Abstract
Nanoconjugates are emerging as promising drug-delivery vehicles because of their multimodular structure enabling them to actively target discrete cells, pass through biological barriers and simultaneously carry multiple drugs of various chemical nature. Nanoconjugates have matured from simple devices to multifunctional, biodegradable, nontoxic and nonimmunogenic constructs, capable of delivering synergistically functioning drugs in vivo. This review mainly concerns the Polycefin family of natural-derived polymeric drug-delivery devices as an example. This type of vehicle is built by hierarchic conjugation of functional groups onto the backbone of poly(malic acid), an aliphatic polyester obtained from the microorganism Physarum polycephalum. Particular Polycefin variants target human brain and breast tumors implanted into animals specifically and actively and could be detected easily by noninvasive imaging analysis. Delivery of antisense oligonucleotides to a tumor-specific angiogenic marker using Polycefin resulted in significant inhibition of tumor angiogenesis and increase of animal survival.
Keywords: biodegradable, brain cancer, breast cancer, imaging analysis, multiple antibodies, multiple drug delivery, multitargeting, Polycefin, poly(malic acid), tumor angiogenesis
Engineering of polymeric biodegradable nanoconjugates represents one of the rapidly emerging and important areas of nanotechnology and nanomedicine. In the context of this review, these nanoconjugates will be discussed mostly in relation to anticancer drug delivery. Polymeric nanoconjugates are characterized by multiple covalently attached functional moieties that can target cellular and molecular markers of diseased tissues specifically to achieve maximum treatment efficacy. An outstanding feature of polymeric nanoconjugates is that they can carry a variety of covalently bound drugs that may act simultaneously on several targets (e.g., mRNA and/or protein). The benefit of such drug carriers is that they deliver more than one prodrug by single conjugate molecule, increasing the probability of coordinate and synergistic action and thus providing an efficient inhibition of multiple aberrant tumor pathways. Owing to the specific targeting of tumor tissue/cells, nanoconjugates provide therapeutically efficacious drug concentrations at the site of treatment and minimal side effects on healthy tissue.
Nanoconjugate delivery systems are significantly different from nonconjugated nano-delivery vehicles, for example, micelles and liposomes, which also combine drugs, targeting and/or other functional moieties but do not form a covalently linked chemical entity. Devices lacking this entity are prone to continuously lose their constituents by spontaneous or damage-induced leakage and this could be a source of toxicity to healthy tissue or disparities in initially balanced drug compositions.
Biodegradability and lack of immunogenicity are other criteria important for treatment that can be met by nanoconjugates. Biodegradability without accumulation of potentially harmful metabolic byproducts in nontargeted tissues is an important feature that enables repeated treatments. Biodegradable moieties can be obtained from natural sources or can be synthesized chemically. Biodegradability involves nanoconjugate metabolism and eventually decomposition to water and carbon dioxide. Absence or low levels of immunogenicity can be met by choosing nanoconjugate platforms and functional moieties that do not elicit an immune response. Despite the realization that drug-delivery systems can be multitargeting, biodegradable and nonimmunogenic, they have still remained scarce until recently.
We shall at first discuss examples of ‘conventional’ nanoconjugates with limited targeting and biodegradability, while introducing general features of this kind of drug carrier, and we will then emphasize the Polycefin system as an example of a biodegradable polymeric platform with multiple functional moieties. Polycefin constitutes a biopolymeric carrier system that enables the attachment of a choice of different drugs and homing modules specifically directed towards tissue/cell and molecular targets, all of them bound covalently to the same nanoconjugate molecule. Such polymers provide the possibility of patient-specific combination therapy programmed by a single molecule of ‘blockbuster drug’. This system offers endless possibilities for developing highly efficient multitargeted drug nanocarriers custom-made for individual patient needs.
The nanoconjugate concept of targeting
An important idea behind the nanoconjugate concept is multitargeting. Small molecules have been designed in modern chemotherapy to stage a simultaneous attack on several molecular targets/markers altered in diseased tissue [1–4]. This type of targeting could be the result of ‘promiscuous’ specificity towards groups of proteins, especially enzymes, such as kinases and phosphatases, or could also be achieved by a mixture of chemotherapeutics with each component attacking a single target specifically. Multi-targeting nanostructures, however, form a single chemical entity with one or more targeting moieties, one or more prodrugs and other auxiliary molecular groups, releasing free drug within the specifically targeted tissue. Tissue and cell targeting follows the urgent need in anticancer therapy to significantly reduce side effects that arise from chemotherapeutic drug toxicity to normal cells and from frequently encountered cancer drug resistance. To overcome this serious drawback of mainstream chemotherapy, nanoconjugates bear groups that target cancer markers of tumor cell-surface antigens, which provide specific homing to tumor tissue. In addition, ‘passive’ targeting of tumor tissue is achieved owing to the enhanced permeability and retention (EPR) [5] effect of nanoconjugates; the efficiency increasing as a function of particle size of less than 200 nm. The EPR effect is due to the usually leaky nature of tumor vasculature and the ability of large molecules to be extravasated passively in those areas. The large physical entity of nanoconjugates as opposed to low-molecular-weight drugs has also proven beneficial for overcoming the multidrug resistance effect [6,7].
The promiscuous type of small-molecule tumor marker-targeting drugs is exemplified by Glivec®/Gleevec® (imatinib mesylate, Novartis, Switzerland), which is a chronic myeloid leukemia drug [8], or AEE788, an oral multiple-receptor tyrosine kinase inhibitor of EGF receptor (EGFR), HER-2 and VEGF receptor (VEGFR) [9]. These compounds show fast kidney clearance that requires high therapeutic concentrations, with side effects, such as cardiotoxicity and other organ toxicity. The majority of small-molecule drug targets are found in fast dividing nontumor tissues as well. Therefore, their tumor specificity is limited.
In addition to passive targeting by the EPR effect, nanoconjugates carry a variety of active targeting units that can enable them to find tumor tissue/cell and molecular tumor markers. Cell-surface targeting agents can home the drug carrier exclusively to the intended tissue/tumor cell [10]. Specific binding of the carrier to target cells enables focused delivery of toxic drugs and leads to the elimination of targeted cells by the same mechanism that would also kill healthy cells in the absence of cell specificity (examples are the DNA-acting drugs cisplatin and doxorubicin).
For successful tumor targeting, drug given systemically may need to pass through some barriers before reaching the tumor cell surface. An important example is the blood–brain barrier (BBB) in the central nervous system. It comprises the system of endothelial cell tight junctions and membrane efflux transporters that limit passage of small molecules, drugs and antibiotics across brain capillary endothelial cells [11, 12]. In brain tumors, this barrier (blood–brain–tumor barrier [BTB]) is partially compromised but still limits the passage of circulating drugs to brain tumors, presenting a serious clinical challenge for chemotherapy [11]. If the delivery vehicle is able to bind to a vascular endothelial surface receptor (e.g., transferrin receptor [TfR]), the drug would be internalized by the endothelial cells and could pass through the BTB/BBB [12]. This targeting principle is being developed actively for potential brain tumor treatment. In this case, drug-delivery system efficacy depends on binding to both the endothelial (through TfR) and tumor cell surface. Ideally, both receptor-binding activities should reside on the same vehicle molecule. The role of this targeting moiety on a nanoconjugate delivery vehicle consists of selectively binding to the tumor tissue/cells and of eventually providing therapeutic concentration of the delivered drug.
Delivery molecules carrying and releasing at once several drugs that are active exclusively against tumor-specific molecules may provide enhanced drug specificity and efficacy. Typical drugs with high specificity are antisense RNA and siRNA to tumor-marker RNA. These technologies have lately attracted a lot of attention and will not be reviewed here, owing to space limitations. Targeting of a single tumor marker may not necessarily be fatal for tumor cell/tissue. Targeting of several different markers at the same time has a potentially more powerful additive/synergistic impact. Simultaneous delivery of several drugs targeting different tumor markers on one carrier molecule has a better chance for a potent combined drug effect than a mixture of nanoconjugates carrying each drug separately. This concept of cell-surface targeting agents and multiple tumor marker-blocking agents on the same drug delivery molecule may lead to the development of the most powerful and patient-specific antitumor treatment. Nanoconjugate drug-delivery systems of this kind are already emerging.
Evolution of targeted nanoconjugate drug delivery
Specific tissue targeting [12] and sustained delivery are the primary goals of nanoconjugate drug delivery. Drug conjugation to synthetic and biological macromolecules was attempted a long time ago [13,14]; in particular, targeted delivery using immunoglobulin conjugates [15], until the principle of a polymer as a platform for targeted drug delivery was put forth [16].
Antibodies are used successfully for disease treatment [17,18] and are also suitable as biodegradable cell surface-targeting platforms for conjugated drug delivery [19]. Immunoconjugates and monoclonal antibodies (mAbs) are among US FDA-approved drugs and some others are in clinical trials [19]. Well known examples include doxorubicin (Doxil®), trastuzumab (Herceptin®) and bevacizumab (Avastin® and Lucentis®). However, because of the potential immunogenicity of antibodies, unless humanized, nonimmunogenic simple polymers may be more appealing as nanoconjugate platforms. Unfortunately, until very recently, polymers could only exploit the passive EPR-effect route for accumulation into tumor tissue.
The introduction by Kopecek of synthetic N-(2-hydroxypropyl) methacrylamide (HPMA) copolymers as drug carriers taking advantage of pinocytosis and endosomal cleavage of a polymer-conjugated oligopeptide spacer was an important step in the development of polymer-based targeted drug delivery [20–23]. It should be noted that the HPMA drug-delivery platform is not biodegradable and is not cleared through the kidneys in cases of high molecular weight (>40 kDa) above the renal threshold. However, efficient sustained drug delivery could be achieved and long-term systemic accumulation avoided by using short-sized HPMA chains (below the threshold of 40 kDa) crosslinked with peptides [24]. Alternative biodegradable nanoconjugate platforms include amino acid-derived polymers, especially poly(L-glutamic acid)s [25,26], poly(malic acid) [27–31], polysaccharides [32] and polyethylene glycol (PEG) [33,34]. Orally administered polysaccharides conjugated with the drug already have been used for targeting colon cancer [35]. A new development in this area is the use of RNA oligonucleotide aptamer-siRNA chimeras that target both cell-surface antigen and mRNA to inhibit the synthesis of tumor-related proteins [36]. It is unknown whether they also use passive (EPR effect) tumor targeting.
