Abstract
Purpose of review
This review aims to summarize the importance of the mammalian NAD-dependent deacetylase SIRT1 as a critical mediator that coordinates metabolic responses to caloric restriction (CR) and the recent progress in the development of SIRT1-targeted CR mimetics. It also discusses possible trade-offs between robustness and frailty in CR and the applicability of CR or SIRT1-targeted CR mimetics to humans.
Recent findings
Loss- and gain-of-function mouse studies have provided genetic evidence that SIRT1 is a key mediator that orchestrates the physiological response to CR. SIRT1-activating compounds function as potential CR mimetics, at least in part, through the activation of SIRT1 in vivo.
Summary
Increasing SIRT1 dosage/activity is effective to provide significant protection from high-fat diet-induced metabolic complications, suggesting that SIRT1 activation likely promotes robustness in the regulation of metabolism. However, CR itself and CR mimicry through systemic SIRT1 activation might also generate frailty in response to unexpected environmental stimuli, such as bacterial and viral infections. It will be of great importance to understand the principles of systemic robustness and its spatial and temporal dynamics for the regulation of aging and longevity in mammals in order to achieve an optimal balance between robustness and frailty in our complex physiological system.
Keywords: SIRT1, sirtuins, caloric restriction, STACs, robustness, frailty
Introduction
The Japanese have long carried an old wisdom for longevity through their dietary culture. Although their life style and diet have rapidly been westernized in the last century, a concept of “hara hachi bunme” or “hara hachi bu”, eating until you are 80% full, has been a traditional dietary control to achieve good health and longevity among the Japanese. Indeed, Ekiken Kaibara (1630-1714), a great philosopher and scientist who enjoyed unusual longevity in the Edo era, has already described this concept in his “Yôjô-kun (The Book of Life-nourishing Principles)” in 1713. More than 200 years later, Clive M. McCay and his colleagues provided an experimental proof for this concept, demonstrating the life span-extending effect of caloric restriction (CR), a dietary regimen low in calories without malnutrition, using rats [1]. Since this discovery, it has been established that CR retards aging and extends life span in a wide variety of organisms, including yeast [2, 3], worms [4, 5], flies [6], rodents [1, 7], and possibly primates [8, 9]. CR also delays the onset and slows the progression of many age-related diseases, such as cancer, kidney disease, autoimmune disease, atherosclerosis, and diabetes [10-13]. Whereas a number of hypotheses have been proposed to explain the anti-aging and life span-extending effects of CR [14], recent evidence suggests that CR activates a highly regulated metabolic program that translates nutritional cues to alterations in transcription, thereby mediating the physiological response to CR [15, 16].
For the past decade, the evolutionarily conserved Sir2 (silent information regulator 2) family of NAD-dependent deacetylases/ADP-ribosyltransferases, also called “sirtuins,” has drawn much attention in the field of aging research as a critical regulator that mediates a variety of physiological responses to CR [17]. Furthermore, small chemical compounds that can activate sirtuins, particularly the mammalian Sir2 ortholog SIRT1, have raised a broad interest in “CR mimetics,” providing a hope to mimic beneficial effects of CR without suffering from a practical difficulty in attempting CR [18, 19•, 20]. Despite these exciting findings, several important questions still remain. Does increased dosage/activity of SIRT1 indeed mimic the physiological response to CR? Does SIRT1 activation always promote robustness at a systemic level? Could SIRT1 activation be effective to achieve good health and longevity in real human life? This short review will focus specifically on the importance of SIRT1, summarizing its function as a critical mediator that coordinates metabolic responses to CR, and will discuss potential pros and cons in the idea of activating SIRT1 as a functional mimicry of CR in mammals.
