Abstract
Purpose
The purpose of this study was to quantify the risk of breast cancer in relation to radiation dose and chemotherapy among survivors of childhood cancer.
Methods
We conducted a case-control study of breast cancer in a cohort of 6,647 women who were 5-year survivors of childhood cancer and who were treated during 1970 through 1986. One hundred twenty patients with histologically confirmed breast cancer were identified and were individually matched to four selected controls on age at initial cancer and time since initial cancer. Medical physicists estimated radiation dose to the breast tumor site and ovaries on the basis of medical records.
Results
The odds ratio for breast cancer increased linearly with radiation dose, and it reached 11-fold for local breast doses of approximately 40 Gy relative to no radiation (P for trend < .0001). Risk associated with breast irradiation was sharply reduced among women who received 5 Gy or more to the ovaries (P = .002). The excess odds ratio per Gy was 0.36 for those who received ovarian doses less than 5 Gy and was 0.06 for those who received higher doses. Radiation-related risk did not vary significantly by age at exposure. Borderline significantly elevated risks were seen for doxorubicin, dactinomycin, dacarbazine, and carmustine.
Conclusion
Results confirm the radiation sensitivity of the breast in girls age 10 to 20 years but do not demonstrate a strong effect of age at exposure within this range. Irradiation of the ovaries at doses greater than 5 Gy seems to lessen the carcinogenic effects of breast irradiation, most likely by reducing exposure of radiation-damaged breast cells to stimulating effects of ovarian hormones.
INTRODUCTION
Breast cancer is an important late adverse effect in women who receive radiotherapy to the chest at a young age.1–10 A recent study estimated a cumulative risk of breast cancer of 29% by age 55 years for women treated with chest irradiation (≥ 40 Gy) for Hodgkin's lymphoma (HL) at age 25 years.10 The relative risk of radiation-related breast cancer is inversely related to age at exposure for persons treated as adolescents or adults1,2,4,5,9,11,12; however, little quantitative information is available concerning risks related to doses received early in childhood and during breast development—when breast tissue is undergoing rapid cell proliferation—and after treatments for childhood cancers other than HL. To help address these gaps in understanding, we conducted a case-control study of new primary breast cancers in a large cohort of childhood cancer survivors.
METHODS
Study Population
The study was conducted as part of the Childhood Cancer Survivor Study (CCSS), a retrospective cohort study of 5-year survivors of childhood cancer (age at diagnosis < 21 years) who were treated at any of 26 collaborating institutions in the United States or Canada (Data Supplement, online only) between January 1, 1970, and December 31, 1986. As of January 1, 2001, 14,361 of 20,245 eligible patients, including 6,647 women, had been located and had agreed to participate in the study. Details of the study design and descriptions of the cohort have been published previously.13,14 The CCSS protocol and contact documents were reviewed and approved by the human participants committee at each participating institution.
Ascertainment of Treatment Information and New Primary Cancers
Therapeutic exposures were ascertained through review of the medical record of each study participant by trained data abstractors who used a standardized protocol. Radiation therapy data were photocopied and were sent to the CCSS Radiation Data Center at The University of Texas M. D. Anderson Cancer Center for dosimetry assessment. Data collected also included the dates of initiation and cessation of treatment for all chemotherapeutic agents, cumulative doses and routes of administration for 28 specific agents, and all surgical procedures.
New primary breast cancers were ascertained through self-report via a baseline questionnaire and follow-up questionnaires sent every 2 to 3 years. All positive responses were screened, and those that represented likely or possible new primary breast cancers were forwarded to the CCSS Pathology Center (Columbus, OH) for verification. A request for a copy of the pathology report was made to the institution of record, and the CCSS pathologist (S.H.) reviewed all reports of possible breast cancers. If the pathology report could not be obtained (n = 4), the patient and/or parent questionnaire response, death certificate, and/or other institutional records were reviewed to determine the presence of a breast cancer. New breast cancers in deceased persons were ascertained through family members and the National Death Index.
Patients and Controls
Eligible patients were women who had a confirmed invasive or in situ primary breast cancer diagnosed before the earlier of either December 31, 2001, or the date of return of the most recent follow-up survey. Overall, 148 breast cancers were identified in 122 women. Nine women first presented with bilateral, synchronous (defined as diagnosis within 1 month of each other) breast cancer, and another 17 were diagnosed subsequently with contralateral breast cancer. In patients with more than one breast cancer, analyses were conducted for the first cancer only. If the tumors were synchronous, priority was given to invasive rather than in situ cancers.
Four controls were selected for each patient, and they were matched on age at diagnosis of first cancer and duration of survival (ie, follow-up; ±2 years). For any case-control sets in which none of the selected controls had the same type of first cancer as the patient, we selected a supplemental control matched on type of initial cancer. Such controls (n = 37) were used only in analyses restricted to controls with the same type of first cancer as the patient.
Two patients, both of whom had an initial HL, and 14 controls were excluded, because we were unable to obtain consent to review medical records, and it was unknown whether they had received radiotherapy. Matched controls for the two excluded patients also were dropped, which provided 120 patients and 464 controls who remained for analysis with respect to radiation treatment.
Radiation Dosimetry
The radiation therapy records for all patients and controls were reviewed to determine therapy details. Abstracted radiotherapy information included dates of therapy, beam energy, field size, field location, number of treatment fractions, and total dose to each field.