Strategy for building safe & efficient multifunctional nanoconjugates
Nanoconjugate engineering principles
A nanoconjugate carries cell-surface and molecular-targeting groups, prodrugs and auxiliary active groups, which provide endosomal uptake by the recipient target cell, endosomal membrane disruption and drug release into the cytoplasm. Bulky groups, such as PEG, protect the delivery system sterically from resorption and enzymatic cleavage. The functional groups, also called modules, of nanoconjugates are typically bound to a polymer backbone (‘platform’) at pendant chemically active moieties, such as -COOH, -OH or -NH2.
Biodegradable platform
Biodegradable and natural-derived polymers, for example, poly(aspartic acid), poly(glutamic acid)s, poly(malic acid) and polysaccharides, should be drug carriers of choice for the prevention of vehicle accumulation in tissues. Polymers can be considered degradable when they have -O-, -NH-, -phosphate, -S- and -S–S- groups in their backbone chain. Such polymers may be hydrolyzed and degraded to water and carbon dioxide. To avoid immunogenicity, the number and kinds of amino acids should be kept low. The conjugate half-life in the body must be such as to achieve a maximum antitumor efficacy and a minimum immune response. Frequently considered biodegradable polymers of synthetic and biological origin have pendant carboxyl groups available for chemical conjugation of drug, targeting and auxiliary modules. These platforms are used for systemic treatment after injection into the bloodstream. Other biopolymers, such as starch or chitosans, have been used as carriers for oral drug delivery [37,38].
Important properties of a biopolymer as a carrier platform are synthetic accessibility, high loading capacity, medium stability (biodegradability), minimal toxicity and immunogenicity. Biodegradable poly(L-glutamic acid), poly(L-aspartic acid) and β-poly(L-malic acid) contain a pendant carboxyl group at their monomer units and have comparable loading capacities (number of possible conjugated groups per gram of polymer). However, the polypeptides (poly(L-glutamic acid) and (poly(L-aspartic acid)) compared with the polyester (β-poly(L-malic acid)) have significant disadvantages, including insolubility in most organic solvents that renders chemistry difficult, a molecular stiffness from hindered rotation around the peptide bonds that enables only minimal relief from steric constraints by large modules, and cleavage by peptidases after application in the circulation. Poly(malic acid) at high doses did not provoke polymer-specific antibodies in mice or rabbits in contrast to the aforementioned polypeptides [39–41]. Importantly, glutamate produced by enzymatic cleavage of poly(glutamic acid) can induce apoptosis in neuronal cells and may contribute to glaucoma [42–45], as well as to lysosomal-storage disorder [46,47]. Poly(γ-D-glutamate) is a toxic component of Bacillus anthracis capsule and induces IgG antibodies [48]. The immunogenic and toxic properties of biopolymers and their monomeric units have to be assessed seriously, especially when considering repeated treatments.
Functional modules
Pendant to the nanoconjugate platform are attached modules with different functions that become sequentially active during delivery:
The cell-targeting modules, such as mAbs or peptides against cell-surface antigens over-expressed in tumors (e.g., TfR [49,50], folate receptor [51–53]), which drive cell uptake by the endosomal pathway;
A protection moiety, frequently PEG [54–56], that guards against enzymatic degradation and resorption by the reticuloendothelial system (RES) [57];
The active drugs or prodrugs that target molecular markers;
The module for drug release within the endosome, usually an enzymatically hydrolysable peptide bond [20] or a pH-dependent, spontaneously hydrolyzing, hydrazone bond [52,53,58];
The endosome escape module activated during maturation of endosomes to lysosomes, consisting of a combination of protonated and thus neutralized carboxylates and hydrophobic groups [31,59–62] or of ionizable moieties that become protonated during maturation [63] or peptides that become lysogenic during endosome acidification [63–65];
The module for drug release in the cytoplasm, if the nanoconjugate carrier is designed for endosomal escape by containing a disulfide link between the platform and the drug that is cleaved by cytoplasmic glutathione [31,58,66];
An optional module for nanoconjugate tissue localization and imaging, typically a fluorescent dye [31].
These modules may vary by structures and/or numbers (e.g., prodrugs), enabling a highly customizable and flexible drug-delivery system that can be modified readily according to particular treatment needs.
The targeting moieties of a nanoconjugate aim at tumor cell-surface markers. Additionally, tumor vascular markers may also be targeted to ensure active receptor-mediated permeation of the BTB or BBB [31]. One such module (e.g., a mAb) will bind to the endothelium on intravenous administration and ensure polymer transcytosis, whereas a second one (or sometimes the same mAb) on the same nanoconjugate molecule binds to the recipient tumor cell surface and achieves endosomal internalization of the carrier system. Examples of such moieties that enable transcytosis are receptor ligands or antibodies that bind to the TfR or receptors for folate, leptin or insulin [12,67,68]. The second targeting module will be directed to tumor-specific targets. By choosing different targets on recipient tumor cells, combination therapy with a single drug-delivery entity should possibly augment the antitumor effect. Examples of tumor cell-surface marker proteins of choice are EGFR and HER-2 [69].
In addition to surface antigen binding, the polymeric nanoconjugate is designed to target one or several (intracellular) molecular tumor markers, such as kinases or phosphatases by specific drugs, or tumor marker-encoding mRNA by antisense oligonucleotide (AON) or siRNA [30,70], all of them attached to the multivalent carrier platform.
Nanoconjugate drug-delivery pathway
A water-soluble nanoconjugate encounters a variety of challenges from the site of, for example, intravenous injection to the drug-targeting site(s) [71]. Its half-life can be limited by soluble and cellular degradative activities in the circulation, kidney clearance and uptake by the liver and spleen as parts of the RES. PEG conjugated to the platform sterically inhibits the access of degrading proteases and nucleases and minimizes recognition and elimination by blood cells [72]. To avoid fast renal clearance, the size of the delivery vehicle should be above a certain threshold. For HPMA nanoconjugates, the molecular weight should be higher than 40–50 kDa [24]. In addition, the size depends on the geometry of module arrangement on the platform [73]. Although increased polymer sizes preclude rapid glomerular clearance, they increase the chance of uptake and clearance by the RES [74,75].
To access the targeted cells, the drug-delivery vehicle needs to pass through the vascular endothelium into the interstitial space. In the brain, the BBB and/or BTB do not allow water-soluble molecules larger than 0.5 kDa to passively cross the blood vessel into brain parenchyma; tumor cells also activate efflux transporters that prevent passage of many systemically administered uncharged and lipophilic agents [11,12,76]. Binding of mAbs to TfRs or insulin receptors that are overexpressed at the surface of tumor cells and tumor endothelium enables the nanocarrier to pass through the BTB by transcytosis [12,31,77–79]. Transcytosis does not appear to cause complex degradation inside endothelial cells [80,81], especially not to cleave disulfide (S–S) bonds [82], thus enabling disulfide conjugation of the drugs to the nanocarrier platform.
Once in the interstitium, the delivery vehicle penetrates through tumor tissue, probably by diffusion, its rate depending on cell adhesion and density [83]. After binding to a surface antigen of recipient tumor cells (e.g., TfR), the nanoconjugate is internalized into early endosomes, which then, by fusion with primary lysosomes, mature into lysosomes with concomitant acidification towards pH 5. Drugs bound to the delivery nanoconjugate by peptide linkers can then be cleaved off the conjugate by enzymes of the maturing endosome, such as cathepsins, penetrate through the endosomal membrane into the cytoplasm and find the molecular target. However, hydrophilic drugs (e.g., AON or siRNA) cannot traverse the endosomal membrane and the nanoconjugate has to first escape the endosome into the cytoplasm using special hydrophobic modules by the mechanism described here (see Other modules). The drug will then be cleaved from the nanoconjugate by reduction of the linker disulfide group with glutathione, a mechanism that works efficiently for AON or siRNA [31].
Polycefin, an example of a multitargeting nanoconjugate
β-poly(L-malic acid), the biodegradable platform
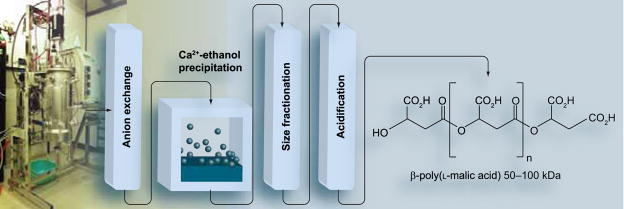
Polymers used as drug-delivery platforms are generally available as biosynthetic or synthetic materials [26–31,84–90]. Current examples of biodegradable polymers include poly(glutamic acid), poly(aspartic acid) and poly(malic acid). In general, chemical synthesis, although enabling large-scale production, frequently suffers from sticky, possibly toxic synthetic impurities or undesired chiral isomers formed during synthesis. We have worked extensively with β-poly(L-malic acid) (PMLA), a linear polyester of L-malic acid with an ester-forming carboxyl group in β-position and a pendant carboxyl group in α-position that remains available for conjugation with different functional groups [90]. This polyanion is synthesized by the slime mold Physarum polycephaliun, which uses PMLA for stockpiling and trafficking of nucleic acid-binding proteins across the plasmodium, a giant polynucleate amoeba-like cell. Large amounts of PMLA are secreted and eventually cleaved enzymatically to L-malate by a hydrolase [91]. PMLA with molecular weight Mn = 50–100 kDa (polydispersity Mw/Mn = 1.3, Mw = weight-averaged molecular weight, Mn = number-averaged molecular weight) from the culture broth of Physarum polycephalum can be highly purified (20 g PMLA from 20 l culture broth) and size-fractionated on Sephadex G25 (Figure 1) [92,93]. The lyophilized polyacid is devoid of material absorbing at 260 and 280 nm. This polymer was used by our group as a backbone for the construction of an anti-cancer drug-delivery system, which we termed Polycefin [31].
Figure 1. Production and purification of poly(malic acid) from Physarum polycephalum.
β-poly(L-malic acid) (PMLA) is produced by plasmodia of the slime mold. The polymer is isolated from the culture broth and purified by passages over DEAE-cellulose, ethanol precipitation of its Ca2+-salt, size fractionation on Sephadex G25 and acidification over Amberlite 120H+. Lyophillized PMLA is dry, white and of Mn (number-averaged molecular weight) 50–100 kDa. The yield from 20 l fermentation of glucose/C02 is 20 g polymer.