SIRT1, a key mediator that orchestrates the physiological response to CR
In experimental model organisms, such as yeast, worms, and flies, it has been demonstrated that Sir2 family proteins play a critical role in the regulation of aging and longevity [17, 21]. Increasing the dosage or activity of Sir2 and its orthologs extends the life spans of those organisms, while deletions or mutations of the Sir2 genes shorten their life spans [22-27]. In certain genetic backgrounds, Sir2 family proteins are also required for the CR-mediated life span extension in those organisms [3, 25, 28-30]. In mammals, there are seven sirtuin family members, named SIRT1 through SIRT7, and the majority of mammalian sirtuin research has so far focused on the function of SIRT1 [17, 31, 32•]. Although it is still unclear whether SIRT1 regulates aging and longevity in mammals, numerous studies have demonstrated that SIRT1 regulates critical metabolic responses to nutritional cues, particularly to low nutritional input, in multiple tissues, as well as cell survival in response to stress and damage, both features common to the response to CR in mammals [17, 31, 32•]. Because many review articles have already addressed the metabolic functions of SIRT1, I will focus on the connection between SIRT1 and caloric restriction in this section.
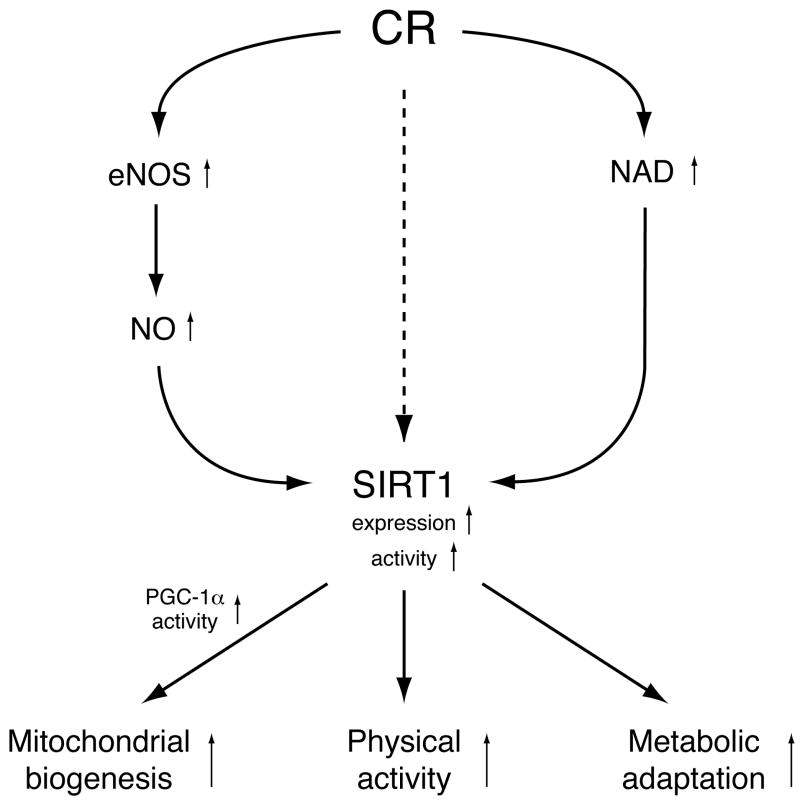
Three loss-of-function mouse studies have so far provided genetic evidence that connects SIRT1 to CR in mice (Fig. 1) [33•-35]. Chen et al. have examined physical activity during CR in wild-type and SIRT1-deficient mice [34]. Whereas wild-type CR mice show a significant increase in physical activity compared to ad libitum-fed controls, SIRT1-deficient mice do not exhibit such an increase. This is not due to a reduced capacity for movement because SIRT1-deficient mice perform as well as or better than wild-type mice in rotarod and treadmill tests, suggesting that SIRT1 plays a critical role in mediating the CR-induced increase in physical activity. Nisoli et al. have shown that the CR-induced enhancement of mitochondrial biogenesis is blunted in endothelial nitric oxide synthase (eNOS)-deficient mice [35]. Interestingly, they have found that NO up-regulates SIRT1 expression, which is also blunted in eNOS-deficient mice. Therefore, it has been proposed that CR induces eNOS and NO, thereby up-regulating SIRT1 and mitochondrial biogenesis. The CR-induced mitochondrial biogenesis might be explained by the ability of SIRT1 to deacetylate and activate peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), a key transcriptional regulator of glucose production and mitochondrial function [36]. Consistent with these findings, Boily et al. have recently provided evidence that SIRT1-deficient mice are metabolically inefficient and unable to adapt to CR normally [33•].
