Abstract
New interest in NAD biology has recently been revived, and enzymes involved in NAD biosynthetic pathways have been identified and characterized in mammals. Among them, nicotinamide phosphoribosyltransferase (Nampt) has drawn much attention in several different fields, including NAD biology, metabolism, and immunomodulatory response. The research history of this protein is peculiar and controversial, and its physiological function has been a matter of debate. Nampt has both intra- and extracellular forms in mammals. Intracellular Nampt (iNampt) is an essential enzyme in the NAD biosynthetic pathway starting from nicotinamide. On the other hand, an extracellular form of this protein has been reported to act as a cytokine named PBEF, an insulin-mimetic hormone named visfatin, or an extracellular NAD biosynthetic enzyme named eNampt. This review article summarizes the research history and reported functions of this unique protein and discusses the pathophysiological significance of Nampt as an NAD biosynthetic enzyme vs. a potential inflammatory cytokine in diverse biological contexts.
Introduction
The concept of an active turnover cycle of NAD was first proposed by Gholson in 1966 [1]. In this short article, he predicted that because this NAD recycling phenomenon represents a considerable expenditure of cellular energy, it argues for “an important, but as yet unknown, function” in cellular metabolism. A decade later, Rechsteiner and his colleagues demonstrated that a half life of NAD is 1.0±0.3 h in cultured cells, providing convincing support for Gholson's concept [2]. They estimated that the rate of NAD biosynthesis is approximately 105 molecules/s/cell, 95% of which replaces the NAD molecules consumed, and only 5% of which maintains the NAD pool size. In a separate study, Rechsteiner and Catanzarite also demonstrated that a half life of NAD in enucleated cells is ∼10 h, strongly indicating that a vast majority of NAD biosynthesis and breakdown occurs primarily in the nucleus [3]. Referring to Gholson's statement, Rechsteiner and his colleagues made an interesting statement in the last paragraph of their Nature paper: “it seems likely that in eukaryotic cells, NAD has some other major function in addition to the classical cytoplasmic role in oxidation and reduction” [2].
Since Gholson's prediction, two major groups of critical enzymes have been discovered and demonstrated to contribute to this rapid turnover cycle of NAD in the nucleus. One is the family of poly(ADP-ribose)polymerases (PARPs) [4, 5], and the other is the Sir2 (silent information regulator 2) protein family of NAD-dependent deacetylases/ADP-ribosyltransferases [6, 7]. PARPs synthesize poly-ADP-ribose on its nuclear target proteins by consuming NAD, while Sir2 proteins couples NAD breakdown to deacetylation or ADP-ribosylation of their target proteins. These unique enzymes have provided substantial proofs for the “important, but as yet unknown, function” [1] of NAD in cellular metabolism. In particular, the discovery of Sir2 NAD-dependent deacetylase activity [8-10] have fueled new interest in NAD biochemistry. For example, human nicotinamide/nicotinic acid mononucleotide adenylyltransferase (Nmnat), a key NAD biosynthetic enzyme whose activity was reported in liver nuclear extracts in 1952 [11], was finally isolated and fully characterized in 2001 [12, 13]. Nicotinamide phosphoribosyltransferase (Nampt), another key NAD biosynthetic enzyme that initiates the NAD biosynthetic pathway from nicotinamide in mammals, was also isolated and characterized for its enzymological features early in this century [14, 15]. Nampt has recently drawn much attention in several different fields, including NAD biology, metabolism, and immune response, because this enzyme has been shown to have a variety of physiological functions [16-18].
In this review article, I will focus on the function of this unique enzyme, Nampt. The research history of Nampt is peculiar and controversial. Therefore, I will first introduce its research history and then summarize its unique functions in different biological contexts. I will also discuss its physiological significance as an NAD biosynthetic enzyme vs. a potential inflammatory cytokine in NAD biology, metabolism, and diseases.
Nampt/PBEF/visfatin: an NAD biosynthetic enzyme, a cytokine, or an insulin-mimetic hormone?
The enzymatic activity of nicotinamide mononucleotide (NMN) pyrophosphorylase, which is now called nicotinamide phosphoribosyltransferase (Nampt; a.k.a. NamPRT) was originally reported by Preiss and Handler in 1957 [19]. In this report, a Km value of this enzyme for nicotinamide was reported to be ∼100 mM, and therefore, the authors suggested that nicotinamide might not be the physiological substrate for this enzyme. However, in 1966, Dietrich et al. partially purified this enzyme from rat liver and reported that its Km for nicotinamide was 2.62 μM [20]. They also detected the highest activity in rat, mouse, and chick livers and cancerous cells, relatively high activity in kidney and heart, and very low activity in brain, skeletal muscle, and lung. Amazingly, what they reported in this paper is very consistent with our findings published almost 40 years later, as described below.
Unfortunately, Nampt has never been purified to homogeneity. An important clue for the identification of the protein came from a different field. In bacterial species, the ability to use nicotinamide as a precursor for NAD biosynthesis historically distinguishes two different strains. One requires pyridine nucleotide sources, such as nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR), for growth in laboratory media, called V-factor-dependent, and the other does not require such sources and can synthesize NAD from nicotinamide, called V-factor-independent. In 2001, Martin et al. cloned the gene that confers the V-factor-independence, named nadV, from Haemophilus ducreyi and demonstrated that bacterial extracts containing the nadV gene product exhibited Nampt enzymatic activity [21]. Surprisingly, the nadV gene product shows a significant homology to a human protein named PBEF (pre-B cell colony-enhancing factor), implying a similar role of PBEF in mammalian NAD biosynthesis [21].