Drugs conjugated to the β-poly(L-malic acid) nanoplatform in use currently
In existing Polycefin variants, the drugs are antisense oligonucleotides (morpholino AONs) to mRNAs of α4 and β1 chains of a tumor vasculature-specific protein, laminin-411 (formerly, laminin-8). It is overexpressed in vessel walls of high-grade glial tumors, invasive breast cancers and their metastases [94,95]. The morpholino AON, unlike other antisense types, are very resistant to nucleases and are highly specific at low concentrations [96].
Laminins are a family of trimeric major structural basement membrane proteins that participate in cell differentiation, migration and proliferation [97]. They act as barriers for tumor cell penetration of surrounding tissues. At the same time, some laminins produced by tumor cells facilitate their migration by integrin receptors and promote tumor invasion [97,98]. Laminin-411 (α4β1γ1), a vascular basement membrane component, has important roles in angiogenesis (capillary maturation) and cell migration. We have documented overexpression of laminin-411 in grade IV human glioma (glioblastoma multiforme [GBM]) and ductal breast carcinoma [99,100]. Because laminins are trimeric proteins, inhibition of synthesis of more than one chain provides a more pronounced blocking of its production than inhibition of only one chain [101]. Moreover, AON inhibition of two laminin-411 chains (α4 + β1) was able to inhibit glioma invasion in vitro [101]. Therefore, our strategy was to block two laminin-411 chains rather than one in a multitargeting approach. Laminin-411 involvement in vessel formation and its overexpression in tumors suggest that its inhibition could reduce tumor neovascularization in vivo. In fact, this was confirmed recently for intracranial GBM implanted in rat brain [30].
Active tumor-targeting module
An antibody to TfR was chosen as the active targeting moiety that could carry Polycefin across the BTB to target glioma cells by binding first to the vascular endothelial TfR, undergo transcytosis, and then react with the tumor cell TfR and its endosomal uptake [31,68,102,103]. TfR is involved in the metabolism of iron mediated by transferrin and is overexpressed on the surface of tumor cells that have a vital need of iron. As described earlier, anti-TfR was used successfully at all stages of drug extravasation and tumor cell targeting. Some nanoconjugate variants contained other targeting antibodies [95], as discussed later.
Other modules
The endosome escape module consists of leucine ethyl ester moieties bound by the amino group to the pendant carboxyl of the PMLA platform. The high abundance of these nonpolar residues enables the formation of lipophilic ‘nests’ that can insert into and destabilize the endosome membrane, enabling leakage of Polycefin into the cytoplasm. Nest formation is increased by charge neutralization at decreased pH during endosome maturation. The drug-releasing module consists of disulfide spacers to liberate the AONs by reduction with cytoplasmic glutathione. The protection module consists of PEG. The reporter module is Alexa Fluor 680 or fluorescein and is optional.
Synthesis
For the chemical synthesis of nanoconjugates, synthetic or natural polymers with pendant carboxyl (-COOH), hydroxyl (-OH) or amino (-NH2) groups for the covalent binding of drugs and auxiliary modules are used. Synthetic platforms of HPMA [21,22,84–89] or PMLA [29] can also be designed that have spacers for module conjugation incorporated by their in situ polymerization. Here, we will focus on the synthesis of a nanoconjugate from a naturally occurring polymer platform.
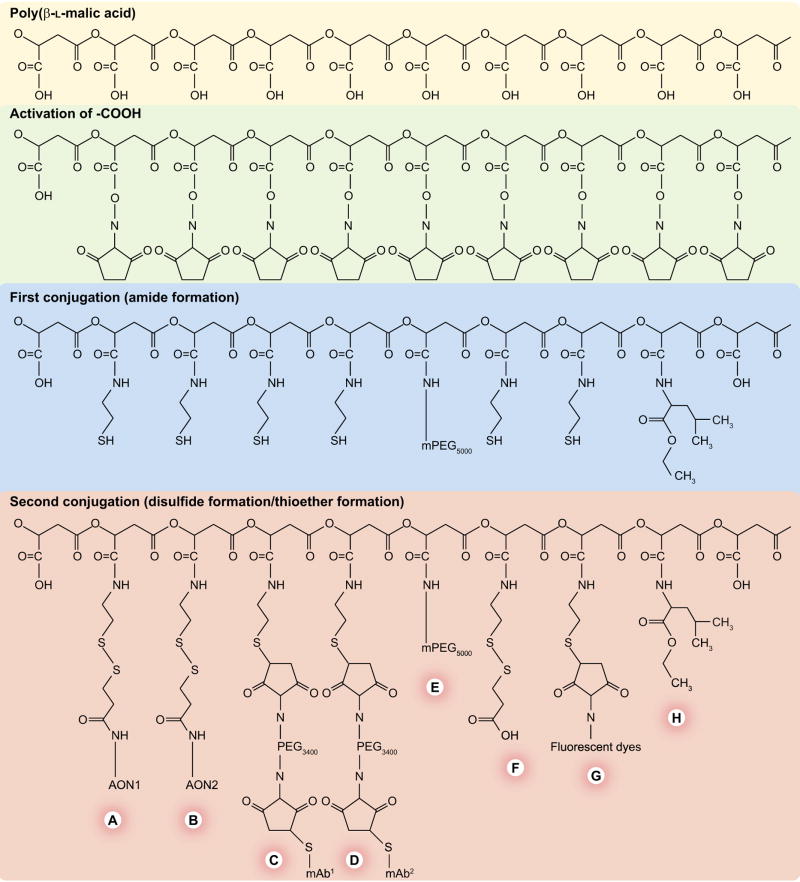
PMLA can be used as a platform for production of a number of different nanoconjugates. A general protocol for the synthesis of Polycefin variants has been established [31]. It involves a limited number of chemical reactions, depending on solubility, concentration and reactivity of the nanoconjugate intermediates and the number and chemical nature of the conjugated modules. Synthetic reactions are carried out in organic solvents or aqueous solutions. Because purification of macromolecular intermediates and products is tedious, the experimental conditions were optimized for completion of chemical reactions. Addition of different modules can be achieved by hierarchical order of sequential conjugations along chemically different reacting groups in the synthesis of Polycefin [31]. A simplified synthesis scheme is shown in Figure 2. In the first set of amide-forming reactions, the protection module (PEG), the endosome escape module (leucine ethylester) and a reactive spacer, 2-mercaptoethylamine, are conjugated to the NHS-ester-activated PMLA platform. In the second set, the targeting antibody and AON drugs are conjugated by a thioether bond and a disulfide bond, respectively. If more drugs or antibodies are needed, they are conjugated together in a mixture with any specific stoichiometry. Purification follows the principle of separation of excess small reactants from macromolecular products by membrane filtration or size-exclusion chromatography. Using this methodology, nanoconjugates can be obtained with predictable composition and stoichiometry [30,31,93,95].
Figure 2. Synthesis of Polycefin nanoconjugate with various functional modules.
Note the hierarchy in the choice of conjugation reactions. The platform consists of biodegradable highly purified β-poly(L-malic acid) from Physarum polycephalum. The carboxyl groups are first activated by esterification with N-hydroxysuccinimide. The hierarchy involves a first series of conjugation reactions with more durable residues, in other words, with polyethylene glycol (PEG), leucine ethylester and the thiol-containing spacer, 2-mercaptoethylamine. In the second series of conjugation reactions, the biochemically fragile molecules, monoclonal antibodies (mAbs) and antisense oligonucleotides (AONs), are conjugated by forming thioether and disulfide bonds, respectively. An optional fluorescent reporter molecule can be similarly conjugated. Nonreacted free thiol groups are then blocked chemically to prevent uncontrolled reactions. The modules are: (A) morpholino AON (to laminin α4 chain) and (B) (to laminin β1 chain) conjugated to the scaffold by disulfide bonds cleavable by the cytoplasmic glutathione to release the free drugs; (C & D) mAbs for cancer cell targeting and receptor-mediated endocytosis; (E) PEG for protection; (F) thiol-masking groups; (G) optional fluorescent reporter dye (fluorescein, Oregon Green or Alexa Fluor 680) for imaging and (H) stretches of conjugated leucine ethyl ester to provide lipophilicity for the disruption of endosomal membranes.
Physical & chemical methods of delivery validation
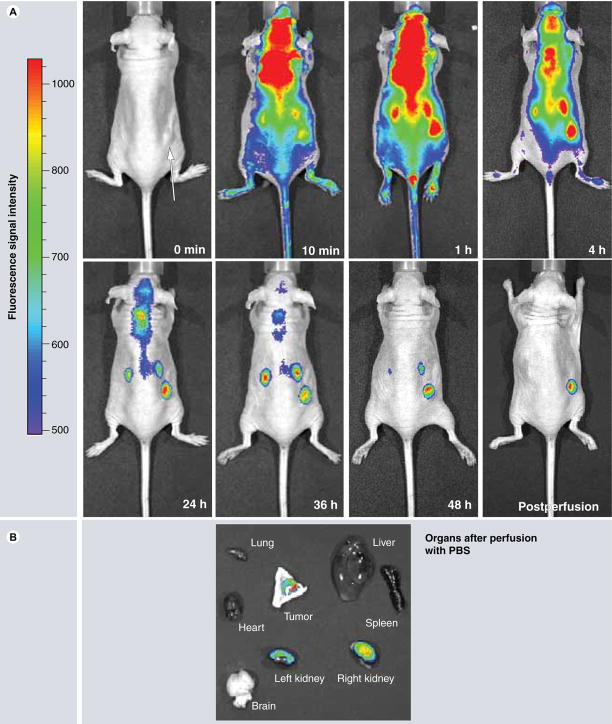
Polycefin distribution and accumulation in cells and tissues was studied routinely by tracking covalently attached fluorescent dye by confocal microscopy in vitro and by fluorescent imaging in vivo [30,31,93,95]. Results of in vivo imaging are presented in Figures 3–6. Polycefin nanoconjugate with covalently bound Alexa Fluor 680 dye was usually injected intravenously and its distribution was followed using a Xenogen IVIS 200 whole-animal fluorescence-imaging system. Mice bearing human brain or breast tumor xenografts have been imaged at different time points (Figures 3 & 5). In some cases, several organs (brain, heart, lung, liver, kidney and spleen) were removed from [euthanized animals for imaging analysis and the drugs remaining in the circulation were eliminated by intra-arterial PBS perfusion for 20 min (Figures 4 & 5).
Figure 3. Time course of whole mouse imaging with Xenogen IVIS 200 system after intravenous Polycefin administration into human brain tumor-bearing mice.