Figure 1.
Genetic linkage between caloric restriction (CR) and SIRT1. CR enhances mitochondrial biogenesis by up-regulating the expression of the endothelial nitric oxide synthase (eNOS), promoting the production of NO, and increasing SIRT1 expression (left). The activation of PGC-1α by SIRT1 likely mediates the effect of CR on mitochondria. CR also enhances physical activity likely by increasing NAD content (right) and up-regulating SIRT1 protein levels (middle) in the brain. SIRT1 is also required for the metabolic adaptation to CR, although the underlying mechanism is unknown.
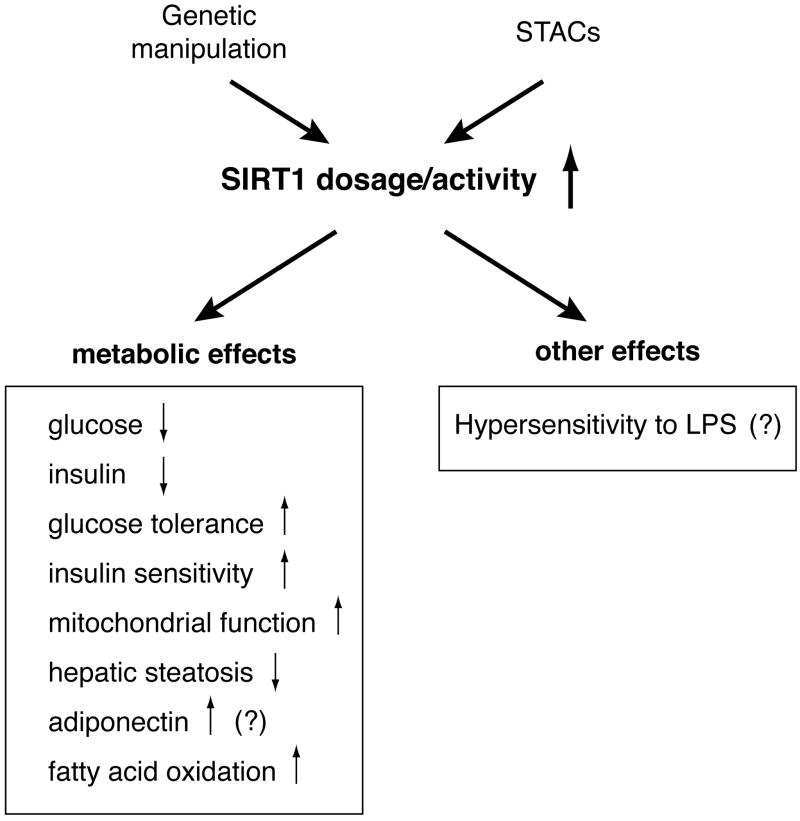
Three gain-of-function mouse studies have also provided supportive evidence (Fig. 2) [37••, 38••, 39••]. Bordone et al. have generated SIRT1-overexpressing transgenic (SIRT1-KI) mice in which the SIRT1 cDNA is knocked into the ubiquitously expressed β-actin locus [38••]. In SIRT1-KI mice, SIRT1 is moderately overexpressed in several tissues including white and brown adipose tissues and brain, but not in liver and muscle, without affecting β-actin protein levels. The SIRT1-KI mice display phenotypes that mimic some of the physiological changes in response to CR, including decreased blood insulin and glucose levels, improved glucose tolerance, reduced fat mass and circulating free fatty acid and leptin levels, reduced total blood cholesterol levels, enhanced oxygen consumption, improved activity in rotarod tests, and delayed reproductive timing. Certain CR-mediated physiological changes, including those in blood levels of triglycerides, high-density lipoprotein cholesterol, insulin-like growth factor 1, adiponectin, and corticosterone, are not recapitulated in SIRT1-KI mice, and whether their life span is extended is not known. Nonetheless, this study currently provides the best evidence for the significance of SIRT1 in the induction of physiological responses to CR. Pfluger et al. and Banks et al. have generated SIRT1-overexpressing transgenic mice using large genomic fragments that contain the entire Sirt1 gene locus [37••, 39••]. Although results from these two groups are not entirely consistent, both SIRT1 transgenic mice show significant protection from the adverse effects of high-fat diet (HFD) on glucose metabolism, which caloric restriction has also been reported to convey. Interestingly, Banks et al. have also found that their SIRT1 transgenic (SirBACO) mice have the increased production of adiponectin likely through the deacetylation of FOXO1 by SIRT1 [37••]. This particular phenotype might provide a potential explanation for the increase in hepatic insulin sensitivity observed in SirBACO mice. On the other hand, Pfluger et al. have found that while their SIRT1 transgenic mice are protected from HFD-induced hepatic inflammation, they show serious hypersensitivity to lipopolysaccharide (LPS) and die much earlier than wild-type mice in response to LPS [39••]. These phenotypes are likely due to the SIRT1-mediated suppression of NFκB activation.
Figure 2.