PBEF was originally isolated as a presumptive cytokine that enhances the maturation of B cell precursors in the presence of interleukin-7 (IL-7) and stem cell factor (SCF) [22]. Although this particular function of PBEF has not been reconfirmed to date, Rongvaux et al. and Revollo et al. have definitively demonstrated that PBEF is indeed mammalian Nampt [14, 15]. Both groups have reported very similar Km values for nicotinamide, 1.24 and 0.92 μM, respectively, comparable to the value that Dietrich et al. reported in 1966 [20]. Rongvaux et al. have also shown that PBEF is able to confer V-factor-independent growth to V-factor-dependent Actinobacillus pleuropneumoniae [15]. Revollo et al. have reconstituted a mammalian NAD biosynthetic pathway from nicotinamide using recombinant Nampt and Nmnat and analyzed enzymological features of this NAD biosynthetic pathway [14].
Interestingly, since the report of PBEF, several groups have shown a cytokine-like function of Nampt/PBEF [17]. The most controversial is the function reported by Fukuhara et al. as a “new visceral fat-derived hormone” named visfatin [23]. Strikingly, visfatin was reported to exert insulin-mimetic effects in cultured cells and to lower plasma glucose levels in mice by binding to and activating the insulin receptor. Their results immediately drew significant attention in the fields of metabolism and diabetes research. However, subsequent studies have produced conflicting results regarding the physiological significance of visfatin function [24-27], partly due to problematic differences in immunoassays and sample treatments [28]. Recently, we have clearly demonstrated that Nampt functions as an intra- and extracellular NAD biosynthetic enzyme and that extracellular Nampt/visfatin does not exert insulin-mimetic effects in vitro or in vivo [29]. Furthermore, most recently, the original visfatin paper has been retracted because of the irreproducibility of the results [30].
This interesting but controversial history of the protein implicates that Nampt likely has multiple functions in different pathophysiological conditions. Reflecting such complex nature of this protein, three different nomenclatures, Nampt, PBEF, and visfatin, have so far been given to this protein. Recently, Nampt has been approved as the official nomenclature of the gene and the protein by both the HUGO Gene Nomenclature Committee (HGNC) and the Mouse Genomic Nomenclature Committee (MGNC). Therefore, Nampt will be used throughout this review.
Nampt-mediated systemic NAD biosynthesis and its importance in β cell function
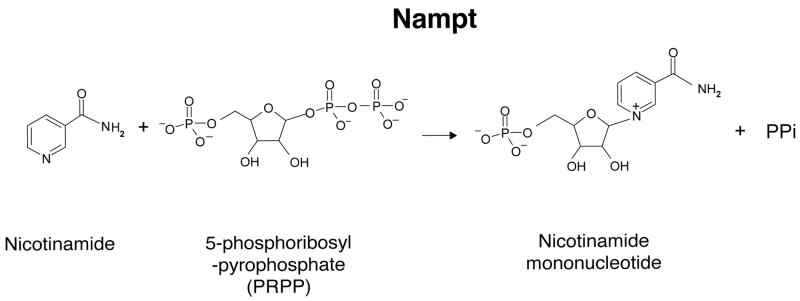
NAD is synthesized from three major precursors – tryptophan, nicotinic acid, and nicotinamide [31, 32]. While lower eukaryotes and invertebrates, such as yeast, worms, and flies, use nicotinic acid (a form of vitamin B3) as a major NAD precursor, mammals predominantly use nicotinamide (another form of vitamin B3) rather than nicotinic acid for NAD biosynthesis [31, 33]. Nampt is the rate-limiting enzyme in the NAD biosynthetic pathway starting from nicotinamide and catalyzes the synthesis of NMN from nicotinamide and 5-phosphoribosyl-pyrophosphate (PRPP) (Fig. (1)) [34]. NMN is then converted to NAD by Nmnat. There are three distinct isoforms of Nmnat, Nmnat1-3, which are localized in nucleus, cytoplasm, and mitochondria, respectively, suggesting that NAD biosynthesis mediated by Nampt and Nmnat might be compartmentalized in each subcellular compartment [18, 31]. The crystal structure of Nampt has also been determined, which firmly establishes that Nampt belongs to a dimeric class of type II phosphoribosyltransferases [35-37].
Fig. (1).
The enzymatic reaction catalyzed by nicotinamide phosphoribosyltransferase (Nampt). PPi, inorganic pyrophospate.
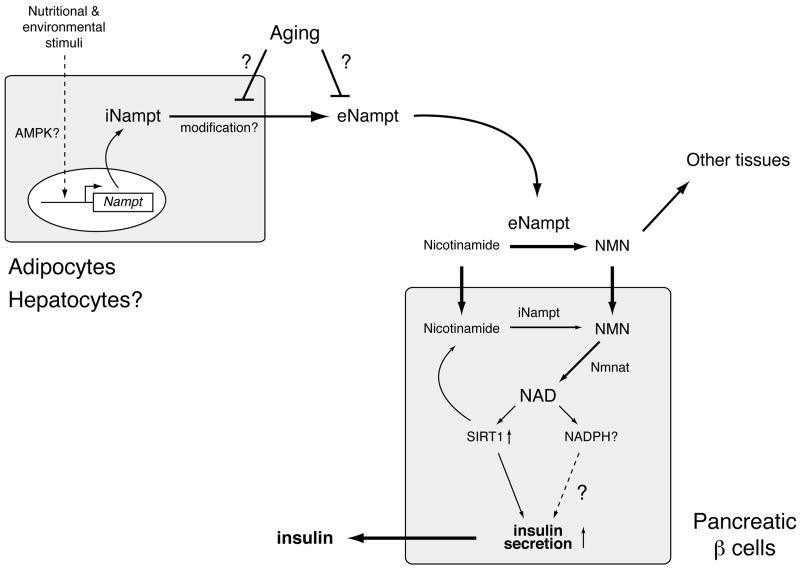
In mammals, Nampt has both intra- and extracellular forms (iNampt and eNampt, respectively; see Fig. (2)) [34]. In mice, the protein expression of iNampt is highest in brown adipose tissue (BAT), liver, and kidney, intermediate in heart, low in white adipose tissue (WAT), lung, spleen, testis, and skeletal muscle, and under a detectable level in pancreas and brain [29], qualitatively consistent with the enzymatic activity in those tissues measured by Dietrich et al. [20]. While the function of iNampt has been firmly established as an NAD biosynthetic enzyme, the function of eNampt has been controversial, as described above. eNampt is positively secreted through a non-classical secretory pathway by fully differentiated mouse and human adipocytes (Fig. (2)) [29], as well as rat and human primary hepatocytes [16]. eNampt appears to be produced through a posttranslational modification, although the nature of the modification is still unknown [29].