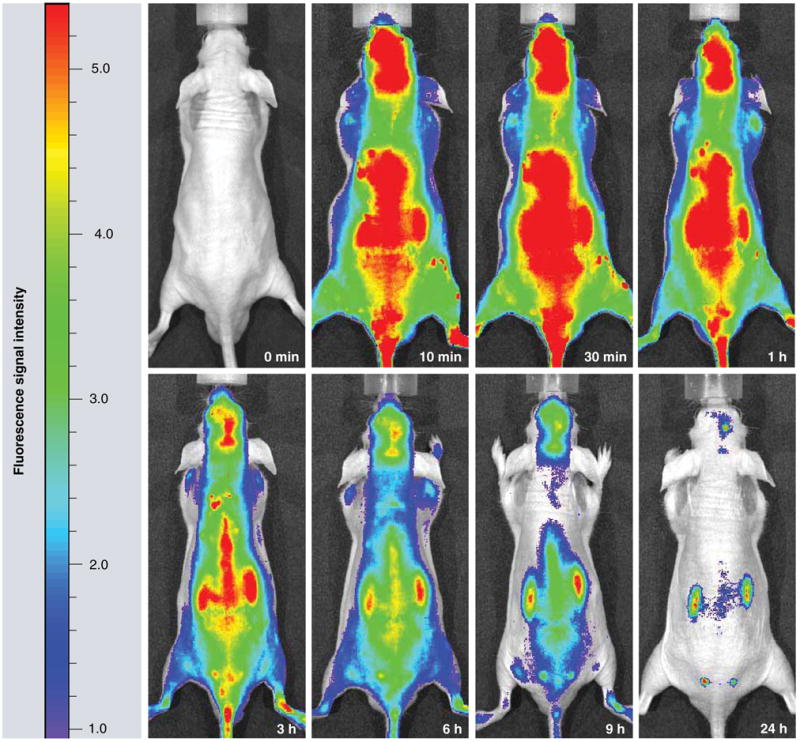
At time zero (0 min), Polycefin was injected into the tail vein. In the next 24 h, it is gradually removed by kidney clearance. By 24 h, only brain tumor and kidneys show significant Polycefin accumulation.
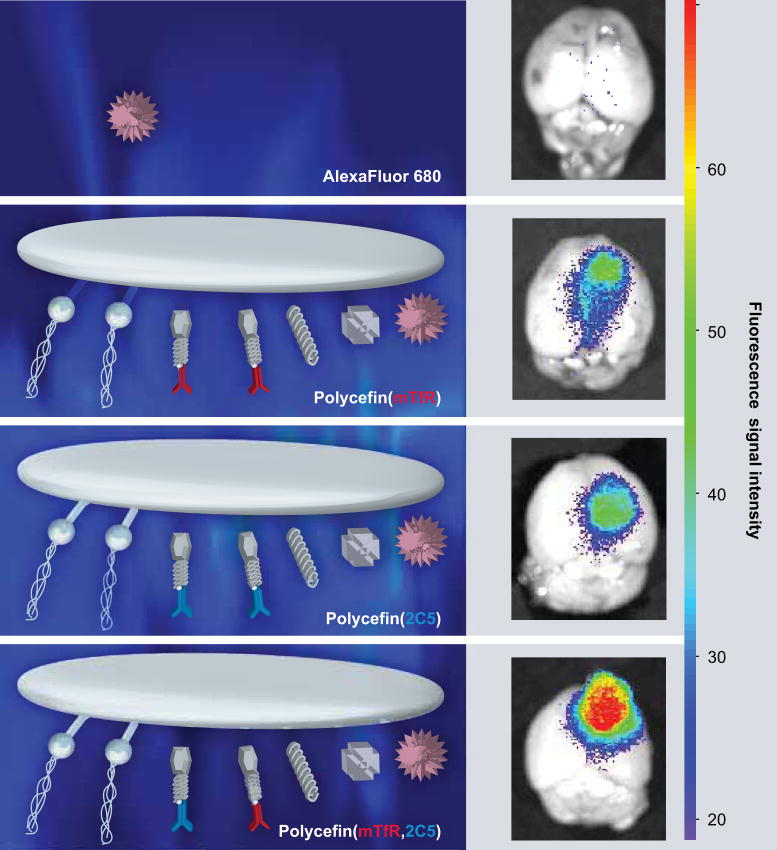
Figure 6. Fluorescence imaging of brain from nude mice bearing human U87MG glioma.
24 h after intravenous injection of free Alexa Fluor 680 and various Alexa Fluor 680-labeled Polycefin variants, only the tumor but not other areas of the brain contain fluorescent drug. Free drug does not show any accumulation. The highest drug accumulation in the tumor is observed with the tandem configuration in Polycefin (mTfR, 2C5) bearing two antibodies targeting mouse endothelium (anti-mTfR) and tumor cell (antinucleosomal monoclonal antibody 2C5 reacting with tumor cell surface antigen). Polycefin variants with one antibody (antimouse TfR or antitumor surface antigen) show less accumulation. A total of 100 μl of Alexa Fluor 680 (0.6 μM) or labeled Polycefin variants at concentrations of 3 μM antisense oligonucleotides were injected intravenously.
mTfR: Mouse transferrin receptor.
Reproduced with permission from [95].
Figure 5. Time course of mouse imaging with Xenogen IVIS 200 system after intravenous Polycefin administration into human brain tumor-bearing mice.
(A) Whole body imaging. Polycefin gradually disappears from circulation over 48 h time course. After 48 h, Polycefin accumulations remain only in the subcutaneous tumor and kidney. After PBS perfusion, most of Polycefin remains in the tumor. (B) Organ imaging 48 h after Polycefin administration. The drug accumulation is detected only in breast tumor and kidney as the drug-clearing organ.
PBS: Phosphate-buffered saline.
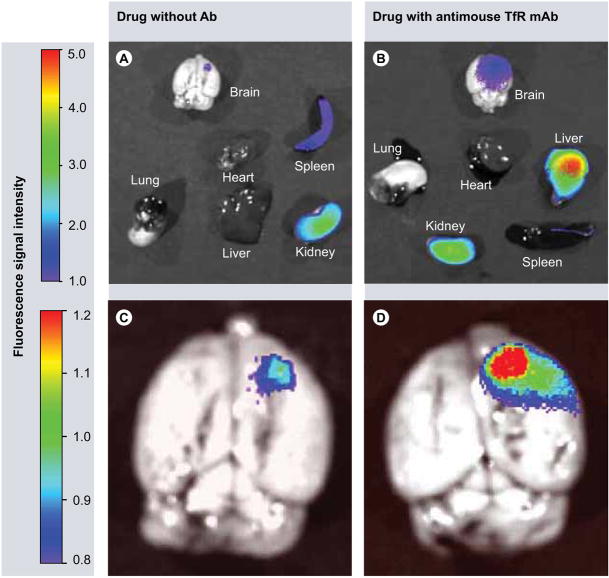
Figure 4. Imaging with different organs 24 h after intravenous administration of Polycefin variants.
(A & C) Imaging of mouse organs after treatment with Polycefin that has no targeting mAb attached. There is a slight accumulation in the brain tumor, some drug in the spleen and the majority of Polycefin is found in the kidney. (B & D) Polycefin variant with antimouse TfR mAb shows significant accumulation in the tumor, liver and kidney. Organs have been perfused with phosphate-buffered saline before imaging to remove unbound drug in the vessels.
mAb: Monoclonal antibody; TfR: Transferrin receptor.
Target-gene expression was tested by immunofluorescence and western blotting and the efficiency of endosomal escape and drug cleavage from the carrier were tested by hemolytic assay and glutathione reduction of S–S bonds, respectively [30,31]. These methods are straightforward and enable rapid and reliable analysis of new Polycefin variants directed against other molecular targets.
Nomenclature
It should be emphasized that a nanobioconjugate, such as Polycefin, is by physical definition not a particle but a macromolecule soluble in body fluid. The term Polycefin denotes a drug-delivery device with PMLA as the backbone to which various functional groups are attached covalently, for example, a mAb to TfR and leucyl ethylester as the active endosomal escape residue [31]. Polycefin variants with substitutions of the originally conjugated groups are indicated by the substituting molecule(s). Recently, versions of Polycefin containing other mAbs instead of anti-TfR antibody were synthesized. Polycefin(mTfR) is the version with mouse antihuman TfR, Polycefin(2C5) is the version with mouse nucleosome-specific mAb 2C5 that reacts with various cancer cells via tumor cell surface-bound nucleosomes [95,104–106] and Polycefin(mTfR,2C5) is the variant with both antibodies attached to one PMLA platform.
Cell-free studies of Polycefin functional modules
The PMLA platform did not contribute significantly to the polymer molecular weight when measured by reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis. Single IgG-conjugated Polycefin had the same electrophoretic mobility as IgG heavy chain [31]. The presence of two immunologically functional mAbs with different specificities on the variant Polycefin(mTfR,2C5) was documented by specific ELISA [95].
AONs conjugated by disulfide bonds to a Polycefin platform were released rapidly in the presence of millimolar concentrations of glutathione prevailing in the cytoplasm [31]. Using a hemolytic assay, it was shown that hydrophobic amino acids that were bound to the platform contributed significantly to membrane rupture necessary for endosomal escape, whereas PEG functioned as a nanoconjugate stabilizer as intended [31].
Polycefin effects in vivo on human tumor xenografts in nude mice & rats
Human glioma grown in rat brain was targeted efficiently with Polycefin-conjugated anti-rat TfR antibody that cross-reacted with human TfR on the surface of human GBM cells, thus enabling efficient transfer through the BTB and uptake by the tumor cells themselves [31]. Specific tumor targeting of Polycefin was also evident with human breast cancer (Figure 5) grown subcutaneously in mice [93].
Targeting of human tumor in mouse brain was also attempted using Polycefin variants with one or two antibodies (Figures 6 & 7). Polycefin(mTfR) facilitated transfer through the mouse BTB but did not have the ability to target human glioma cells owing to lack of cross-reactivity of anti-mouse mAb with human TfR [31,95]. Some Polycefin accumulation in the tumor was, however, observed (Figure 6). A similar degree of accumulation was seen with Polycefin(2C5) bearing only antitumor antibody (Figure 6). Significantly more efficient tumor targeting and higher drug accumulation was observed using Polycefin(mTfR,2C5) (Figures 6 & 7) with tandem mouse mTfR and antinucleosome 2C5 antibodies [95]. In this case, the first antibody could bind to the tumor endothelium and the second one reacted with a tumor cell-surface receptor (Figure 7). The drug component of Polycefin was aimed at inhibiting laminin-411 synthesis and thus tumor-specific angiogenesis. Indeed, angiogenesis in treated glioma was reduced substantially (Figure 8A) after Polycefin administration [30]. Inhibition of tumor angiogenesis was accompanied by a significantly prolonged survival of treated rats (Figure 8B) [30,31]. After intravenous Polycefin treatment, animals developed massive necrosis at the tumor site (Figure 9), apparently as a result of blocking tumor angiogenesis by targeting the synthesis of laminin-411.