The CR-mimetic effects of gain-of-function or chemical activation of SIRT1 in mice. The phenotypes that have not been confirmed by either genetic or pharmacological studies are shown with question marks. Details are described in text.
These findings collectively support the notion that SIRT1 is a key player that mediates the physiological response to CR. In particular, increasing SIRT1 dosage is effective to provide significant protection from HFD-induced metabolic complications, indicating that SIRT1 activation likely promotes robustness in the regulation of metabolism. Given that increased SIRT1 dosage causes hypersensitivity to LPS, however, systemic SIRT1 activation might also generate frailty in response to unexpected environmental stimuli, such as bacterial infection. Effective trade-offs between robustness and frailty have been suggested in a highly optimized tolerance (HOT) architecture underlying complex biological networks, such as metabolism [40-42]. Therefore, it will be of great importance to carefully assess the effects of SIRT1 activation on the trade-offs between robustness and frailty at a systemic level.
Sirtuin activating compounds (STACs) : Do they mimic physiological effects of CR?
Provided that SIRT1 plays a key role in mediating metabolically beneficial effects of CR, the idea of developing chemical compounds that activate SIRT1 has naturally attracted researchers in the field of sirtuin biology. David Sinclair and his colleagues have reported that a group of polyphenolic compounds (STACs), such as resveratrol, fisetin, and butein, can activate the catalytic activity of Sir2 and its orthologs and extend the life spans of yeast, worms, and flies [23, 27]. Furthermore, they have reported that in HFD-fed mice, resveratrol (22.4 mg/kg/day) conveys increased insulin sensitivity, improved motor function, improved hepatic pathology, enhanced mitochondrial biogenesis, increased AMP-activated protein kinase (AMPK) and PGC-1α activity, and increased survival [43]. Lagouge et al. have also reported that resveratrol (400 mg/kg/day) prevents HFD-induced obesity, increases the aerobic capacity of the skeletal muscle, enhances endurance in running, and improves insulin sensitivity [44]. These effects are associated with an induction of genes required for oxidative phosphorylation and mitochondrial biogenesis likely caused by the deacetylation and activation of PGC-1α by activated SIRT1. More recently, Pearson, Baur et al. have shown that resveratrol (100, 400, and 2400 mg/kg of food) induces gene expression profiles similar to those induced by every-other-day feeding, which is known to convey physiological effects similar to those in CR, particularly in liver and skeletal muscle [45••]. Resveratrol treatment also slows down age-associated transcriptional changes in liver and skeletal muscle and delays functional decline in old mice. Nonetheless, resveratrol fails to increase the life span of standard diet-fed mice, while it does increase the life span of HFD-fed mice. These results support the idea that resveratrol can be used as a potential CR mimetic possibly through the activation of SIRT1 in mammals.