Fig. (2).
A model for the secretion of eNampt and the regulation of glucose-stimulated insulin secretion by Nampt-mediated systemic NAD biosynthesis in pancreatic β cells. Nampt functions as an intra- and extracellular NAD biosynthetic enzyme (iNampt and eNampt). eNampt, which appears to be modified posttranslationally, is positively secreted by fully differentiated adipocytes and possibly by hepatocytes. The expression of the Nampt gene is regulated in response to a variety of nutritional and environmental stimuli, and AMP-activated protein kinase (AMPK) might be involved in this regulation. Nicotinamide, a form of vitamin B3, is converted to nicotinamide mononucleotide (NMN) by iNampt in the cell and also by eNampt in blood circulation. Circulating NMN is distributed to tissues and organs and transported to the inside of cells likely through an unidentified transporter and rapidly converted to NAD by Nmnat. In β cells, Nampt-mediated NAD biosynthesis promotes glucose-stimulated insulin secretion by activating Sirt1 and possibly by increasing other metabolic signals, such as NADPH. Nampt-mediated systemic NAD biosynthesis also declines with advanced age, resulting in reduced Sirt1 activity and insulin secretion in aged β cells. The secretion or the enzymatic activity of eNampt might be affected by aging.
We have recently demonstrated that eNampt exhibits robust, even higher NAD biosynthetic activity compared to iNampt and that NAD biosynthesis mediated by iNampt and eNampt plays a critical role in the regulation of glucose-stimulated insulin secretion (GSIS) in pancreatic β cells (Fig. (2)) [29]. Nampt heterozygous (Nampt+/-) female mice show impaired glucose tolerance due to a defect in GSIS. Primary Nampt+/- islets also show defects in NAD biosynthesis and GSIS. Remarkably, administration of NMN, a product of the Nampt reaction, can completely ameliorate these defects in GSIS in Nampt+/- mice and islets, strongly indicating that the observed defects are due to a lack of Nampt-mediated NAD biosynthesis [29]. Furthermore, FK866, a potent chemical inhibitor of Nampt [38], significantly inhibits NAD biosynthesis and GSIS in isolated wild-type primary islets, and again, administration of NMN restores normal NAD biosynthesis and GSIS in FK866-treated wild-type primary islets [29]. Therefore, these findings indicate that pancreatic β cells require Nampt-mediated NAD biosynthesis to maintain normal NAD biosynthesis and GSIS. Reduced plasma levels of eNampt and NMN have been detected in Nampt+/- females, but not in males. This disparity in plasma eNampt and NMN levels between genders explains why only Nampt+/- females show defects in glucose metabolism and why NMN administration can restore normal function in those females, although the reason for this sex-dependent disparity is currently unknown [29].
Based on these findings, it has been proposed that Nampt-mediated systemic NAD biosynthesis plays a critical role in the regulation of β cell function and that NMN might function as an essential plasma metabolite that can modulate GSIS in β cells (Fig. (2)) [16, 29]. Intriguingly, individuals homozygous for either of two single nucleotide polymorphism variants in the Nampt gene promoter region have lower fasting plasma insulin levels [39], implying the connection between Nampt-mediated NAD biosynthesis and insulin secretion in humans. Although further investigation will be necessary to test this model, the finding that Nampt-mediated systemic NAD biosynthesis is a critical contributor to β cell function provides new insights into the physiological significance of Nampt in the regulation of glucose metabolism and its potential role in the pathophysiology of β cell dysfunction [16].
Nampt and sirtuins: A connection to metabolism, stress response, and aging
In 2000, Leonard Guarente and I made a surprising discovery that yeast and mammalian Sir2 proteins have NAD-dependent deacetylase activity and that this activity is essential for the longevity control in yeast [8]. This discovery immediately attracted many scientists to “an important, but as yet unknown, function” [1] of NAD-dependent Sir2 family deacetylases. Since this first discovery, Sir2 family proteins, called “sirtuins,” have been emerging as evolutionarily conserved, critical regulators for aging and longevity in a wide variety of experimental organisms, and sirtuin biology has been rapidly evolving in many different research areas from bacteria to humans [6, 7, 40]. Sirtuins couple NAD breakdown to deacetylation and/or ADP-ribosylation on lysine residues of target proteins. Nicotinamide, acetyl-ADP-ribose, and deacetylated proteins are produced in their NAD-dependent deacetylation reactions. Numerous target proteins, including histones and a variety of transcription factors, have already been reported for sirtuins in different species [7]. In mammals, there are seven sirtuin family members, named Sirt1 through Sirt7 [7, 40, 41]. Sirt1 is the mammalian ortholog of the founder protein Sir2 in yeast, and the majority of mammalian sirtuin research has so far focused on the function of Sirt1, revealing many critical roles of Sirt1 in the regulation of glucose and lipid metabolism and cellular stress responses. Reported enzymatic activities, subcellular localizations, and functions of mammalian sirtuins are summarized in Table 1.