The overall approach in determining organ doses in persons treated with radiation was detailed in a previous report.15 Breast doses were determined to the most specific tumor location possible for each case-control set. Records reviewed to determine the location of the subsequent breast cancer included operative notes, pathology reports, radiology reports, self-reported diagrams, and any correspondence in the available medical record relevant to the breast tumor. By using all available photographs, diagrams, and notes on blockings and field shaping, the proximity of each radiation field to the specific breast tumor location of interest was determined for each patient in a matched set. Location was set as in-beam unblocked, in-beam blocked, on the field edge, on the block edge, near the field edge, or out of the beam; dose was calculated accordingly. There were 13 patient sets with unknown tumor locations. If the tumor location was unknown but the breast received a relatively uniform dose, an average dose to the breast was calculated and was used in the analysis. If the tumor location was unknown and the breast received a wide range of dose (eg, blocked to unblocked from a mantle field), the breast dose was set to unknown.
Breast development was taken into account for dosimetry by changing the size of the breast for dose calculations on the basis of stage of development. We reviewed all treatment records for photographs and notes on development to determine the stage of breast development at the time of treatment for each patient treated with radiation; however, photographs were available only from a few institutions and mostly for patients who had HL, so breast development for the majority of patients could not be established by using the treatment records. We determined breast development for the remaining patients by using the median age of entry into a Tanner stage, taking ethnicity into account,16 and adjusting this determination on the basis of early or late menarche. For dosimetric purposes, the Tanner stages were divided into three categories: nipple/bud (Tanner 1 or 2), underdeveloped (Tanner 3), and developed (Tanner 4 or 5).
Radiation dose was calculated by summing every dose from the first to last radiation treatment given on or before a date 5 years before the age at breast cancer diagnosis and a corresponding date for matched controls. This was done to account for the minimum latency of most radiation-related solid cancers. Additionally, doses to left and right ovaries were estimated for each patient. If these doses differed, the minimum dose was used.
Each patient in a case-control set was given an organ dose score to reflect the quality of the dosimetry provided. This score reflects both the quality of the radiotherapy record information and the precision or certainty of the breast tumor location.
Statistical Analysis
We used SAS procedure PHREG (SAS Institute, Cary, NC) and the Epicure module PECAN (Hirosoft International, Seattle, WA)17,18 programs to fit conditional regression models, to estimate odds ratios (ORs), to model dose-response relations, to perform likelihood ratio tests, and to calculate 95% likelihood-based CIs. Statistical tests were two-sided and were based on an α level of .05. Radiation dose-response models considered were simplifications of the following general model: excess OR (EOR) = [β1D + β2D2][exp(β3D + β4D2], in which the EOR is the OR minus 1), D is dose, and β1 through β4 are regression coefficients. The exponential term allows for the possible effects of cell killing at high doses. The model EOR = β1D corresponds to a straight-line, dose-response relation, in which β1 equals the slope (ie, EOR per Gy). Risk estimates were adjusted for type of first cancer (ie, HL, bone or soft tissue sarcoma, other). Because data were compatible with a straight-line, dose-response relation (Results), effect modification was evaluated by using the model EOR = [β1D][exp(βizi)], in which zi equals the modifier(s) under consideration. We evaluated possible modification of β1 by age at diagnosis of first cancer, attained age, time since first cancer, year of initial cancer diagnosis, radiation dose to the ovaries, and chemotherapy for the first cancer.
RESULTS
Among the 120 breast cancers included in the present analysis, 80% were invasive cancers and 20% were in situ cancers. Tumor laterality was equally distributed (ie, 51% were right-sided tumors), and most occurred in the upper outer quadrant. The breast cancer was the second cancer for 113 women, and it was a subsequent cancer for seven women. HL was the initial cancer in 65.0% of patients with breast cancer and in 40.1% of controls (Table 1). Bone and soft tissue sarcomas were the next-most common types of first cancer among patients. The median age at diagnosis of the first cancer among the patients was 16.0 years, and the median age at diagnosis of the breast cancer was 35.9 years (range, 20.9 to 49.6 years). Only six patients (5%) occurred among women whose first cancer was diagnosed before age 10 years (range, 6 to 9 years). The median interval from first cancer to breast cancer was 19.4 years (range, 6.7 to 29.6 years).
Table 1.