However, while resveratrol is often baldly referred to as a SIRT1 activator, it is still not clear to what extent the pharmacological effects of resveratrol depend on SIRT1. For example, Dasgupta and Milbrandt have reported that resveratrol activates AMPK and thereby mitochondrial biogenesis in a LKB1 kinase-dependent but SIRT1-independent manner [46]. Resveratrol has also been known as an anti-oxidant [47]. Therefore, it is very likely that resveratrol has a number of “off-target” effects as a plant-derived “xenohormetic” compound [48]. This pleiotropic feature of resveratrol may not necessarily be bad as far as this compound can mimic the beneficial effects of CR and overall promote systemic robustness. Nonetheless, to overcome this specificity problem of resveratrol, more potent and specific STACs have been developed. Milne, Lambert, Schenk et al. have reported new SIRT1-activating non-polyphenolic compounds that are 1,000-fold more potent than resveratrol [49]. They have also shown that these new STACs improve glucose homeostasis and insulin sensitivity in diet-induced and genetically obese rodent models, implicating a novel therapeutic intervention for the treatment of type 2 diabetes. Most recently, Feige et al. have further examined the pharmacological effects of SRT1720, one of those new STACs, on glucose and cholesterol homeostasis, endurance and locomoter functions, hepatic oxidative functions, and energy expenditure in HFD-fed mice [50••]. Whereas SRT1720 does not have the direct action on AMPK, this compound can improve conditions in the aforementioned functions at the doses of 100-500 mg/kg/day in HFD-fed mice. Interestingly, SRT1720 induces the expression of genes required for fatty acid oxidation likely through the deacetylation of PGC-1α, FOXO1, and p53 and indirect AMPK activation, which is different from the effects of resveratrol. Given that nutritionally deprived conditions trigger a metabolic switch towards lipid oxidation, this compound appears to activate metabolic pathways that can also be activated by low nutritional cues, such as caloric restriction.
These findings suggest that resveratrol and new STACs might function as potential CR mimetics, at least in part, through the activation of SIRT1 in vivo (Fig. 2). These drugs might also be effective to treat age-associated metabolic complications, such as type 2 diabetes, especially when individuals suffer from diet-induced obesity. Then, the real question is what trade-offs resveratrol and other STACs make in terms of the balance between robustness and frailty in our system. More generally, what are the overall effects of CR on the balance between robustness and frailty at a systemic level? I will discuss this issue in the following section.
Possible trade-offs between robustness and frailty in caloric restriction
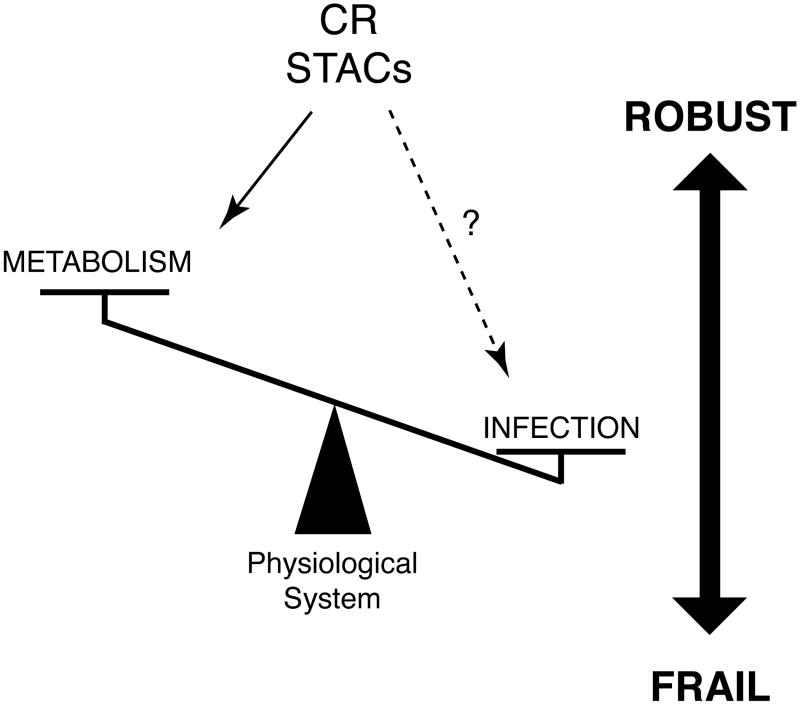
The theory of highly optimized tolerance (HOT) architecture indicates that systems that have evolved to comprise a greater scale of complexity are optimized for specific perturbations but are also inevitably susceptible to unexpected perturbations [40, 51]. Such trade-offs are an inherent, unavoidable feature of complex systems, and biological systems are not an exception. Our metabolic control system is highly robust in response to short- and long-term alterations in food availability, and caloric restriction apparently activates multiple metabolic regulatory pathways and keeps the entire system robust even under a low nutritional condition (Fig. 3). For example, CR mice usually maintain significantly lower blood insulin levels due to increased insulin sensitivity but show remarkably increased postprandial insulin levels compared to ad libitum-fed mice [52, our unpublished findings], indicating that the insulin-secreting