Figure 7. Schematic illustrating Polycefin reaction with different cells in a human U87MG brain tumor xenograft model in mice.
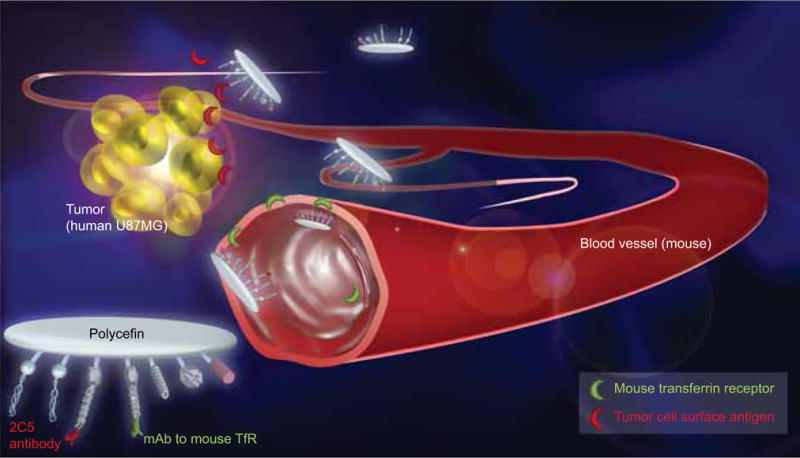
Polycefin contains mAbs to mouse TfR and tumor cell surface antigen. Binding of antimouse TfR to mouse endothelium causes drug internalization and transcytosis, whereby it becomes available to glioma cells that bind Polycefin through the attached 2C5 antibody. mAb: Monoclonal antibody; TfR: Transferrin receptor.
Figure 8. In vivo target inhibition by Polycefin and animal survival.
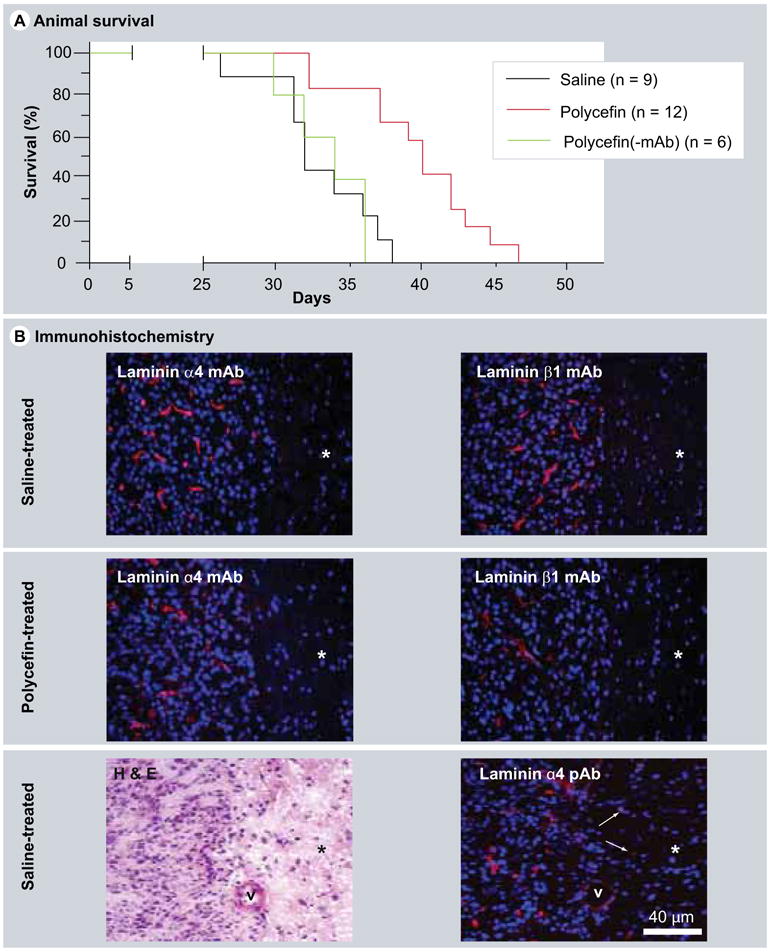
(A) Survival of Polycefin-treated (0.5 mg/kg body weight) and control rats. After intracranial administration of four doses of Polycefin, the animal survival time was significantly increased (p < 0.0004) compared with saline-treated or Polycefin(-mAb)-treated rats. (B) Immunofluorescent analysis of xenotransplanted brain tumors in rats with antihuman mAbs to laminin α4 or β1 chains. After Polycefin treatment, the number of tumor vessels positive for either laminin chain was markedly diminished. Therefore, Polycefin inhibited the expression of both its targets and their incorporation into basement membrane by human tumor cells. Asterisks denote tumor-adjacent (normal) brain area. This area has significantly decreased cellularity (revealed by blue nuclear staining with 4′,6-diamidino-2-phenylindole [DAPI]) compared with highly cellular tumor at the left. No vascular staining is observed in the tumor-adjacent area with both mAbs because the antibodies only recognized human laminin chains produced in this case by tumor astrocytes. Left lower panel, a H&E-stained tumor section showing a sharp boundary between highly cellular tumor (to the left) and surrounding brain parenchyma (asterisk) with significantly fewer cells. Right lower panel, staining of a serial section with a pAb to laminin α4 chain recognizing human and rat protein that reveals all vessels. Note increased vascularity and cellularity of the tumor as opposed to hypocellular surrounding tissue (asterisk) that has only scattered vessels (arrows), v, large blood vessel marked for orientation purposes.
H&E: Hematoxylin and eosin; mAb: Monoclonal antibody; pAb: Polyclonal antibody.
Reproduced with permission from [30].
Figure 9. Human brain tumor in a nude mouse without and after intravenous Polycefin treatment.
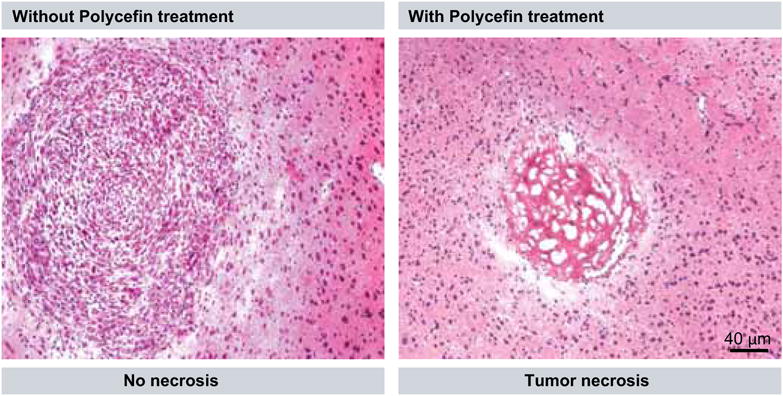
(A) Multicellular glioma structure developed after inoculation of U87MG cell line intracranially into the nude mouse that died at day 32. (B) Massive necrosis that replaced glioma structure after eight intravenous treatments with Polycefin of a nude mouse sacrificed at day 74.
These data offer promise for the future use of Polycefin with a specific combination of targeting antibodies and antitumor drugs for the treatment of various tumors. Specific variants of the nanoconjugate may be directed against patient-specific tumor markers, providing individualized cancer therapy.
Conclusions & future perspective
The results reported here for Polycefin nanoconjugates support the idea that tandem configurations of platform-conjugated antibodies or other entities with appropriate target specificities and combinations of different molecular-drug species on one carrier are feasible and may enhance the efficacy of tumor treatment significantly.
To simplify Polycefin structure, an alternative approach may use single inhibitors that act on multiple pathways simultaneously by blocking the production and/or function of groups of enzymes, such as tyrosine kinases, or master regulatory molecules, such as protein kinase CK2. In the first group of drugs, SU11248 [2], AEE788 [9] and RAD001 [107] are being considered seriously as potent anticancer drugs. Each of them inhibits several targets associated with tyrosine kinase-dependent signaling pathways abnormal in many tumors. Serine-threonine protein kinase CK2, as a representative of the second group, acts on more than 300 protein substrates inside the cell and its single inhibitors can block cell proliferation, migration, tumor growth and angiogenesis and can increase cancer cell apoptosis [108–112]. The respective inhibitor drugs are potential candidates for future Polycefin variants or similar nanoconjugates.
The future Polycefin compounds may not only be directed against cancer cells but could also be used in other pathological conditions requiring specific drug delivery. Such conditions may include neovascularization in the eye as exemplified by widespread vision-threatening diseases, such as age-related macular degeneration (AMD) and proliferative diabetic retinopathy (PDR). FDA-approved aptamer (pegaptanib/Macugen® from Pfizer, Inc., NY, USA) and antibody-based (ranibizumab/Lucentis® from Genentech, Inc., CA, USA) anti-VEGF drugs are already being used to treat the wet (neovascular) form of AMD, with beneficial effects to patients after intravitreal injections [113]. It is possible that the addition of such drugs to neovasculature-targeting nanoconjugates may increase their efficacy and specificity with reduction of side effects. Anti-PDR drug development is rather slow because this disease appears to be more multifactorial than AMD and may require multitargeting approach feasible for nanoconjugates. A Polycefin variant with a conjugated CK2 inhibitor or inhibitors of several angiogenic pathways may prove to be a promising candidate for PDR therapy.
In the cardiovascular field, Polycefin nanoconjugates that bear inhibitors of antiangiogenic molecules, such as thrombospondins, may be used. These drug-delivery systems may be beneficial for patients with myocardial infarction who need enhanced angiogenesis at the site of injury for efficient tissue repair and relief of ischemia [114]. Some small-molecule effectors, such as ONO-1301 (a synthetic prostacyclin agonist), have been developed that activate angiogenic growth factor signaling pathways and promote angiogenesis in the ischemic heart [115].
The success of nanoconjugate drug delivery, as manifested by recent FDA approval and clinical trials, as well as the development of second-generation nanoconjugate multidrug-delivery systems have been reviewed recently [116].