Table 1. Mammalian sirtuin family members.
| Enzymatic activity | Subcellular localization | Function | |
|---|---|---|---|
| Sirt1 | Deacetylase | Nuclear, Cytoplasmic | Glucose production (liver) Cholesterol regulation (liver) Fatty acid mobilization (WAT) Adipokine regulation (WAT) Fatty acid oxidation (skeletal muscle) Insulin secretion (pancreatic β cells) Neuroprotection (brain) Regulation of cellular differentiation Stress resistance&apoptosis control Mediator for caloric restriction? |
| Sirt2 | Deacetylase | Cytoplasmic, Nuclear | Tublin deacetylation Cell cycle control |
| Sirt3 | Deacetylase | Mitochondrial, Nuclear | Mitochondrial protein deacetylation Acetate metabolism regulation Thermogenesis (BAT)? |
| Sirt4 | ADP-ribosyltransferas e | Mitochondrial | Amino acid-stimulated insulin secretion (pancreatic β cells) |
| Sirt5 | Deacetylase | Mitochondrial | Unknown |
| Sirt6 | ADP-ribosyltransferase Deacetylase | Nuclear | Base excision repair Telomeric chromatin structure |
| Sirt7 | unknown | Nucleolar | Pol I transcription? |
Because sirtuins absolutely require NAD for their enzymatic activities, NAD biosynthesis plays a critical role in the regulation of their functions (Fig. (3)) [16, 18]. We have demonstrated that increased dosage of iNampt enhances total cellular NAD levels and thereby augments the transcriptional regulatory activity of Sirt1 and also that increasing the dosage of iNampt and Sirt1 induces common gene expression changes in mouse fibroblasts [14]. In human vascular smooth muscle cells (SMCs), van der Veer et al. have provided similar lines of evidence for the connection between Nampt and Sirt1 [42]. Up-regulation of iNampt is accompanied with SMC maturation. While knockdown of iNampt reduces NAD biosynthesis and impairs SMC survival and maturation, overexpression of iNampt increases cellular NAD levels, enhances Sirt1 activity, and promotes SMC maturation. Increased dosage of iNampt also enhances the capacity of SMCs to mature and form nascent endothelial channels in vivo. In another study, van der Veer et al. have also shown that iNampt promotes cellular life span of SMCs through the Sirt1-mediated deacetylation and degradation of p53 [43]. In cardiac myocytes, Pillai et al. have reported that increased dosage of iNampt protects them from PARP-induced cell death through increased NAD production and enhanced Sirt1 activity [44]. Most recently, Fulco et al. have demonstrated that glucose restriction inhibits differentiation of skeletal myoblasts through the AMP-activated protein kinase (AMPK)-dependent induction of Nampt expression and the resultant activation of Sirt1, suggesting that the AMPK-Nampt-Sirt1 pathway might function as a key signaling pathway in response to reduced nutrient availability [45]. Therefore, these findings suggest that Nampt-mediated NAD biosynthesis plays a critical role in the regulation of Sirt1 activity in different cell types and regulates a wide variety of important biological processes, including cellular differentiation, stress response, and metabolism, in mammals.
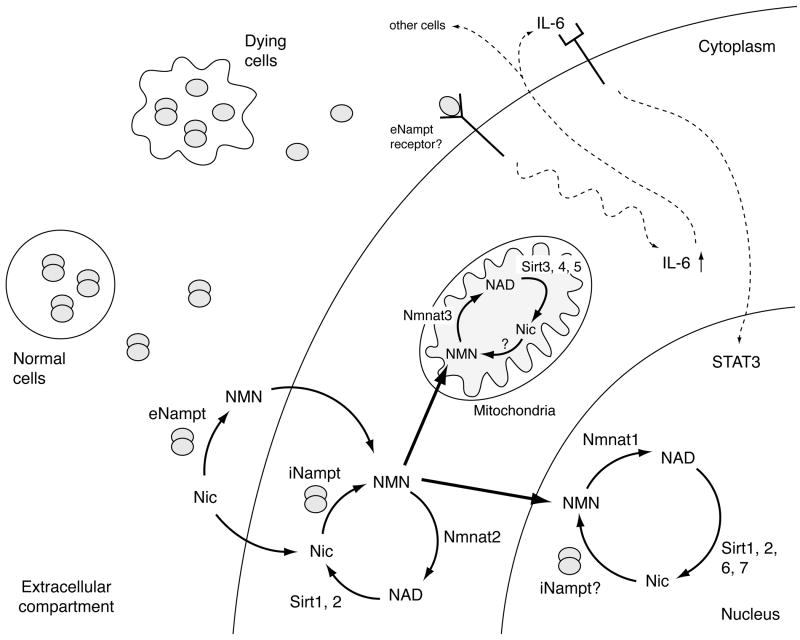
Fig. (3).
The connection between Nampt-mediated NAD biosynthesis and mammalian sirtuins in each subcellular compartment, and a possible dual function of Nampt as an NAD biosynthetic enzyme and a cytokine. There are seven mammalian sirtuin family members, Sirt1-7, and they have distinct subcellular localizations (see Table 1). Although how NAD biosynthesis is regulated in each subcellular compartment is still poorly understood, it is likely that NAD biosynthesis plays a critical role in the regulation of mammalian sirtuin functions in these compartments. As depicted in this scheme, Nampt normally forms a dimer and functions as an NAD biosynthetic enzyme. Nampt produces NMN both intra- and extracellularly, and NMN might also be transported to the nucleus and mitochondria. It is still unclear whether iNampt is directly involved in nuclear and mitochondrial NAD biosynthesis. eNampt has been reported to function as a cytokine, and what regulates this dual role of eNampt is currently unknown. However, based on recent findings, a monomer of Nampt appears to retain a cytokine-like function that stimulates IL-6 production and protects macrophage from cell death. In this scheme, it is speculated that monomers of Nampt might be released from dying cells due to apoptosis or necrosis. See details in texts.
iNampt also plays an important role in NAD biosynthesis and sirtuin activation in mitochondria (Fig. (3)). Yang et al. have shown that iNampt expression is induced by a variety of cellular stress and nutrient restriction [46]. While increasing iNampt levels does not prevent depletion of total cellular NAD under genotoxic stress, it maintains mitochondrial NAD levels and protects cells against cell death induced by genotoxicity through mitochondrial sirtuins, Sirt3 and Sirt4. Although Nmnat3 is localized in mitochondria [31], whether Nampt goes into mitochondria under stress conditions remains unclear. The idea that NAD biosynthesis might be compartmentalized inside the cell has long been discussed [18, 47]. For example, it has been suggested that the rapid turnover of NAD occurs in a compartment in which the NAD does not equilibrate with the pyridine nucleotide involved in glycolysis, such as NADH [47]. Because each mammalian sirtuin member exhibits its specific subcellular localization and consumes NAD in its own compartment (e.g. nucleus, cytoplasm, or mitochondria; see Fig. (3)), it will be of great importance to examine how NAD turnover is regulated in each subcellular compartment.