function of pancreatic β cells is significantly enhanced in CR mice. Interestingly, this is exactly the phenotype of pancreatic β cell-specific SIRT1-overexpressing (BESTO) mice [53], providing another line of indirect but supportive evidence that SIRT1 mediates the enhancement of metabolic robustness in caloric restriction. Whereas caloric restriction promotes metabolic robustness, it has also been reported that calorically restricted animals show reduced natural killer cell function and increased mortality in response to influenza virus infection (Fig. 3) [54, 55••, 56]. This potentially critical trade-off between robustness in metabolism and frailty in infection might have been unrecognized because laboratory animals are usually maintained in a specific pathogen free (SPF) environment. Furthermore, as discussed in the previous section, SIRT1 transgenic mice show serious hypersensitivity and early death in response to LPS [39••], implying that SIRT1 might be involved in this specific trade-off under caloric restriction (Fig. 3). Therefore, it will be of great importance to examine whether SIRT1 activation indeed increases frailty to infectious pathogens, such as viruses and bacteria.
Figure 3.
Possible trade-offs between robustness in metabolism and frailty in infection in CR. Trade-offs between robustness and frailty are an inherent, unavoidable feature of complex systems, such as biological systems. Whereas CR promotes metabolic robustness, it appears to increase frailty to infections pathogens. Given that sirtuin (SIRT1 in particular) activating compounds (STACs) function as a potential CR mimetics, STACs might also convey similar trade-offs. Therefore, it will be critical to seek an optimal balance between robustness in metabolism and frailty in infection in our complex physiological system.
Then, a critical question is whether caloric restriction or SIRT1-targeted CR mimetics could be effective to achieve good health and longevity for humans. The answer to this question will not be available for a while. However, I would like to provide interesting food for thought. Multiple independent studies have so far reported that all-cause mortality and body mass index (BMI) show the U-shape association in men and women, namely that while there is a significant increase in all-cause mortality among people with a BMI of higher than ∼25, people with a BMI of lower than ∼20 also show increased mortality even after adjusting possible confounding factors [57-59, 60••, 61••]. One explanation for this phenomenon is that the low BMI group includes people who have undiagnosed pre-existing disease conditions that actually cause low BMI and higher mortality [58-60••]. This explanation might be supported by the finding that people who have chronic obstructive pulmonary diseases (COPDs) are indeed included in this group [59]. However, another potential explanation is that people with lower BMI might have higher frailty to infectious diseases due to the trade-off between robustness in metabolism and frailty in infection. Indeed, it has been reported that major causes of death in this low BMI group include tuberculosis and pneumonia [59]. If this is the case, an optimal balance between increasing metabolic robustness and decreasing frailty in infection might be achieved in a metabolic condition that can maintain BMI values associated with lowest all-cause mortality. Therefore, for people who have high BMI values associated with high mortality, caloric restriction or SIRT1-targeted CR mimetics would probably be effective to maximize metabolic robustness and to minimize frailty to infectious pathogens. However, we should carefully examine whether the same intervention could also achieve similar total benefits even for people who maintain a healthy or lower range of BMI.