Descriptive Demographic and Clinical Characteristics of Patients With Breast Cancer and Controls
| Characteristic | Patients (n = 120)* |
Matched Controls (n = 464)* |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Type of first cancer | ||||
| Leukemia | 7 | 5.8 | 75 | 16.2 |
| CNS | 3 | 2.5 | 41 | 8.8 |
| Hodgkin's lymphoma | 78 | 65.0 | 188 | 40.5 |
| NHL | 4 | 3.3 | 29 | 6.3 |
| Kidney | 3 | 2.5 | 4 | 0.9 |
| Neuroblastoma | 1 | 0.8 | 1 | 0.2 |
| Soft tissue sarcoma | 9 | 7.5 | 53 | 11.4 |
| Bone sarcoma | 15 | 12.5 | 73 | 15.7 |
| Age at first cancer, years† | ||||
| < 5 | 0 | 0.0 | 6 | 1.3 |
| 5-9 | 6 | 5.0 | 18 | 3.9 |
| 10-14 | 32 | 26.7 | 134 | 28.9 |
| 15-20 | 82 | 68.3 | 306 | 65.9 |
| Years since first cancer† | ||||
| 5.0-14.9 | 27 | 22.5 | 104 | 22.4 |
| 15.0-19.9 | 39 | 32.5 | 148 | 31.9 |
| 20.0-24.9 | 33 | 27.5 | 130 | 28.0 |
| 25.0-32.0 | 21 | 17.5 | 82 | 17.7 |
| Attained age, years | ||||
| 18.0-34.9 | 53 | 44.2 | 224 | 48.3 |
| 35.0-39.9 | 46 | 38.3 | 159 | 34.3 |
| 40.0-44.9 | 14 | 11.7 | 57 | 12.3 |
| 45.0-51.0 | 7 | 5.8 | 24 | 5.2 |
| Year of first cancer diagnosis | ||||
| 1970-1974 | 58 | 48.3 | 191 | 41.2 |
| 1975-1979 | 45 | 37.5 | 175 | 37.7 |
| 1980-1986 | 17 | 14.2 | 98 | 21.2 |
| Year of breast cancer diagnosis | NA | |||
| 1983-1989 | 17 | 14.2 | ||
| 1990-1995 | 42 | 35.0 | ||
| 1996-2001 | 61 | 50.8 | ||
Abbreviations: NHL, non-Hodgkin's lymphoma; NA, not applicable.
Two patients and 24 controls with missing information about radiotherapy for initial cancer were excluded.
Matching variable.
Frequency of use of radiation therapy and radiation dose to the breast varied widely among controls by type of initial cancer (data not shown). One third of patients with bone sarcoma were given radiation therapy compared with 92% of patients with HL. Among irradiated controls, median dose to the affected region of the breast in the corresponding patient ranged from less than 0.25 Gy for patients with leukemia, central nervous system cancer, or soft tissue sarcoma to 15.1 Gy for patients with Wilms tumor and to 26.8 Gy for patients with HL. Among controls younger than 10 years at the time of diagnosis of the initial cancer, 62% received radiotherapy, and the median radiation dose among those exposed was 0.2 Gy (mean, 4.0 Gy). The median age at the end of follow-up varied from 28 years for controls diagnosed with Wilms tumor to 36 years for survivors of HL or bone sarcoma.
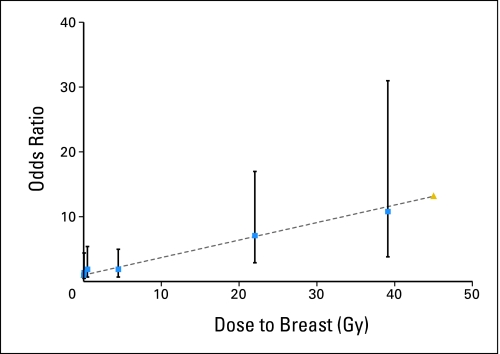
The OR associated with having received any radiotherapy for the initial cancer was 2.7 (95% CI, 1.4 to 5.6; Table 2). A linear dose-response model fit the data well, and there was little evidence of either upward curvature at lower doses or downward curvature at higher doses (Fig 1). The slope of the linear model was 0.27 per Gy (95% CI, 0.10 to 0.67) and was highly significantly different from zero (P < .0001). The estimated relative risks were 6.4 at 20 Gy and 11.8 at 40 Gy. The slope increased to 0.34 per Gy (95% CI, 0.10 to 1.06) when analysis was restricted to persons with the highest quality of radiotherapy information. The dose-response pattern was qualitatively similar, though less precise, when analysis included only controls with the same type of initial cancer as the patient (data not shown). Results also were similar when analysis was restricted to breast cancer as the second primary cancer only. The EORs per Gy were 0.34 (95% CI, 0.12 to 0.96) for invasive cancers and 0.10 (95% CI, 0.00 to 0.96) for in situ cancers.
Table 2.
Breast Cancer Risk by Any Radiation Treatment for the Initial Cancer and by Radiation Dose to the Affected Region of the Breast
| Treatment or Dose | Mean Radiation Dose (Gy)* | Patients |
Controls |
Analysis |
|||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | OR† | 95% CI | ||
| Any radiation | |||||||
| No | 0.0 | 13 | 10.8 | 136 | 29.3 | 1.0 | Referent |
| Yes | 13.4 | 107 | 89.2 | 328 | 70.7 | 2.7 | 1.4 to 5.4 |
| Dose category, Gy‡ | |||||||
| 0 | 0.0 | 13 | 12.2 | 127 | 32.7 | 1.0 | Referent |
| > 0-0.13 | 0.1 | 6 | 5.6 | 49 | 12.6 | 1.4 | 0.5 to 4.4 |
| 0.14-1.29 | 0.5 | 7 | 6.5 | 48 | 12.3 | 1.9 | 0.7 to 5.4 |
| 1.30-11.39 | 4.5 | 11 | 10.3 | 55 | 14.1 | 1.9 | 0.7 to 5.0 |
| 11.40-29.99 | 22.0 | 34 | 31.8 | 56 | 14.4 | 7.1 | 2.9 to 17 |
| 30.00-60.00 | 39.1 | 36 | 33.6 | 54 | 13.9 | 10.8 | 3.8 to 31 |
| Total No. of patients with any dose | 107 | 100.0 | 389 | 100.0 | P < .0001§ | ||
Abbreviation: OR, odds ratio.
