
- Kawawaki D, Shibata T, Goda N, Doya K, Kawato M. Anterior and superior lateral occipito-temporal cortex responsible for target motion prediction during overt and covert visual pursuit. Neurosci Res. 2006;54:112–123. doi: 10.1016/j.neures.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Kiani R, Hanks TD, Shadlen MN. Bounded integration in parietal cortex underlies decisions even when viewing duration is dictated by the environment. J Neurosci. 2008;28:3017–3029. doi: 10.1523/JNEUROSCI.4761-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Shadlen MN. Neural correlates of decision in the dorsolateral prefrontal cortex of the macaque. Nature Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- Kim YG, Badler JB, Heinen SJ. Trajectory interpretation by supplementary eye field neurons during ocular baseball. J Neurophysiol. 2005;94:1385–1391. doi: 10.1152/jn.00109.2005. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. The control of voluntary eye movements: new perspectives. Neuroscientist. 2005;11:124–137. doi: 10.1177/1073858404271196. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements. 4th New York: Oxford Univ. Press; 2006. [Google Scholar]
- Lu X, Matsuzawa M, Hikosaka O. A neural correlate of oculomotor sequences in supplementary eye field. Neuron. 2002;34:317–325. doi: 10.1016/s0896-6273(02)00657-8. [DOI] [PubMed] [Google Scholar]
- Mann SE, Thau R, Schiller PH. Conditional task-related responses in monkey dorsomedial frontal cortex. Exp Brain Res. 1988;69:460–468. doi: 10.1007/BF00247300. [DOI] [PubMed] [Google Scholar]
- Missal M, Heinen SJ. Facilitation of smooth pursuit initiation by electrical stimulation in the supplementary eye fields. J Neurophysiol. 2001;86:2413–2425. doi: 10.1152/jn.2001.86.5.2413. [DOI] [PubMed] [Google Scholar]
- Missal M, Heinen SJ. Supplementary eye fields stimulation facilitates anticipatory pursuit. J Neurophysiol. 2004;92:1257–1262. doi: 10.1152/jn.01255.2003. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sakai K, Hikosaka O. Neuronal activity in medial frontal cortex during learning of sequential procedures. J Neurophysiol. 1998;80:2671–2687. doi: 10.1152/jn.1998.80.5.2671. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J Neurosci. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CR, Gettner SN, Ventura V, Carta R, Kass RE. Neuronal activity in macaque supplementary eye field during planning of saccades in response to pattern and spatial cues. J Neurophysiol. 2000;84:1369–1384. doi: 10.1152/jn.2000.84.3.1369. [DOI] [PubMed] [Google Scholar]
- Purushothaman G, Bradley DC. Neural population code for fine perceptual decisions in area MT. Nat Neurosci. 2005;8:99–106. doi: 10.1038/nn1373. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neuronal activity related to visually guided saccadic eye movements in the supplementary motor area of rhesus monkeys. J Neurophysiol. 1991;66:530–558. doi: 10.1152/jn.1991.66.2.530. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. J Neurophysiol. 1987;57:179–200. doi: 10.1152/jn.1987.57.1.179. [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M, Amador N, Sanchez H, Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature. 1997;390:398–401. doi: 10.1038/37114. [DOI] [PubMed] [Google Scholar]
- Seidmann E, Zohary E, Newsome WT. Temporal gating of neural signals during performance of a visual discrimination task. Nature. 1998;394:72–75. doi: 10.1038/27906. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Rev. 2000;32:413–448. doi: 10.1016/s0165-0173(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Zaksas D, Pasternak T. Directional signals in the prefrontal cortex and in area MT during a working memory for visual motion task. J Neurosci. 2006;26:11726–11742. doi: 10.1523/JNEUROSCI.3420-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