Poly(malic acid) as a polymer with multiple functional groups offers great potential for future production of tandem-configured drug-carrier systems. The Polycefin system with the combination of anti-TfR to direct the conjugate across the BTB and 2C5 antibody to target tumor cells represents a promising nanoplatform for brain tumor treatment. Further studies of this platform should provide a better understanding of the optimal targeting mechanisms and conditions. A tandem-type Polycefin might serve in future as a potential therapeutic modality for the treatment of patients with brain tumors and in experimental cancer studies. The demonstrated versatility, multitargeting ability and biodegradable nature of the PMLA platform warrant further development of Polycefin nanoconjugates for the treatment of various diseases, including cancer, ocular neovascular diseases and cardiovascular ischemic conditions.
Executive summary
Nanoconjugates for drug delivery
Nanoconjugates are tailored macromolecules harboring covalently bound biologically active modules to target specific tissue/cells and disease-marker molecules.
Unlike micelles, liposomes or sponge-like drug carriers, nanoconjugates do not leak drug on their delivery pathway and thus do not display toxicity towards healthy tissue/cells.
Targeting
One nanoconjugate molecule can harbor multiple targeting molecules and drugs, also enabling recognition and penetration of biological barriers after systemic administration in addition to targeting cells and molecular markers.
Synergistic therapeutic effect is thus possible and should be more effective than monotherapy for tumor treatment because of several drugs or molecular inhibitors present on the nanoconjugate at the same time.
Biodegradability
Biodegradability is an essential feature of successful nanoconjugates used for drug delivery in order to avoid deposition of material in host tissue after repeated application.
Polycefin family
Nanoconjugates of the poly(malic acid) platform have proven to be nontoxic, nonimmunogenic and biodegradable.
Polycefin nanoconjugate delivery devices allow putting together a covalent conjugate of high loads of biologically functioning modules at the multiple pendant carboxyl groups of the polyester platform.
Multitargeting by Polycefin-conjugated monoclonal antibodies
Blood–brain barrier, blood–tumor barrier and tumor marker molecules are targeted with β-poly(L-malic acid) (PMLA)-conjugated monoclonal antibodies and antisense oligonucleotides (AONs).
In the future, a variety of other targeting groups/drugs can be added provided they have chemically functional moieties for the conjugation with the carboxylic groups of the PMLA platform.
Tumor imaging & tumor treatment
The accumulation of Polycefin in tumor tissue after systemic application to experimental animals can be followed by tracking platform-conjugated fluorescent dyes. Xenogeneic animal models with inoculated human glioma and breast tumor cells show tumor-specific Polycefin accumulation.
The nanoconjugate with AONs to tumor-specific laminins inhibits tumor angiogenesis, tumor growth and prolongs survival of glioma-bearing animals.
Acknowledgments
The authors are indebted to Natalya M Khazenzon (Cedars-Sinai) for expert assistance through the course of this work and to Reinhard Sterner and Sonja Fuchs, Regensburg University, Germany, for technical support for β-poly(L-malic acid) production.
Financial & competing interests disclosure: This work was supported by grants from NIH (CA123495 to JY Ljubimova, EY13431 to AV Ljubimov), The Skirball Program in Molecular Ophthalmology, Winnick Family Foundation Research Scholar award and M01 RR00425 (to AV Ljubimov), a grant from Arrogene Inc., as well a grant from the Department of Neurosurgery, Cedars-Sinai Medical Center. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Zinner RG, Herbst RS. Pemetrexed in the treatment of advanced non-small-cell lung cancer: a review of the clinical data . Clin Lung Cancer. 2004;5 (Suppl 2):S67–S74. doi: 10.3816/clc.2004.s.006. [DOI] [PubMed] [Google Scholar]
- 2.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinopoulos PA, Vandoros GP, Papavassiliou AG. FK228 (depsipeptide): a HDAC inhibitor with pleiotropic antitumor activities. Cancer Chemother Pharmacol. 2006;58:711–715. doi: 10.1007/s00280-005-0182-5. [DOI] [PubMed] [Google Scholar]
- 4.Montemurro F, Valabrega G, Aglietta M. Lapatinib: a dual inhibitor of EGFR and HER2 tyrosine kinase activity. Expert Opin Biol Ther. 2007;7:257–268. doi: 10.1517/14712598.7.2.257. [DOI] [PubMed] [Google Scholar]
- 5•.Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. Basic article for understanding the importance of enhanced permeability and retention in drug delivery. [DOI] [PubMed] [Google Scholar]
- 6.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers for overcoming drug resistance in cancer. Adv Drug Deliv Rev. 2002;13:759–779. doi: 10.1016/s0169-409x(02)00047-9. [DOI] [PubMed] [Google Scholar]
- 7.Luo Y, Prestwich GD. Cancer-targeted polymeric drugs. Curr Cancer Drug Targets. 2002;2:209–226. doi: 10.2174/1568009023333836. [DOI] [PubMed] [Google Scholar]
- 8.Heaney NB, Holyoake TL. Therapeutic targets in chronic myeloid leukaemia. Hematol Oncol. 2007;25:66–75. doi: 10.1002/hon.813. [DOI] [PubMed] [Google Scholar]
- 9.Younes MN, Park YW, Yazici YD, et al. Concomitant inhibition of epidermal growth factor and vascular endothelial growth factor receptor tyrosine kinases reduces growth and metastasis of human salivary adenoid cystic carcinoma in an orthotopic nude mouse model. Mol Cancer Ther. 2006;5:2696–2705. doi: 10.1158/1535-7163.MCT-05-0228. [DOI] [PubMed] [Google Scholar]
- 10.Khandare JJ, Minko T. Antibodies and peptides in cancer therapy. Crit Rev Ther Drug Carrier Syst. 2006;23:401–436. doi: 10.1615/critrevtherdrugcarriersyst.v23.i5.20. [DOI] [PubMed] [Google Scholar]
- 11.Badruddoja MA, Black KL. Improving the delivery of therapeutic agents to CNS neoplasms: a clinical review. Front Biosci. 2006;11:1466–1478. doi: 10.2741/1896. [DOI] [PubMed] [Google Scholar]
- 12•.Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–558. doi: 10.1016/s0896-6273(02)01054-1. Important article about the role of the blood–brain barrier (BBB) that has to be overcome for drug delivery. [DOI] [PubMed] [Google Scholar]
- 13.Jatzkewitz H. Peptamin (glycyl-L-leucylmescaline) bound to blood plasma expander (polyvinylpyrrolidone) as a new depot form of a biologically active primary amine (Mescaline) Z Naturforsch. 1955;10b:27–31. [Google Scholar]
- 14.Panarin EF, Ushakov SN. Synthesis of polymer salts and amidopenicillines. Khim Pharm Zhur. 1968;2:28–311. [Google Scholar]
- 15.Mathé G, Lo TB, Bernard J. Effect sur la leucémie L1210 de la souris d’une combinaison par diazotation d’améthoptérine et de γ-globulines de hamsters porteurs de cette leucémie par hétérogreffe. C R Hebd Seances Acad Sci. 1958;246:1626–1628. [PubMed] [Google Scholar]
- 16•.Ringsdorf H. Structure and properties of pharmacologically active polymers. J Polym Sci Polym Symp. 1975;51:135–153. Important article introducing polymers as platforms of nanoconjugates. [Google Scholar]
- 17.Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat Rev Drug Discov. 2003;2:52–62. doi: 10.1038/nrd984. [DOI] [PubMed] [Google Scholar]
- 18.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 19.Schrama D, Reisfeld RJ, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5:147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 20•.Kopecek J, Kopecková P, Minko T, et al. HPMA copolymer-anticancer drug conjugates: design, activity, and mechanism of action. Em J Pharm Biopharm. 2000;50:61–81. doi: 10.1016/s0939-6411(00)00075-8. Important introduction into the field of N-(2-hydroxypropyl) methacrylamide-based nanoconjugates. [DOI] [PubMed] [Google Scholar]
- 21.Kopecek J, Kopeckova P, Minko T, et al. Water soluble polymers in tumor targeted delivery. J Control Release. 2001;74:147–158. doi: 10.1016/s0168-3659(01)00330-3. [DOI] [PubMed] [Google Scholar]
- 22.Duncan R. Drug–polymer conjugates: potential for improved chemotherapy. Anticancer Drugs. 1992;3:175–210. doi: 10.1097/00001813-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Nori A, Kopecek J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv Drug Deliv Rev. 2005;57:609–636. doi: 10.1016/j.addr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Sprincl L, Exner J, Sterba O, et al. New types of synthetic infusion solutions III Elimination and retention of poly(N-(2-hydroxypropyl)methacrylamide) in a test organism. J Biomed Mater Res. 1976;10:953–963. doi: 10.1002/jbm.820100612. [DOI] [PubMed] [Google Scholar]
- 25.Haider M, Megeed Z, Ghandehari H. Genetically engineered polymers: status and prospects for controlled release. J Contol Release. 2004;95:1–26. doi: 10.1016/j.jconrel.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Li C. Poly(L-glutamic acid)–anticancer drug conjugates. Adv Drug Deliv Rev. 2002;54:695–713. doi: 10.1016/s0169-409x(02)00045-5. [DOI] [PubMed] [Google Scholar]
- 27.Ouchi T, Fujino A, Tanaka K, et al. Synthesis and antitumor activity of conjugates of poly(α-malic acid) and 5-fluoroacils bound via ester, amide or carbamoyl bonds. J Control Release. 1990;12:143–153. [Google Scholar]
- 28.Abdellaouri K, Boustta M, Vert M, et al. Metabolic-derived artificial polymers designed for drug targeting, cell penetration and bioresorption. Eur J Pharm Sci. 1998;6:61–73. doi: 10.1016/s0928-0987(97)00069-9. [DOI] [PubMed] [Google Scholar]
- 29.Cammas S, Béar M-M, Moine L, et al. Polymers of malic acid and 3-alkylmalic acid as synthetic PHAs in the design of biocompatible hydrolysable devices. Int J Biol Macromol. 1999;25:273–282. doi: 10.1016/s0141-8130(99)00042-2. [DOI] [PubMed] [Google Scholar]