Mean dose among controls.
Adjusted for type of first cancer (ie, Hodgkin's lymphoma, bone or soft tissue sarcoma, other).
Women with unknown dose were excluded. Analyses by radiation doses were based on 107 patients and 389 controls. Dose categories correspond to quintiles among irradiated controls. Percentages by dose category were based on women with known doses to the breast.
Test for trend.
Fig 1.
Breast cancer risk by radiation dose to the breast.
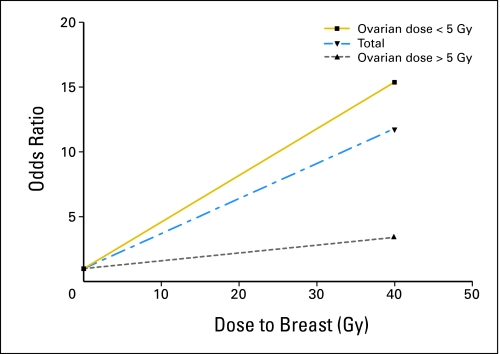
When the linear slope of the radiation dose-response curve for breast cancer was allowed to vary according to whether radiotherapy for the initial cancer delivered a dose of ≥ 5 Gy to the ovaries, a significant difference in slope was observed (P = .002; Table 3; Fig 2). The slopes were 0.36 per Gy for those who received ovarian doses less than 5 Gy and 0.06 per Gy for those who received higher doses. These findings were little changed with adjustment for nulliparity or use of hormone replacement therapy. The mean age at menopause for postmenopausal women with ovarian doses ≥ 5 Gy was 22 years. The slope of the radiation dose-response curve did not vary significantly by age at diagnosis of the initial cancer (Table 3). The association with age at exposure remained nonsignificant when analysis was restricted to women with ovarian doses less than 5 Gy. Likelihood ratio tests for modification of the slope by year of initial cancer, time since initial cancer, and attained age also were not significant (Table 3).
Table 3.
EOR for Breast Cancer per Gy of Radiation Dose to Site of Breast Tumor by Selected Demographic and Clinical Characteristics
| Characteristic | No. of Patients | No. of Controls | Analysis |
P* | |
|---|---|---|---|---|---|
| EOR/Gy | 95% CI | ||||
| Overall | 107 | 389 | 0.27 | 0.10 to 0.67 | < .0001 |
| Age at initial cancer, years | .43 | ||||
| < 13 | 15 | 74 | 0.29 | 0.06 to 1.09 | |
| 13-15 | 37 | 125 | 0.44 | 0.14 to 1.36 | |
| 16-20 | 55 | 190 | 0.21 | 0.06 to 0.62 | |
| Type of initial cancer† | |||||
| HL | 67 | 145 | Model fit failed‡ | Unknown | |
| All others | 40 | 244 | 0.21 | 0.09 to 0.51 | |
| Year of initial cancer | |||||
| 1970-1974 | 53 | 175 | 0.33 | 0.12 to 0.90 | .57 |
| 1975-1979 | 41 | 156 | 0.24 | 0.08 to 0.66 | |
| 1980-1986 | 13 | 86 | 0.19 | −0.07 to 0.64 | |
| Years since first cancer | .25 | ||||
| 5.00-4.9 | 24 | 85 | 0.39 | 0.09 to 1.77 | |
| 15.0-19.9 | 32 | 115 | 0.35 | −0.13 to 1.71§ | |
| 20.0-24.9 | 31 | 115 | 0.26 | 0.06 to 1.01 | |
| 25.0-32.0 | 20 | 74 | 0.13 | −0.09 to 0.70§ | |
| Attained age, years | .17 | ||||
| 18-34 | 46 | 184 | 0.33 | 0.11 to 0.92 | |
| 35-39 | 41 | 134 | 0.56 | 0.15 to 1.93 | |
| 40-44 | 14 | 52 | 0.02 | −0.06 to 0.09‖ | |
| 45-50 | 6 | 19 | 0.70 | −1.56 to 2.95‖ | |
| Radiation dose to ovaries, Gy | .002 | ||||
| < 5 | 99 | 342 | 0.36 | 0.14 to 0.93 | |
| ≥ 5 | 8 | 47 | 0.06 | −0.06 to 0.27§ | |
Abbreviations: EOR, excess odds ratio; HL, Hodgkin's lymphoma.
Test for trend or heterogeneity.
Includes supplemental controls.
Parameter estimates did not converge.
Wald-type lower confidence bound. Likelihood-based bound was not obtained.
Wald-type CI.
Fig 2.
Fitted breast cancer risk by radiation dose to the breast and ovary.
Overall, chemotherapy for the initial cancer was not associated significantly with breast cancer risk when analyses were adjusted for radiation dose to the breast and ovary (Tables 3 and 4). Alkylating agents as a group also were not associated significantly with risk. Carmustine was significantly associated with breast cancer on the basis of a small number of exposed patients. Nonsignificantly reduced ORs were seen for mechlorethamine and procarbazine. The positive associations with dactinomycin and dacarbazine approached statistical significance. There also was a borderline-significant positive association for anthracyclines as a group, attributable mostly to doxorubicin. None of the listed agents showed significant associations with dose.
Table 4.