Conclusion
In the past decade, significant progress has been made in understanding the molecular mechanism for the anti-aging effects of caloric restriction in mammals, and SIRT1 and possibly other sirtuins play a critical role in mediating these physiological effects of CR. SIRT1-targeted CR mimetics are currently providing hope to achieve good health and longevity even for humans. However, further investigation will be absolutely necessary to understand the principles of systemic robustness and its spatial and temporal dynamics in the regulation of aging and longevity in mammals in order to achieve an optimal balance between robustness and frailty in our complex physiological system. Thus, until we become ready to intervene in the complex regulatory system for aging and longevity in humans, Ekiken Kaibara's concept of “hara hachi bunme” might be the best approach to enjoy our daily lives.
Acknowledgments
I thank all members of the Imai lab for their insightful comments on this review article. I apologize to those whose work is not cited due to the focus of this review and space limitations. This work was supported by grant from the National Institute on Aging (AG024150), Ellison Medical Foundation, and Longer Life Foundation to S. I.
References
- 1.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 2.Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. Faseb J. 2000;14:2135–7. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- 3.Lin SJ, Defossez PA, Guarente L. Life span extension by calorie restriction in S. cerevisiae requires NAD and SIR2. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 4.Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 5.Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc R Soc Lond B Biol Sci. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 7.Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 8.Ramsey JJ, Colman RJ, Binkley NC, et al. Dietary restriction and aging inrhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 9.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 10.Weindruch RH, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: Longevity, cancer, immunity, and lifetime energy intake. J Nutrit. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 11.Bronson RT, Lipman RD. Reduction in rate of occurrence of age related lesions in dietary restricted laboratory mice. Growth Dev Aging. 1991;55:169–184. [PubMed] [Google Scholar]
- 12.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes Rev. 2004;5:13–19. doi: 10.1111/j.1467-789x.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 14.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 16.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Imai S, Guarente L. Sirtuins: A universal link between NAD, metabolism, and aging. In: Guarente L, Partridge L, Wallace D, editors. The Molecular Biology of Aging. New York: Cold Spring Habor Laboratory Press; 2007. pp. 39–72. [Google Scholar]
- 18.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- •19.Milne JC, Denu JM. The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol. 2008;12:11–17. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]; This paper and [20] are recent review articles that summarize the importance of sirtuins as broad therapeutic targets for age-associated diseases.
- 20.Westphal CH, Dipp MA, Guarente L. A therapeutic role for sirtuins in diseases of aging? Trends Biochem Sci. 2007;32:555–560. doi: 10.1016/j.tibs.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 22.Astrom SU, Cline TW, Rine J. The Drosophila melanogaster sir2+ gene Is nonessential and has only minor effects on position-effect variegation. Genetics. 2003;163:931–937. doi: 10.1093/genetics/163.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 24.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 27.Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 28.Anderson RM, Bitterman KJ, Wood JG, et al. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin SJ, Kaeberlein M, Andalis AA, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech Ageing Dev. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Dali-Youcef N, Lagouge M, Froelich S, et al. Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- •32.Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7:104–112. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]; This is another review article that summarizes the physiological importance of mammalian sirtuins in the regulation of metabolism.
- •33.Boily G, Seifert EL, Bevilacqua L, et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS ONE. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides supportive evidence that SIRT1 functions as a key mediator for the physiological response to caloric restriction.
- 34.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 35.Nisoli E, Tonello C, Cardile A, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 36.Rodgers JT, Lerin C, Haas W, et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- ••37.Banks AS, Kon N, Knight C, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; This recent study demonstrates that increasing SIRT1 dosage provides significant protection from the adverse effects of high-fat diet in mice and also that it increases adiponectin production through the deacetylation of FOXO1.