- 30.Fujita M, Khazenzon NM, Ljubimov AV, et al. Inhibition of laminin-8 in vivo using a novel poly(malic acid)-based carrier reduces glioma angiogenesis. Angiogenesis. 2006;9:183–191. doi: 10.1007/s10456-006-9046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Lee B-S, Fujita M, Khazenzon NM, et al. E. Polycefin, a new prototype of a multifunctional nanoconjugate based on poly(β-L-malic acid) for drug delivery. Bioconjug Chem. 2006;17:317–326. doi: 10.1021/bc0502457. Important article introducing the Polycefin concept of targeted drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugahara S, Okuno S, Yano T, et al. Characteristics of tissue distribution of various polysaccharides as drug carriers: influences of molecular weight and anionic charge on tumor targeting. Biol Pharm Bull. 2001;24:535–543. doi: 10.1248/bpb.24.535. [DOI] [PubMed] [Google Scholar]
- 33.Tomlinson R, Heller J, Brocchini S, et al. Polyacetal–doxorubicin conjugates designed for pH-dependent degradation. Bioconjug Chem. 2003;14:1096–1106. doi: 10.1021/bc030028a. [DOI] [PubMed] [Google Scholar]
- 34.Fleming AB, Haverstick K, Saltzman WM. In vitro cytotoxicity and in vivo distribution after direct delivery of PEG–camptothecin conjugates to the rat brain. Bioconjug Chem. 2004;15:1364–1375. doi: 10.1021/bc034180o. [DOI] [PubMed] [Google Scholar]
- 35.Sinha VR, Kumria R. Polysaccharides in colon-specific drug delivery. Int J Pharm. 2001;214:19–38. doi: 10.1016/s0378-5173(01)00720-7. [DOI] [PubMed] [Google Scholar]
- 36.McNamara JO, II, Andrechek ER, Wang Y, et al. Cell type-specific delivery of siRNA aptamer-siRNA chimeras. Nat Biotechnol. 2006;24:1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 37.Deres S, Gdalevsky GY, Gilboa I, et al. Bioadhesive grafted starch copolymers as platform for peroral drug delivery: a study of theophylline release. J Control Release. 2004;94:391–399. doi: 10.1016/j.jconrel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Roldo M, Hornof M, Caliceti P, et al. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: synthesis and in vitro evaluation. Eur J Pharm Biopharm. 2004;57:115–121. doi: 10.1016/s0939-6411(03)00157-7. [DOI] [PubMed] [Google Scholar]
- 39.Murphy PD, Sage HJ. Variation in the size of antibody sites for the poly-L-aspartate hapten during the immune response. J Immunol. 1970;105:460–470. [PubMed] [Google Scholar]
- 40.Wang Y, Dias JA, Nimec Z, et al. The properties and function of γ-glutamyl hydrolase and poly-γ-glutamate. Adv Enzyme Regul. 1993;33:207–218. doi: 10.1016/0065-2571(93)90019-a. [DOI] [PubMed] [Google Scholar]
- 41.Maurer PH, Gerulat BF, Pinchuck P. Antigenicity of polypeptides (poly-α-amino acids) XI Quantitative relationships among polymers and rabbit antisera. J Biol Chem. 1964;239:922–929. [PubMed] [Google Scholar]
- 42.Ruiz F, Alvarez G, Ramos M, et al. Cyclosporin A targets involved in protection against glutamate excitotoxicity. Eur J Pharmacol. 2000;404:29–39. doi: 10.1016/s0014-2999(00)00584-7. [DOI] [PubMed] [Google Scholar]
- 43.Kumar DM, Perez E, Cai ZY, et al. Role of nonfeminizing estrogen analogues in neuroprotection of rat retinal ganglion cells against glutamate-induced cytotoxicity. Free Radic Biol Med. 2005;38:1152–1163. doi: 10.1016/j.freeradbiomed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Bhavnani BR. Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens. BMC Neurosci. 2006;7:49–71. doi: 10.1186/1471-2202-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta N, Yucel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007;18:110–114. doi: 10.1097/ICU.0b013e3280895aea. [DOI] [PubMed] [Google Scholar]
- 46.Kishore BK, Lambricht P, Laurent G, et al. Mechanism of protection afforded by polyaspartic acid against gentamicin-induced phospholipidosis II Comparative in vitro and in vivo studies with poly-L-aspartic, poly-L-glutamic and poly-D-glutamic acids. J Pharmacol Exp Ther. 1990;255:875–885. [PubMed] [Google Scholar]
- 47.Kishore BK, Maldague P, Tulkens PM, et al. Poly-D-glutamic acid induces an acute lysosomal thesaurismosis of proximal tubules and a marked proliferation of interstitium in rat kidney. Lab Invest. 1996;74:1013–1023. [PubMed] [Google Scholar]
- 48.Schneerson R, Kubler-Kielb J, Liu TY, et al. Poly(γ-D-glutamic acid) protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: a potential addition to the anthrax vaccine. Proc Natl Acad Sci USA. 2003;100:8945–8950. doi: 10.1073/pnas.1633512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardridge WM. Vector-mediated drug delivery to the brain. Adv Drug Deliv Rev. 1999;36:299–321. doi: 10.1016/s0169-409x(98)00087-8. [DOI] [PubMed] [Google Scholar]
- 50.Qian ZM, Li H, Sun H, et al. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 51.Leamon CP, Reddy JA. Folate-targeted chemotherapy. Adv Drug Deliv Rev. 2004;56:1127–1141. doi: 10.1016/j.addr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Bae Y, Jang WD, Nishiyama N, et al. Multifunctional polymeric micelles with folate-mediated cancer cell targeting and pH-triggered drug releasing properties for active intracellular drug delivery. Mol Biosyst. 2005;1:242–250. doi: 10.1039/b500266d. [DOI] [PubMed] [Google Scholar]
- 53.Chytil P, Etrych T, Konak C, et al. Properties of HPMA copolymer-doxorubicin conjugates with pH-controlled activation: effect of polymer chain modification. J Control Release. 2006;115:26–36. doi: 10.1016/j.jconrel.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 54.Maruyama K, Takahashi N, Toshiaki T, et al. Immunoliposomes bearing polyethyleneglycol-coupled Fab′ fragment show prolonged circulation time and high extravasation into targeted solid tumors in vivo. FEBS Lett. 1997;413:177–180. doi: 10.1016/s0014-5793(97)00905-8. [DOI] [PubMed] [Google Scholar]
- 55.Greenwald RB, Choe YH, McGuire J, et al. Effective drug delivery by PEGylated drug conjugates. Adv Drug Deliv Rev. 2003;55:217–250. doi: 10.1016/s0169-409x(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 56.Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev. 2003;55:403–419. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 57•.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. Important for understanding the function of PEG in drug delivery. [DOI] [PubMed] [Google Scholar]
- 58.West KR, Otto S. Reversible covalent chemistry in drug delivery. Curr Drug Discov Technol. 2005;2:123–160. doi: 10.2174/1570163054866882. [DOI] [PubMed] [Google Scholar]
- 59•.Philippova OE, Hourdet D, Audebert R, et al. pH-Responsive gels of hydrophobically modified poly(acrylic acid) Macromolecules. 1997;30:8278–8285. Basics for understanding the role of ionizing carboxyls and of lipophilic residues of the nanoconjugate in endosomal escape. [Google Scholar]
- 60.Murthy N, Robichaud JR, Tirrell DA, et al. The design and synthesis of polymers for eukaryotic membrane disruption. J Control Release. 1999;61:137–143. doi: 10.1016/s0168-3659(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 61.Turk MJ, Reddy JA, Chmielewski JA, et al. Characterization of a novel pH-sensitive peptide that enhances drug release from folate-targeted liposomes at endosomal pHs. Biochim Biophys Acta. 2002;1559:56–68. doi: 10.1016/s0005-2736(01)00441-2. [DOI] [PubMed] [Google Scholar]
- 62.Rozema DB, Ekena K, Lewis DL, et al. Endosomolysis by masking of a membrane-active agent (EMMA) for cytoplasmic release of macromolecules. Bioconjug Chem. 2003;14:51–57. doi: 10.1021/bc0255945. [DOI] [PubMed] [Google Scholar]
- 63.Merdan T, Kopecek J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Deliv Rev. 2002;54:715–758. doi: 10.1016/s0169-409x(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 64.Mastrobattista E, Koning GA, Bloois L, et al. Functional characterization of an endosome-disruptive peptide and its application in cytosolic delivery of immunoliposome-entrapped proteins. J Biol Chem. 2002;277:27135–27143. doi: 10.1074/jbc.M200429200. [DOI] [PubMed] [Google Scholar]
- 65.Cho YW, Kim JD, Park K. Polycation gene delivery systems: escape from endosomes to cytosol. J Pharm Pharmacol. 2003;55:721–734. doi: 10.1211/002235703765951311. [DOI] [PubMed] [Google Scholar]
- 66.Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 67.Reddy JA, Allagadda VM, Leamon CP. Targeting therapeutic and imaging agents to folate receptor positive tumors. Curr Pharm Biotechnol. 2005;6:131–150. doi: 10.2174/1389201053642376. [DOI] [PubMed] [Google Scholar]
- 68•.Daniels TR, Delgado T, Helguera G, et al. The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin Immunol. 2006b;121:159–176. doi: 10.1016/j.clim.2006.06.006. Important article about the use of transferrin receptor in drug delivery. [DOI] [PubMed] [Google Scholar]
- 69.Solit DB, Rosen N. Targeting HER2 in prostate cancer: where to next? J Clin Oncol. 2007;3:241–243. doi: 10.1200/JCO.2006.08.8187. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Zhang YF, Bryant J, et al. Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin Cancer Res. 2004;10:3667–3677. doi: 10.1158/1078-0432.CCR-03-0740. [DOI] [PubMed] [Google Scholar]
- 71.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 72.Papisov MI. Theoretical considerations of RES-avoiding liposomes: molecular mechanics and chemistry of liposome interactions. Adv Drug Deliv Rev. 1998;32:119–138. doi: 10.1016/s0169-409x(97)00135-x. [DOI] [PubMed] [Google Scholar]
- 73.Seymour LW, Duncan R, Strohalm J, et al. Effect of molecular weight of N-(2-Hydroxypropyl)methacrylamide copolymers on body distribution and rate of excretion after subcutaneous, intraperitoneal, and intravenous administration to rats. J Biomed Mater Res. 1987;21:1341–1358. doi: 10.1002/jbm.820211106. [DOI] [PubMed] [Google Scholar]