Nampt-mediated systemic NAD biosynthesis might also be involved in a progressive age-associated decline in β cell function, which has been suggested to be one of the major contributing factors to the pathogenesis of type 2 diabetes [16]. In pancreatic β cell-specific Sirt1-overexpressing (BESTO) mice, Sirt1 significantly promotes GSIS and improves glucose tolerance in vivo [48]. However, we have recently found that aged BESTO mice completely lose all phenotypes of enhanced β cell function and that Sirt1 activity is significantly reduced in aged β cells [49]. This seems to be due to a reduction in plasma NMN levels over age, and consistent with this notion, administration of NMN restores enhanced GSIS and improved glucose tolerance in aged BESTO females. Although the reason for the observed sex-dependent difference in response to NMN administration, which is similar to the case of Nampt+/- males [29], still remains unclear, these findings suggest that Nampt-mediated systemic NAD biosynthesis declines over age, resulting in reduced Sirt1 activity and GSIS in aged β cells (Fig. (2)). Considering pleiotropic functions of Sirt1 and other sirtuins in mammals, it is conceivable that the age-associated decline in Nampt-mediated systemic NAD biosynthesis affects multiple functions of other cell types that are sensitive to changes in NAD biosynthesis, such as neurons, through the reduction of Sirt1 and other sirtuin activities (Fig. (2)). If this is the case, the activation of Sirt1 in such cell types by increasing systemic NAD biosynthesis, as well as the use of small chemical Sirt1 activators [50], might be an effective therapeutic approach for the prevention and treatment of age-associated diseases caused by functional deficits in those cell types, such as type 2 diabetes and dementia [16].
Is eNampt important for the pathophysiology of obesity and type 2 diabetes?
Since the first report of visfatin [23], numerous reports have been published for circulating eNampt/visfatin levels in a variety of pathophysiological metabolic conditions [17, 25, 27]. However, reported results have been conflicting regarding the potential connection between eNampt levels and metabolic disorders [24-27]. In the original visfatin paper, Fukuhara et al. reported a correlation between amounts of visceral fat and levels of plasma eNampt in humans and also observed increased plasma eNampt levels in diabetic KKAy and high fat diet-fed mice [23]. A number of papers have provided similar lines of evidence that support a potential connection between plasma eNampt levels and anthoromopetric and metabolic parameters in patients with obesity and type 1, 2, and gestational diabetes [51-54]. Contrarily, other reports have provided opposite results or no support to this potential connection [55, 56]. These paradoxical results appear to be due, at least in part, to significant variability in immunoassays for the detection of plasma eNampt. For example, Körner et al. has recently found that in a commercially available immunoassay kit, which has been used in the majority of clinical studies, the detection of eNampt is compromised by an unknown protein with much higher molecular weight than eNampt [28].
Recently, Retnakaran et al. have developed a new ELISA kit with increased sensitivity and specificity for the quantitative measurement of eNampt in human serum, plasma or cell culture supernatants [57]. They have found that circulating eNampt levels detected by this new assay are independently associated with type 2 diabetes but not with other metabolic parameters, including anthropometric parameters, lipid profile, fasting glucose, and homeostasis model assessments of insulin resistance (HOMA-IR) and β cell function (HOMA-B). Instead, they have found that eNampt levels are associated with circulating resistin levels, although the physiological significance of this association is still unclear. Körner et al. have also used an ELISA kit from the same company for their small cohort of obese patients and have found that there is no correlation between plasma eNampt levels and anthropometric and metabolic parameters, although there is a tendency of increased eNampt levels in visceral obese patients [28].
These conflicting reports strongly indicate that an extra caution is necessary to properly assess a potential connection between circulating eNampt levels and metabolic disorders. A new assay system with higher sensitivity, specificity, and linearity needs to be developed for the accurate measurement of plasma eNampt levels, and the development of such a system is currently underway. Therefore, the association between plasma eNampt levels and major metabolic disorders, such as obesity and type 2 diabetes, is still unclear, and further careful investigation will be required to address this important problem.
Another face of Nampt: Its cytokine-like function and potential importance in immunomodulatory responses
As described above, human Nampt was originally identified as a putative cytokine named PBEF [22]. Several groups have since reported cytokine-like functions of eNampt/PBEF. Jia et al. have shown that eNampt/PBEF expression is up-regulated by inflammatory stimuli, including lipopolysaccharide (LPS), IL-1β, and TNF-α, in neutrophils and monocytes in vitro and in neutrophils harvested from critically ill septic patients [58]. They have also demonstrated that secreted eNampt functions as an inhibitor of neutrophil apoptosis in response to a variety of inflammatory stimuli. Interestingly, this anti-apoptotic function of eNampt somewhat requires the presence of iNampt. Ognjanovic et al. have reported that Nampt is expressed in normal fetal membranes and up-regulated in severely infected membranes or in amniotic epithelial cells exposed to inflammatory stimuli [59, 60]. They have also reported that eNampt treatment induces the expression of IL-6 and IL-8 in amnion-like epithelial cells and fetal membrane explants. Furthermore, several studies have suggested that Nampt plays an important role in the pathogenesis of acute lung injury [61-63]. Recently, Hong et al. have demonstrated that Nampt+/- mice are protected from severe lung injury and exhibit significantly reduced levels of lung injury-associated gene expression [62]. Interestingly, the production of IL-6 is significantly reduced in lung injury-induced Nampt+/- mice compared to controls. The function of Nampt as an inflammatory cytokine has also been implicated in other acute and chronic inflammatory conditions, including atherosclerosis, myocardial infarction, rheumatoid arthritis, inflammatory bowel disease, and psoriasis [17].