Breast Cancer Risk in Relation to Chemotherapy for Childhood Cancer
| Chemotherapy | No. of Patients | No. of Controls | Analysis |
||
|---|---|---|---|---|---|
| OR | 95% CI | P | |||
| Any | |||||
| No | 40 | 126 | 1.00 | ||
| Yes | 66 | 259 | 0.90 | 0.53 to 1.54 | .70 |
| Any alkylating agent | |||||
| No | 53 | 185 | 1.00 | ||
| Yes | 53 | 200 | 0.93 | 0.56 to 1.55 | .79 |
| Alkylating agent score* | |||||
| 0 | 53 | 163 | 1.00 | ||
| 1 | 12 | 63 | 0.67 | 0.30 to 1.51 | .17 |
| 2 | 12 | 36 | 1.40 | 0.58 to 3.39 | .33 |
| 3 | 17 | 37 | 1.15 | 0.55 to 2.41 | .67 |
| Mechlorethamine, mg/m2 | |||||
| No | 92 | 343 | 1.00 | ||
| Yes | 14 | 42 | 0.81 | 0.37 to 1.76 | .60 |
| > 0-44 | 5 | 12 | 0.71 | 0.20 to 2.53 | .87 |
| > 44-63.7 | 3 | 13 | 0.41 | 0.09 to 1.75 | .41 |
| ≥ 63.7 | 3 | 9 | 0.61 | 0.10 to 3.59 | .93 |
| Procarbazine, mg/m2 | |||||
| No | 77 | 305 | 1.00 | ||
| Yes | 29 | 80 | 0.70 | 0.37 to 1.35 | .29 |
| > 0-4,178.6 | 9 | 20 | 0.69 | 0.26 to 1.86 | .67 |
| > 4,178.6-< 7,000 | 8 | 26 | 0.69 | 0.23 to 2.02 | .68 |
| ≥ 7,000 | 7 | 12 | 0.92 | 0.28 to 3.09 | .77 |
| BCNU | |||||
| No | 100 | 370 | 1.00 | ||
| Yes | 6 | 15 | 3.71 | 1.12 to 12.30 | .03 |
| CCNU | |||||
| No | 100 | 375 | 1.00 | ||
| Yes | 6 | 10 | 1.73 | 0.55 to 5.44 | .35 |
| Cyclophosphamide | |||||
| No | 67 | 238 | 1.00 | ||
| Yes | 39 | 147 | 1.37 | 0.79 to 2.38 | .26 |
| Dacarbazine | |||||
| No | 100 | 379 | 1.00 | ||
| Yes | 6 | 6 | 3.49 | 0.96 to 12.68 | .06 |
| Dactinomycin | |||||
| No | 87 | 332 | 1.00 | ||
| Yes | 19 | 53 | 2.40 | 0.96 to 5.96 | .06 |
| Bleomycin | |||||
| No | 95 | 362 | 1.00 | ||
| Yes | 11 | 23 | 1.82 | 0.77 to 4.28 | .17 |
| Any anthracycline | |||||
| No | 76 | 263 | 1.00 | ||
| Yes | 30 | 122 | 1.86 | 0.99 to 3.48 | .05 |
| Daunorubicin | |||||
| No | 104 | 363 | 1.00 | ||
| Yes | 2 | 22 | 0.84 | 0.17 to 4.22 | .84 |
| Doxorubicin, mg/m2 | |||||
| No | 78 | 279 | 1.00 | ||
| Yes | 28 | 106 | 1.87 | 0.98 to 3.55 | .06 |
| > 0-198.8 | 7 | 19 | 2.09 | 0.71 to 6.14 | .46 |
| > 198.8-< 350.8 | 8 | 18 | 1.93 | 0.64 to 5.87 | .55 |
| ≥ 350.8 | 7 | 53 | 1.28 | 0.44 to 3.73 | .70 |
NOTE. OR estimates were adjusted for radiation dose to breast and ovary and for type of first cancer (ie, Hodgkin's lymphoma, sarcoma, other). Analyses for mechlorethamine and procarbazine dose categories were also adjusted for cumulative dose of doxorubicin, whereas the odds ratios for no/yes were adjusted for doxorubicin (no/yes). Data in table are for never/ever (no/yes) and by tertiles of dose for selected drugs. Doses were unknown for some patients known to have received a drug.
Abbreviations: OR, odds ratio; BCNU, bischloroethylnitrosourea (carmustine); CCMU, cyclohexylchloroethylnitrosourea (lomustine).
Calculated according to the method described by Tucker et al.19 Dose scores were assigned to individual alkylating agents on the basis of the distributions of doses (mg/m2) to each agent, and these scores then were summed across agents.
When analysis was not adjusted for radiation dose to the breast, the highest risks were seen among women who had an initial HL or Wilms tumor (data not shown). However, when analysis was adjusted for radiation dose and age at irradiation, differences by type of first cancer were no longer statistically significant.