- 74.Gaur U, Sahoo SK, De TK, et al. Biodistribution of fluoresceinated dextran using novel nanoparticles evading reticuloendothelial system. Int J Pharm. 2000;202:1–10. doi: 10.1016/s0378-5173(99)00447-0. [DOI] [PubMed] [Google Scholar]
- 75.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 76.Taylor EM. The impact of efflux transporters in the brain on the development of drugs for CNS disorders. Clin Pharmacokinet. 2002;41:81–92. doi: 10.2165/00003088-200241020-00001. [DOI] [PubMed] [Google Scholar]
- 77.Coloma MJ, Lee HJ, Kurihara A, et al. Transport across the primate blood–brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm Res. 2000;17:266–274. doi: 10.1023/a:1007592720793. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Pardridge WM. Delivery of β-galactosidase to mouse brain via the blood–brain barrier transferrin receptor. J Pharmacol Exp Ther. 2005;313:1075–1081. doi: 10.1124/jpet.104.082974. [DOI] [PubMed] [Google Scholar]
- 79•.Pardridge WM. Blood-brain-barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3:90–105. doi: 10.1124/mi.3.2.90. Important article on the role of the BBB in drug delivery to the brain. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi K, Suzuki K, Ichikim Y, et al. Transcytosis of lipid microspheres by human endothelial cells. Pharmacology. 1996;53:37–47. doi: 10.1159/000139413. [DOI] [PubMed] [Google Scholar]
- 81.Lu W, Tan YZ, Hu KL, et al. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood-brain barrier. Int J Pharm. 2005;295:247–260. doi: 10.1016/j.ijpharm.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 82.Kang YS, Voigt K, Bickel U. Stability of the disulfide bond in an avidin-biotin linked chimeric peptide during in vivo transcytosis through brain endothelial cells. J Drug Target. 2000;8:425–434. doi: 10.3109/10611860008997918. [DOI] [PubMed] [Google Scholar]
- 83.Grantab R, Sivananthan S, Tannock IF. The penetration of anticancer drugs through tumor tissue as a function of cellular adhesion and packing density of tumor. Cancer Res. 2006;66:1033–1039. doi: 10.1158/0008-5472.CAN-05-3077. [DOI] [PubMed] [Google Scholar]
- 84.Kopecek J, Baziliva H. Poly[n-(2-hydroxypropyl)methacrylamide]. 1 Radical polymerization and copolymerization. Europ Polym J. 1973;9:7–14. [Google Scholar]
- 85.Duncan R, Seymour LC, Scarlett L, et al. Fate of N-(2-hydroxypropyl)methacrylamide copolymers with pendent galactosamine residues after intravenous administration to rats. Biochim Biophys Acta. 1986;880:62–71. doi: 10.1016/0304-4165(86)90120-0. [DOI] [PubMed] [Google Scholar]
- 86.Omelyanenko V, Kopeckova P, Prakash RK, et al. Biorecognition of HPMA copolymer-adriamycin conjugates by lymphocytes mediated by synthetic receptor binding epitopes. Pharm Res. 1999;16:1010–1019. doi: 10.1023/a:1018975414165. [DOI] [PubMed] [Google Scholar]
- 87.Kunath K, Kopceckova P, Minko T, et al. HPMA copolymer-anticancer drug-OV-TL16 antibody conjugates 3 The effect of free and polymer-bound adriamycin on the expression of some genes in the OVCAR-3 human ovarian carcinoma cell line. Eur J Pharm Biopharm. 2000;49:11–15. doi: 10.1016/s0939-6411(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 88.Etrych T, Jelinkova M, Rihova B, et al. New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: synthesis and preliminary in vitro and in vivo biological properties. J Control Release. 2001;73:89–102. doi: 10.1016/s0168-3659(01)00281-4. [DOI] [PubMed] [Google Scholar]
- 89.Jensen KD, Kopeckova P, Kopecek J. Antisense oligonucleotides delivered to the lysosome escape and actively inhibit hepatitis B virus. Bioconjug Chem. 2002;13:975–984. doi: 10.1021/bc025559y. [DOI] [PubMed] [Google Scholar]
- 90.Lee B-S, Vert M, Holler E. Water-soluble aliphatic polyesters: poly(malic acid)s. In: Doi V, Steinbuechel A, editors. Biopolymers. 3a. Wiley-VCH; New York, USA: 2002. pp. 75–103. [Google Scholar]
- 91.Korherr C, Roth M, Holler E. Poly(β-L-malate) hydrolase from plasmodia of Physarum polycephalum. Can J Microbiol. 1995;41 (Suppl 1):192–199. [Google Scholar]
- 92•.Lee B-S, Holler E. Effects of culture conditions on β-poly(L-malate) production by Physarum polycephalum. Appl Microbiol Biotechnol. 1999;51:647–652. Important article on biogenic production of long-chain polymalic acid. [Google Scholar]
- 93.Ljubimova JY, Fujita M, Khazenzon NM, et al. Nanoconjugate based on polymalic acid for tumor targeting. Chem Biol Interact. 2008;171:195–203. doi: 10.1016/j.cbi.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94•.Ljubimova JY, Lakhter AJ, Loksh A, et al. Overexpression of α4 chain-containing laminins in human glial tumors identified by gene microarray analysis. Cancer Res. 2001;61:5601–5610. Important article on the discovery of tumor-specific vascular laminin changes in the brain. [PubMed] [Google Scholar]
- 95••.Fujita M, Lee B-S, Khazenzon MN, et al. Brain tumor tandem targeting using a combination of monoclonal antibodies attached to biopoly(β-L-malic acid) J Control Release. 2007;122:356–363. doi: 10.1016/j.jconrel.2007.05.032. First article on tissue/cell multitargeting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Summerton J, Weller D. Morpholino antisense oligomers: design, preparation and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- 97.Hallmann R, Horn N, Selg M, et al. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 98.Fujiwara H, Gu J, Sekiguchi K. Rac regulates integrin-mediated endothelial cell adhesion and migration on laminin-8. Exp Cell Res. 2004;292:67–77. doi: 10.1016/j.yexcr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 99.Ljubimova JY, Fugita M, Khazenzon NM, et al. Association between laminin-8 and glial tumor grade, recurrence, and patient survival. Cancer. 2004;101:604–612. doi: 10.1002/cncr.20397. [DOI] [PubMed] [Google Scholar]
- 100.Fujita M, Khazenzon NM, Bose S, et al. Overexpression of β1 chain-containing laminins in capillary basement membranes of human breast cancer and its metastases. Breast Cancer Res. 2005;7:411–421. doi: 10.1186/bcr1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khazenzon NM, Ljubimov AV, Lakhter AJ, et al. Antisense inhibition of laminin-8 expression reduces invasion of human gliomas in vitro. Mol Cancer Ther. 2003;2:985–994. [PubMed] [Google Scholar]
- 102.Broadwell RD, Baker-Cairns BJ, Frieden PM, et al. Transcytosis of protein through the mammalian cerebral epithelium and endothelium III Receptor mediated transcytosis through the blood–brain barrier of blood-borne transferrin and antibody against the transferrin receptor. Exp Neurol. 1996;142:47–65. doi: 10.1006/exnr.1996.0178. [DOI] [PubMed] [Google Scholar]
- 103.Daniels TR, Delgado T, Rodriguez J, et al. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 104.Torchilin VP, Lukyanov AN. Peptide and protein drug delivery to and into tumors: challenges and solutions. Drug Discov Today. 2003;8:259–266. doi: 10.1016/s1359-6446(03)02623-0. [DOI] [PubMed] [Google Scholar]
- 105.Iakoubov LZ, Torchilin VP. A novel class of antitumor antibodies: nucleosome-restricted antinuclear autoantibodies (ANA) from healthy aged nonautoimmune mice. Oncol Res. 1997;9:439–446. [PubMed] [Google Scholar]
- 106.Gupta B, Torchilin VP. Monoclonal antibody 2C5-modified doxorubicin-loaded liposomes with significantly enhanced therapeutic activity against intracranial human brain U-87 MG tumor xenografts in nude mice. Cancer Immunol Immunother. 2007;56:1215–1223. doi: 10.1007/s00262-006-0273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yao JC. Molecular targeted therapy for carcinoid and islet-cell carcinoma. Best Pract Res Clin Endocrinol Metab. 2007;21:163–172. doi: 10.1016/j.beem.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 108.Ljubimov AV, Caballero S, Aoki AM, et al. Involvement of protein kinase CK2 in angiogenesis and retinal neovascularization. Invest Ophthalmol Vis Sci. 2004;45:4583–4591. doi: 10.1167/iovs.04-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ahmad KA, Wang G, Slaton J, et al. Targeting CK2 for cancer therapy. Anticancer Drugs. 2005;16:1037–1043. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- 110.Kramerov AA, Saghizadeh M, Pan H, et al. Expression of protein kinase CK2 in astroglial cells of normal and neovascularized retina. Am J Pathol. 2006;168:1722–1736. doi: 10.2353/ajpath.2006.050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pagano MA, Cesaro L, Meggio F, et al. Protein kinase CK2: a newcomer in the ‘druggable kinome’. Biochem Soc Trans. 2006;34 (Pt6):1303–1306. doi: 10.1042/BST0341303. [DOI] [PubMed] [Google Scholar]
- 112.Wang G, Ahmad KA, Unger G, et al. CK2 signaling in androgen-dependent and -independent prostate cancer. J Cell Biochem. 2006;99:382–391. doi: 10.1002/jcb.20847. [DOI] [PubMed] [Google Scholar]
- 113.Takeda AL, Colquitt JL, Clegg AJ, et al. Pegaptanib and ranibizumab for neovascular age-related macular degeneration: a systematic review. Br J Ophthalmol. 2007;91:1177–1182. doi: 10.1136/bjo.2007.118562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chatila K, Ren G, Xia Y, et al. The role of the thrombospondins in healing myocardial infarcts. Cardiovasc Hematol Agents Med Chem. 2007;5:21–27. doi: 10.2174/187152507779315813. [DOI] [PubMed] [Google Scholar]
- 115.Nakamura K, Sata M, Iwata H, et al. A synthetic small molecule, ONO-1301, enhances endogenous growth factor expression and augments angiogenesis in the ischaemic heart. Clin Sci (Lond) 2007;112:607–616. doi: 10.1042/CS20060301. [DOI] [PubMed] [Google Scholar]
- 116.Duncan R, Vicent MJ, Greco F, et al. Polymer-drug conjugates: towards a novel approach for the treatment of endocrine-related cancer. Endocr Relat Cancer. 2005;12:S189–S199. doi: 10.1677/erc.1.01045. [DOI] [PubMed] [Google Scholar]