Unfortunately, in those previous studies, it has been unclear whether the NAD biosynthetic enzymatic activity of Nampt is required for or separable from the reported effects of eNampt as an inflammatory cytokine. Busso et al. have recently shown that pharmacological inhibition of Nampt by FK866 reduces pro-inflammatory cytokine secretion in inflammatory cells in vitro and in joints affected with rheumatoid arthritis in vivo [64]. Whereas these results suggest that Nampt enzymatic activity is required for the secretion of pro-inflammatory cytokines, whether eNampt still functions as a cytokine remains unanswered. Most recently, Li et al. have demonstrated for the first time that eNampt protects macrophages from ER stress-induced apoptosis through its non-enzymatic activity that triggers secretion of IL-6 and consequentially activates the pro-survival signal transducer STAT3 in a IL-6-mediated autocrine/paracrine manner (Fig. (3)) [65]. Interestingly, this anti-apoptotic effect of eNampt does not require dimerization of the protein, which is necessary to form enzymatically active Nampt. Therefore, while the dimer form is necessary for enzymatic activity, the monomer form, which might come from necrotic or apoptotic cells in inflammation, may retain cytokine-like activity (Fig. (3)).
Finally, for the reported insulin-mimetic activity of eNampt under the name of visfatin, there has been no evidence supporting the direct binding of eNampt/visfatin to the insulin receptor. There are only two reports that have so far provided indirect support for the connection between eNampt and insulin signaling [66, 67]. Xie et al. have reported that eNampt exerts an insulin-like activity on osteoblasts, although they do not provide evidence for the interaction with the insulin receptor [67]. Song et al. have recently found that eNampt mediates glucose uptake in mesangial cells [66]. Paradoxically, whereas FK866 inhibits this eNampt-mediated glucose uptake, knockdown of the insulin receptor also inhibits this effect. One possible explanation for these results is that there might be some cross-talk between eNampt-mediated and insulin signaling pathways. More detailed molecular analysis will be necessary for this controversial insulin-mimetic activity of eNampt/visfatin.
Conclusions and perspectives
With our current knowledge of this protein, it is fair to conclude that the major function of this unique protein is Nampt as an NAD biosynthetic enzyme. This notion has been firmly supported by the biochemical, structural, and physiological analyses of Nampt and also by the fact that the NAD biosynthetic function of Nampt is highly conserved between bacteria and mammals [33, 34]. Nonetheless, because of its complicated, controversial research history, the major function of this protein as an NAD biosynthetic enzyme has been underrepresented in publications. On the other hand, it is also very intriguing that this protein is positively secreted as eNampt and appears to have a dual function as an NAD biosynthetic enzyme and an inflammatory cytokine. Therefore, in future studies, both functions need to be carefully addressed, and it will be of great importance to elucidate the physiological relevance of each function in various biological contexts.
As an NAD biosynthetic enzyme, Nampt plays a critical role in the regulation of NAD biosynthesis at a systemic level and thereby controls the functions of mammalian sirtuin family members, as well as other NAD-consuming enzymes, such as PARPs, in each subcellular compartment. Therefore, Nampt-mediated NAD biosynthesis is likely involved in a variety of critical biological processes, including metabolism, stress response, and aging. To address the physiological importance of Nampt-mediated NAD biosynthesis, it will be critical to examine 1) the requirement and the importance of this particular NAD biosynthesis pathway in different cell types, tissues, or organs and 2) the subcellular and extracellular compartmentalization of NAD biosynthesis for the regulation of distinct sirtuin functions. Most recently, Rongvaux et al. have generated a mouse strain lacking Nampt expression in the T and B lymphocyte lineage and have reported that Nampt expression is required for B and T lymphocyte development [68]. Such an approach with tissue-specific knockout and transgenic mice will provide important clues to address the first problem. On the other hand, to address the second problem, it will be important to elucidate molecular mechanisms by which Nampt is sorted to each subcellular compartment or secreted to an extracellular compartment. Additionally, an accurate methodology for the measurement of NAD precursors and metabolites in these compartments will need to be established to study the dynamics of NAD biosynthesis at systemic or subcellular levels.
To dissect the dual function of eNampt, it may be important to pay careful attention to its structural characteristics. Nampt needs to be dimerized to function as an NAD biosynthetic enzyme [29], and plasma eNampt has been reported to be mostly a dimer [28]. However, Li et al. have also found that Nampt mutants that cannot form a dimer are still capable of mediating a cytokine-like function [65]. Therefore, to definitively demonstrate any cytokine-like activity of this protein, it is critical to examine whether mutant proteins that do not form a dimer or do not possess significant NAD biosynthetic activity can still mediate a cytokine-like activity of interest. Such mutants have already been identified [29, 37, 65], and thus it will be of great interest to examine whether those mutants still possess any reported cytokine-like activity. In the case that eNampt definitively functions as a cytokine, it will also be of great importance to identify a receptor for eNampt and elucidate its signaling pathway.
Nampt has recently been shown to have an important connection to a wide variety of diseases, including obesity, type 2 diabetes, and inflammatory diseases. Therefore, there will be potential applications of chemical Nampt inhibitors and NAD metabolites (as stimulants of NAD biosynthesis) for the prevention and treatment of those diseases. The field of NAD biology has now been revived, and the exploration of this new world will provide new opportunities to improve the quality of our lives through this new century.
Acknowledgments
I thank all members of the Imai lab for their helpful discussions and comments. I apologize to those whose work is not cited due to space limitations. This work was supported by grants from the National Institute on Aging (AG024150), the Ellison Medical Foundation, and the Longer Life Foundation to S. I.