DISCUSSION
To our knowledge, this is the largest study to date of breast cancer after childhood cancer that includes detailed treatment information and radiation dosimetry. We observed a highly significant, linear relation between radiation dose and breast cancer risk. Women who received local breast doses of 40 Gy were at an overall 11-fold risk relative to nonirradiated patients. However, the slope of the dose-response curve was modified by dose to the ovaries. Among women whose treatment for the initial cancer delivered a sterilizing dose to the ovaries of greater than 5 Gy, the slope of the dose-response curve was reduced. A breast dose of 40 Gy was associated with only a 3.4-fold risk of breast cancer. We did not observe significant associations between breast cancer risk and prior treatment with alkylating agents, but there was some indication of a positive association for anthracyclines (ie, for doxorubicin). Risk was highest among those whose first cancer was HL; however, after analysis was adjusted for radiation dose and age at exposure, type of first cancer was not significantly associated with breast cancer risk. We did not detect a significant effect of age at exposure on radiation-related risk, perhaps in part because of the limited range in age at diagnosis of patients with HL, the lower doses to the breast for first cancers diagnosed at younger ages, and the younger ages at the end of follow-up. The relative risk of breast cancer also was not significantly associated with attained age. A constant relative risk would imply an increasing excess absolute risk with age.
Our findings concerning effects of breast and ovarian irradiation resemble those reported by Travis et al9 for breast cancer after irradiation for HL between the ages of 13 and 30 years (median, 22 years). Unlike those investigators, we did not observe a significant inverse association with alkylating agents, even though some of the same agents were involved. However, the point estimates we observed for any use of procarbazine or mechlorethamine each were between 0.7 and 0.8, and lower 95% CI bounds were less than 0.40, which indicates that the results of the two studies are not statistically inconsistent. Furthermore, Travis et al9 noted that the apparent protective effect of alkylating agents became stronger with increasing numbers of cycles of chemotherapy and was most pronounced in the small group of patients who received nine or more cycles. As with pelvic irradiation, a protective effect of selected alkylating agents is thought to be related to ablation of ovarian function and the resulting suppression of hormonal stimulation of breast tissue. Ovaries in adolescent girls have a greater reserve of eggs and follicles relative to older women and are less likely to experience loss of function for a given dose of alkylating agents or radiation.20 In a previous study of CCSS survivors, the risk of therapy-induced acute ovarian failure increased with age at exposure.21 Previous studies of second primary breast cancer after irradiation for HL in young women have reported EORs per Gy of 0.065 and 0.159; these are somewhat lower than what was seen in this study for irradiation at younger ages.
Our results pertain to second breast cancer risks for persons diagnosed with childhood cancer between 1970 and 1986, but treatments have changed over time. Most notable from the standpoint of second primary breast cancer are the reductions in radiation dose and field size for the treatment of children with HL.22,23 To the extent that our findings suggest a linear dose-response relation, with no evidence of a downturn in risk at the highest doses, one might predict that newer treatments for HL would be associated with reduced risk for breast cancer. There has been a general reduction in the use of radiation therapy for other childhood cancers as well, including for non-Hodgkin's lymphoma, acute lymphoblastic leukemia, and central nervous system cancers.22
Strengths of this study include its large number of breast cancer patients relative to previous studies of childhood cancer survivors and the inclusion of detailed radiation dosimetry. Limitations include uncertainty about the precise anatomic location of the breast cancer and of the tumor progenitor cell at the time of irradiation for childhood cancer. We attempted to take stage of breast development at the time of exposure into account, but we did not have complete information about this. There were small numbers of patients who received radiation in the low to intermediate dose range, which compromised our ability to describe the shape of the dose-response curve over this interval. The strong correlations among type of first cancer, age at first cancer, and radiation dose to the breast limited our ability to evaluate variation in radiation sensitivity before and after menarche.
These results largely support those from a recent study of breast cancer risk in relation to treatment of HL in young women and adolescents.9 The risk of breast cancer after treatment for a first cancer at a young age appears to be a balance between direct, dose-dependent effects of irradiation of the breast and opposing effects of high-dose ovarian irradiation or repeated systemic chemotherapy with alkylating agents. These data point to an important role of hormonal stimulation on radiation-related breast cancer. The cohort is still relatively young, and the largest part of the radiation-related absolute excess occurrence of breast cancer may yet be seen.
Acknowledgment
The Acknowledgment and Appendix are included in the full-text version of this article; they are available online at www.jco.org. They are not included in the PDF version (via Adobe® Reader®).
Supported by National Cancer Institute Grant No. U24 CA55727; the Children's Cancer Research Fund; the Lance Armstrong Foundation Grant No. 147149; and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Presented in part at the Spring Meeting of the Children's Oncology Group, March 23, 2006, Chicago, IL.
We thank the survivors who have contributed to the Childhood Cancer Survivor Study (CCSS), the data abstractors and survey interviewers, the investigators of the institutions that participated in the CCSS (Data Supplement, online only), Pauline Mitby for her contributions to the data collection, and Rita Weathers for assistance with radiation dosimetry programming.
Appendix
Study background.
The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived 5 or more years after diagnosis of childhood cancer. The CCSS is a retrospectively ascertained cohort of 20,346 childhood cancer survivors diagnosed before age 21 years between 1970 and 1986 and of approximately 4,000 siblings of survivors, who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The CCSS is currently funded by National Cancer Institute U24 Resource Grant No. U24 CA55727, awarded to St Jude Children's Research Hospital. Currently, CCSS investigators are in the process of expanding the cohort to include an additional 14,000 childhood cancer survivors diagnosed before age 21 years between 1987 and 1999. For information on how to access and utilize the CCSS resource, visit http://www.stjude.org/ccss.
CCSS institutions and providers.