- ••38.Bordone L, Cohen D, Robinson A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]; This study currently provides the best evidence for the significance of SIRT1 in the induction of the physiological response to CR in mice.
- ••39.Pfluger PT, Herranz D, Velasco-Miguel S, et al. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study also demonstrates the importance of SIRT1 for the protection from high-fat diet-induced metabolic complications. It also reports that SIRT1 transgenic mice show hypersensitivity and early death in response to lipopolysaccharide challenge.
- 40.Carlson JM, Doyle J. Highly optimized tolerance: Robustness and design in complex systems. Phys Rev Lett. 2000;84:2529–2532. doi: 10.1103/PhysRevLett.84.2529. [DOI] [PubMed] [Google Scholar]
- 41.Csete M, Doyle J. Bow ties, metabolism and disease. Trends Biotechnol. 2004;22:446–450. doi: 10.1016/j.tibtech.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Zhou T, Carlson JM, Doyle J. Mutation, specialization, and hypersensitivity in highly optimized tolerance. Proc Natl Acad Sci USA. 2002;99:2049–2054. doi: 10.1073/pnas.261714399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- ••45.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides detailed transcriptional and physiological profiles of resveratrol-treated mice under standard and high-fat diet conditions.
- 46.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Labinskyy N, Csiszar A, Veress G, et al. Vascular dysfunction in aging: potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr Med Chem. 2006;13:989–96. doi: 10.2174/092986706776360987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howitz KT, Sinclair DA. Xenohormesis: sensing the chemical cues of other species. Cell. 2008;133:387–391. doi: 10.1016/j.cell.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••50.Feige JN, Lagouge M, Canto C, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]; This is the most recent report for the detailed transcriptional and physiological effects of SRT1720, one of newly developed sitruin activating compounds (STACs).
- 51.Kitano H. Towards a theory of biological robustness. Mol Syst Biol. 2007;3:137. doi: 10.1038/msb4100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson A, Liu F, Adamo ML, et al. The role of insulin and insulin-like growth factor-I in mammalian ageing. Best Pract Res Clin Endocrinol Metab. 2004;18:393–406. doi: 10.1016/j.beem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Moynihan KA, Grimm AA, Plueger MM, et al. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Gardner EM. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol A Biol Sci Med Sci. 2005;60:688–694. doi: 10.1093/gerona/60.6.688. [DOI] [PubMed] [Google Scholar]
- ••55.Ritz BW, Aktan I, Nogusa S, Gardner EM. Energy restriction impairs natural killer cell function and increases the severity of influenza infection in young adult male C57BL/6 mice. J Nutr. 2008;138:2269–2275. doi: 10.3945/jn.108.093633. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study illustrates an important case that caloric restriction increases frailty to viral infection in mice.
- 56.Roecker EB, Kemnitz JW, Ershler WB, Weindruch R. Reduced immune responses in rhesus monkeys subjected to dietary restriction. J Gerontol A Biol Sci Med Sci. 1996;51:B276–279. doi: 10.1093/gerona/51a.4.b276. [DOI] [PubMed] [Google Scholar]
- 57.Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 58.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 59.Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- •60.Klenk J, Nagel G, Ulmer H, et al. Body mass index and mortality: results of a cohort of 184,697 adults in Austria. Eur J Epidemiol. 2009;24:83–91. doi: 10.1007/s10654-009-9312-4. [DOI] [PubMed] [Google Scholar]
- •61.Hozawa A, Okamura T, Oki I, et al. Relationship between BMI and all-cause mortality in Japan: NIPPON DATA80. Obesity (Silver Spring) 2008;16:1714–1717. doi: 10.1038/oby.2008.237. [DOI] [PubMed] [Google Scholar]; These two recent reports illustrate the U-shaped association between all-cause mortality and body-mass index (BMI) in men and women, consistent with the results from other larger studies, such as [57-59].