References
- 1.Gholson RK. The pyridine nucleotide cycle. Nature. 1966;212:933–934. doi: 10.1038/212933a0. [DOI] [PubMed] [Google Scholar]
- 2.Rechsteiner M, Hillyard D, Olivera BM. Magnitude and significance of NAD turnover in human cell line D98/AH2. Nature. 1976;259:695–696. doi: 10.1038/259695a0. [DOI] [PubMed] [Google Scholar]
- 3.Rechsteiner M, Catanzarite V. The biosynthesis and turnover of nicotinamide adenine dinucleotide in enucleated culture cells. J Cell Physiol. 1974;84:409–422. doi: 10.1002/jcp.1040840309. [DOI] [PubMed] [Google Scholar]
- 4.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 5.Sugimura T, Miwa M. Poly(ADP-ribose): Historical perspective. Mol Cell Biochem. 1994;138:5–12. doi: 10.1007/BF00928437. [DOI] [PubMed] [Google Scholar]
- 6.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 7.Imai S, Guarente L. Sirtuins: A universal link between NAD, metabolism, and aging. In: Guarente L, Partridge L, Wallace D, editors. The Molecular Biology of Aging. New York: Cold Spring Habor Laboratory Press; 2007. pp. 39–72. [Google Scholar]
- 8.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 9.Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, et al. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci USA. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, et al. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci USA. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogeboom GH, Schneider WC. Cytochemical studies VI. The synthesis of diphosphopyridine nucleotide by liver cell nuclei. J Biol Chem. 1952;197:611–620. [PubMed] [Google Scholar]
- 12.Emanuelli M, Carnevali F, Saccucci F, Pierella F, Amici A, Raffaelli N, et al. Molecular cloning, chromosomal localization, tissue mRNA levels, bacterial expression, and enzymatic properties of human NMN adenylyltransferase. J Biol Chem. 2001;276:406–412. doi: 10.1074/jbc.M008700200. [DOI] [PubMed] [Google Scholar]
- 13.Schweigler M, Hennig K, Lerner F, Niere M, Hirsch-Kauffmann M, Specht T, et al. Characterizationof recombinant human nicotinamide mononucleotide adenylyl transferase (NMNAT), a nuclear enzyme essential for NAD synthesis. FEBS Lett. 2001;492:95–100. doi: 10.1016/s0014-5793(01)02180-9. [DOI] [PubMed] [Google Scholar]
- 14.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 15.Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–3234. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 16.Imai S, Kiess W. Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Front Biosci. 2008 doi: 10.2741/3428. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008;83:804–816. doi: 10.1189/jlb.0807581. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Lavu S, Sinclair DA. Nampt/PBEF/Visfatin: a regulator of mammalian health and longevity? Exp Gerontol. 2006;41:718–726. doi: 10.1016/j.exger.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preiss J, Handler P. Enzymatic synthesis of nicotinamide mononucleotide. J Biol Chem. 1957;225:759–770. [PubMed] [Google Scholar]
- 20.Dietrich LS, Fuller L, Yero IL, Martinez L. Nicotinamide mononucleotide pyrophosphorylase activity in animal tissues. J Biol Chem. 1966;241:188–191. [PubMed] [Google Scholar]
- 21.Martin P, Shea R, Mulks M. Identification of a plasmid-encoded gene from Haemophilus ducreyi which confers NAD independence. J Bacteriol. 2001;183:1168–1174. doi: 10.1128/JB.183.4.1168-1174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 24.Arner P. Visfatin--a true or false trail to type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:28–30. doi: 10.1210/jc.2005-2391. [DOI] [PubMed] [Google Scholar]
- 25.Sethi JK. Is PBEF/visfatin/Nampt an authentic adipokine relevant to the metabolic syndrome? Curr Hypertens Rep. 2007;9:33–38. doi: 10.1007/s11906-007-0007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethi JK, Vidal-Puig A. Visfatin: the missing link between intra-abdominal obesity and diabetes? Trends Mol Med. 2005;11:344–347. doi: 10.1016/j.molmed.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens JM, Vidal-Puig AJ. An update on visfatin/pre-B cell colony-enhancing factor, an ubiquitously expressed, illusive cytokine that is regulated in obesity. Curr Opin Lipidol. 2006;17:128–131. doi: 10.1097/01.mol.0000217893.77746.4b. [DOI] [PubMed] [Google Scholar]
- 28.Körner A, Garten A, Bluher M, Tauscher R, Kratzsch J, Kiess W. Molecular characteristics of serum visfatin and differential detection by immunoassays. J Clin Endocrinol Metab. 2007;92:4783–4791. doi: 10.1210/jc.2007-1304. [DOI] [PubMed] [Google Scholar]
- 29.Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, et al. Nampt/PBEF/visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Retraction. Science. 2007;318:565b. doi: 10.1126/science.318.5850.565b. [DOI] [PubMed] [Google Scholar]
- 31.Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Enzymology of NAD+ homeostasis in man. Cell Mol Life Sci. 2004;61:19–34. doi: 10.1007/s00018-003-3161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magni G, Amici A, Emanuelli M, Raffaelli N, Ruggieri S. Enzymology of NAD+ synthesis. Adv Enzymol Relat Areas Mol Biol. 1999;73:135–182. doi: 10.1002/9780470123195.ch5. [DOI] [PubMed] [Google Scholar]
- 33.Rongvaux A, Andris F, Van Gool F, Leo O. Reconstructing eukaryotic NAD metabolism. Bioessays. 2003;25:683–690. doi: 10.1002/bies.10297. [DOI] [PubMed] [Google Scholar]
- 34.Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol. 2007;23:164–170. doi: 10.1097/MOG.0b013e32801b3c8f. [DOI] [PubMed] [Google Scholar]
- 35.Khan JA, Tao X, Tong L. Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat Struct Mol Biol. 2006;13:582–588. doi: 10.1038/nsmb1105. [DOI] [PubMed] [Google Scholar]