St Jude Children's Research Hospital, Memphis, TN: Leslie L. Robison, PhD (Member, CCSS Steering Committee; Project Principal Investigator [National Cancer Institute Grant No. U24 CA55727]), Melissa Hudson, MD (Institutional Principal Investigator; Member, CCSS Steering Committee), Greg Armstrong, MD (Member, CCSS Steering Committee); Children's Healthcare of Atlanta/Emory University Atlanta, GA: Lillian Meacham, MD (Institutional Principal Investigator), Ann Mertens, PhD (Member, CCSS Steering Committee); Children's Hospitals and Clinics of Minnesota Minneapolis, St Paul, MN: Joanna Perkins, MD (Institutional Principal Investigator), Maura O'Leary, MD (Former Institutional Principal Investigator); Children's Hospital and Medical Center, Seattle, WA: Debra Friedman, MD, MPH (Institutional Principal Investigator), Thomas Pendergrass, MD (Former Institutional Principal Investigator); Children's Hospital, Denver, CO: Brian Greffe, MD (Institutional Principal Investigator), Lorrie Odom, MD (Former Institutional Principal Investigator); Children's Hospital Los Angeles, CA: Kathy Ruccione, RN, MPH (Institutional Principal Investigator); Children's Hospital, Oklahoma City, OK: John Mulvihill, MD (Member, CCSS Steering Committee); Children's Hospital of Philadelphia, PA: Jill Ginsberg, MD (Institutional Principal Investigator), Anna Meadows, MD (Member, CCSS Steering Committee); Children's Hospital of Pittsburgh, PA: Jean Tersak, MD (Institutional Principal Investigator), A. Kim Ritchey, MD (Former Institutional Principal Investigator), Julie Blatt, MD (Former Institutional Principal Investigator); Children's National Medical Center, Washington, DC: Gregory Reaman, MD (Institutional Principal Investigator), Roger Packer, MD (Member, CCSS Steering Committee); Cincinnati Children's Hospital Medical Center: Stella Davies, MD, PhD (Member, CCSS Steering Committee); City of Hope, Los Angeles, CA: Smita Bhatia, MD (Institutional Principal Investigator); Dana-Farber Cancer Institute/Childrens Hospital, Boston, MA: Lisa Diller, MD (Institutional Principal Investigator), Holcombe Grier, MD (Former Institutional Principal Investigator), Frederick Li, MD (Former Member, CCSS Steering Committee); Fred Hutchinson Cancer Research Center, Seattle, WA: Wendy Leisenring, ScD (Institutional Principal Investigator; Member, CCSS Steering Committee), John Potter, MD, PhD (Member, CCSS Steering Committee; Former Institutional Principal Investigator); Hospital for Sick Children, Toronto, ON: Mark Greenberg, MBChB. (Institutional Principal Investigator), Paul C. Nathan, MD (Former Institutional Principal Investigator); International Epidemiology Institute, Rockville, MD: John Boice, ScD (Member, CCSS Steering Committee); Mayo Clinic, Rochester, MN: Vilmarie Rodriguez, MD (Institutional Principal Investigator), W. Anthony Smithson, MD (Former Institutional Principal Investigator), Gerald Gilchrist, MD (Former Institutional Principal Investigator); Memorial Sloan-Kettering Cancer Center New York: Charles Sklar, MD (Institutional Principal Investigator; (Member, CCSS Steering Committee), Kevin Oeffinger, MD (Member, CCSS Steering Committee); Miller Children's Hospital: Jerry Finklestein, MD(Former Institutional Principal Investigator); National Cancer Institute, Bethesda, MD: Barry Anderson, MD (Member, CCSS Steering Committee), Peter Inskip, ScD (Member, CCSS Steering Committee); Nationwide Children's Hospital, Columbus, Ohio: Amanda Termuhlen, MD (Institutional Principal Investigator), Frederick Ruymann, MD (Former Institutional Principal Investigator), Stephen Qualman, MD (Former Member, CCSS Steering Committee), Sue Hammond, MD (Member, CCSS Steering Committee); Riley Hospital for Children, Indianapolis, IN: Terry A. Vik, MD (Institutional Principal Investigator), Robert Weetman, MD (Former Institutional Principal Investigator); Roswell Park Cancer Institute, Buffalo, NY: Daniel M. Green, MD (Institutional Principal Investigator; (Member, CCSS Steering Committee); St. Louis Children's Hospital, MO: Robert Hayashi, MD (Institutional Principal Investigator), Teresa Vietti, MD (Former Institutional Principal Investigator); Stanford University School of Medicine, Stanford, CA: Neyssa Marina, MD (Institutional Principal Investigator), Sarah S. Donaldson, MD (Member, CCSS Steering Committee), Michael P. Link, MD (Former Institutional Principal Investigator); Texas Children's Hospital, Houston, TX: Zoann Dreyer, MD (Institutional Principal Investigator); University of Alabama, Birmingham, AL: Kimberly Whelan, MD, MSPH (Institutional Principal Investigator), Jane Sande, MD (Former Institutional Principal Investigator), Roger Berkow, MD (Former Institutional Principal Investigator); University of Alberta, Edmonton, AB: Yutaka Yasui, PhD (Member, CCSS Steering Committee); University of California, Los Angeles, CA: Jacqueline Casillas, MD MSHS (Institutional Principal Investigator), Lonnie Zeltzer, MD (Member, CCSS Steering Committee; Former Institutional Principal Investigator); University of California, San Francisco, CA: Robert Goldsby, MD (Institutional Principal Investigator), Arthur Ablin, MD (Former Institutional Principal Investigator); University of Michigan, Ann Arbor, MI: Raymond Hutchinson, MD (Institutional Principal Investigator); University of Minnesota, Minneapolis, MN: Joseph Neglia, MD, MPH (Institutional Principal Investigator; Member, CCSS Steering Committee); University of Southern California: Dennis Deapen, Dr. P.H. (Member, CCSS Steering Committee); University of Washington, Seattle, WA: Norman Breslow, PhD (Former Member, CCSS Steering Committee); University of Texas, Southwestern Medical Center at Dallas, TX: Dan Bowers, MD (Institutional Principal Investigator), Gail Tomlinson, MD (Former Institutional Principal Investigator), George R. Buchanan, MD (Former Institutional Principal Investigator); The University of Texas M. D. Anderson Cancer Center, Houston, TX: Louise Strong, MD (Institutional Principal Investigator; Member, CCSS Steering Committee), Marilyn Stovall, MPH, PhD (Member, CCSS Steering Committee).