- 36.Kim MK, Lee JH, Kim H, Park SJ, Kim SH, Kang GB, et al. Crystal Structure of Visfatin/Pre-B Cell Colony-enhancing Factor 1/Nicotinamide Phosphoribosyltransferase, Free and in Complex with the Anti-cancer Agent FK-866. J Mol Biol. 2006;362:66–77. doi: 10.1016/j.jmb.2006.06.082. [DOI] [PubMed] [Google Scholar]
- 37.Wang T, Zhang X, Bheda P, Revollo JR, Imai S, Wolberger C. Structure of Nampt/PBEF/visfatin, a mammalian NAD(+) biosynthetic enzyme. Nat Struct Mol Biol. 2006;13:661–662. doi: 10.1038/nsmb1114. [DOI] [PubMed] [Google Scholar]
- 38.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- 39.Bailey SD, Loredo-Osti JC, Lepage P, Faith J, Fontaine J, Desbiens KM, et al. Common Polymorphisms in the Promoter of the Visfatin Gene (PBEF1) Influence Plasma Insulin Levels in a French-Canadian Population. Diabetes. 2006;55:2896–2902. doi: 10.2337/db06-0189. [DOI] [PubMed] [Google Scholar]
- 40.Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7:104–112. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 42.van der Veer E, Nong Z, O'Neil C, Urquhart B, Freeman D, Pickering JG. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25–34. doi: 10.1161/01.RES.0000173298.38808.27. [DOI] [PubMed] [Google Scholar]
- 43.van der Veer E, Ho C, O'Neil C, Barbosa N, Scott R, Cregan SP, et al. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007;282:10841–10845. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- 44.Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2α deacetylase activity. J Biol Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 45.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, et al. Nutrient-sensitive mitochondrial NAD(+) levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernofsky C. Physiology aspects of pyridine nucleotide regulation in mammals. Mol Cell Biochem. 1980;33:135–143. doi: 10.1007/BF00225285. [DOI] [PubMed] [Google Scholar]
- 48.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, et al. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in β cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 52.Haider DG, Holzer G, Schaller G, Weghuber D, Widhalm K, Wagner O, et al. The adipokine visfatin is markedly elevated in obese children. J Pediatr Gastroenterol Nutr. 2006;43:548–549. doi: 10.1097/01.mpg.0000235749.50820.b3. [DOI] [PubMed] [Google Scholar]
- 53.Krzyzanowska K, Krugluger W, Mittermayer F, Rahman R, Haider D, Shnawa N, et al. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin Sci (Lond) 2006;110:605–609. doi: 10.1042/CS20050363. [DOI] [PubMed] [Google Scholar]
- 54.Lopez-Bermejo A, Chico-Julia B, Fernandez-Balsells M, Recasens M, Esteve E, Casamitjana R, et al. Serum visfatin increases with progressive beta-cell deterioration. Diabetes. 2006;55:2871–2875. doi: 10.2337/db06-0259. [DOI] [PubMed] [Google Scholar]
- 55.Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–2916. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 56.Pagano C, Pilon C, Olivieri M, Mason P, Fabris R, Serra R, et al. Reduced plasma visfatin/pre-B cell colony-enhancing factor in obesity is not related to insulin resistance in humans. J Clin Endocrinol Metab. 2006;91:3165–3170. doi: 10.1210/jc.2006-0361. [DOI] [PubMed] [Google Scholar]
- 57.Retnakaran R, Youn BS, Liu Y, Hanley AJG, Lee NS, Park JW, et al. Correlation of circulating full-length visfatin (PBEF/Nampt) with metabolic parameters in subjects with and without diabetes: a cross-sectional study. Clin Endocrinol. 2008 doi: 10.1111/j.1365-2265.2008.03264.x. [DOI] [PubMed] [Google Scholar]
- 58.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26:107–117. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- 60.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol. 2002;187:1051–1058. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 61.Bajwa EK, Yu CL, Gong MN, Thompson BT, Christiani DC. Pre-B-cell colony-enhancing factor gene polymorphisms and risk of acute respiratory distress syndrome. Crit Care Med. 2007;35:1290–1295. doi: 10.1097/01.CCM.0000260243.22758.4F. [DOI] [PubMed] [Google Scholar]
- 62.Hong SB, Huang Y, Moreno-Vinasco L, Sammani S, Moitra J, Barnard JW, et al. Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury. Am J Respir Crit Care Med. 2008;178:605–617. doi: 10.1164/rccm.200712-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med. 2005;171:361–70. doi: 10.1164/rccm.200404-563OC. [DOI] [PubMed] [Google Scholar]
- 64.Busso N, Karababa M, Nobile M, Rolaz A, Van Gool F, Galli M, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS ONE. 2008;3:e2267. doi: 10.1371/journal.pone.0002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Zhang Y, Dorweiler B, Cui D, Wang T, Woo CW, et al. Extracellular Nampt promotes macrophages survival via a non-enzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem. 2008 doi: 10.1074/jbc.M805866200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song HK, Lee MH, Kim BK, Park YG, Ko GJ, Kang YS et al. Visfatin: A new player in mesangial cell physiology and diabetic nephropathy. Am J Physiol Renal Physiol. 2008 doi: 10.1152/ajprenal.90231.2008. Epub on Sep. 3. [DOI] [PubMed] [Google Scholar]
- 67.Xie H, Tang SY, Luo XH, Huang J, Cui RR, Yuan LQ, et al. Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int. 2007;80:201–210. doi: 10.1007/s00223-006-0155-7. [DOI] [PubMed] [Google Scholar]
- 68.Rongvaux A, Galli M, Denanglaire S, Van Gool F, Drèze PL, Szpirer C, et al. Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J Immunol. 2008;181:4685–4695. doi: 10.4049/jimmunol.181.7.4685. [DOI] [PubMed] [Google Scholar]