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Peter D. Inskip, Leslie L. Robison, Joseph P. Neglia
Financial support: Peter D. Inskip, Leslie L. Robison
Administrative support: Leslie L. Robison
Provision of study materials or patients: Leslie L. Robison
Collection and assembly of data: Peter D. Inskip, Leslie L. Robison, Marilyn Stovall, Susan A. Smith, Sue Hammond, Ann C. Mertens, Joseph P. Neglia
Data analysis and interpretation: Peter D. Inskip, Leslie L. Robison, Marilyn Stovall, John A. Whitton, Sarah S. Donaldson, Anna T. Meadows, Joseph P. Neglia
Manuscript writing: Peter D. Inskip, Leslie L. Robison, Marilyn Stovall, Lisa Diller, Lisa Kenney, Sarah S. Donaldson, Anna T. Meadows, Joseph P. Neglia
Final approval of manuscript: Peter D. Inskip, Leslie L. Robison, Marilyn Stovall, Susan A. Smith, Sue Hammond, Ann C. Mertens, John A. Whitton, Lisa Diller, Lisa Kenney, Sarah S. Donaldson, Anna T. Meadows, Joseph P. Neglia
REFERENCES
- 1.Hancock SL, Tucker MA, Hoppe RT. Breast cancer after treatment of Hodgkin's disease. J Natl Cancer Inst. 1993;85:25–31. doi: 10.1093/jnci/85.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin's disease. N Engl J Med. 1996;334:745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 4.van Leeuwen FE, Klokman WJ, van't Veer MB, et al. Long-term risk of second malignancy in survivors of Hodgkin's disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18:487–497. doi: 10.1200/JCO.2000.18.3.487. [DOI] [PubMed] [Google Scholar]
- 5.van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin's disease. J Natl Cancer Inst. 2003;95:971–980. doi: 10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- 6.Aisenberg AC, Finkelstein DM, Doppke KP, et al. High risk of breast carcinoma after irradiation of young women with Hodgkin's disease. Cancer. 1997;79:1203–1210. doi: 10.1002/(sici)1097-0142(19970315)79:6<1203::aid-cncr20>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Metayer C, Lynch CF, Clarke EA, et al. Second cancers among long-term survivors of Hodgkin's disease diagnosed in childhood and adolescence. J Clin Oncol. 2000;18:2435–2443. doi: 10.1200/JCO.2000.18.12.2435. [DOI] [PubMed] [Google Scholar]
- 8.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141:590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 9.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin's disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 10.Travis LB, Hill D, Dores G, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97:1428–1437. doi: 10.1093/jnci/dji290. [DOI] [PubMed] [Google Scholar]
- 11.Swerdlow AJ, Barber JA, Vaughan Hudson G, et al. Risk of second malignancy after Hodgkin's disease in a collaborative British cohort: The relation to age at treatment. J Clin Oncol. 2000;18:498–509. doi: 10.1200/JCO.2000.18.3.498. [DOI] [PubMed] [Google Scholar]
- 12.Friedman DL, Rovo A, Leisenring W, et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: A report from the FHCRC and the EBMT-Late Effect Working Party. Blood. 2008;111:939–944. doi: 10.1182/blood-2007-07-099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 14.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 15.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 16.Sun SS, Schubert CM, Chumlea WC, et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110:911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- 17.Lubin JH. A computer program for the analysis of matched case-control studies. Computers Biomed Res. 1981;14:138–143. doi: 10.1016/0010-4809(81)90031-8. [DOI] [PubMed] [Google Scholar]
- 18.Preston DL, Lubin JH, Pierce DA, et al. Epicure Users Guide. Seattle, WA: Hirosoft International; 1993. [Google Scholar]
- 19.Tucker MA, Meadows AT, Boice JD, Jr, et al. Leukemia after therapy with alkylating agents for childhood cancer. J Natl Cancer Inst. 1987;78:459–464. [PubMed] [Google Scholar]
- 20.Sklar C. Maintenance of ovarian function and risk of premature menopause related to cancer treatment. J Natl Cancer Inst Monogr. 2005;34:25–27. doi: 10.1093/jncimonographs/lgi018. [DOI] [PubMed] [Google Scholar]
- 21.Chemaitilly W, Mertens AC, Mitby P, et al. Acute ovarian failure in the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2006;91:1723–1728. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- 22.Kalapurakal JA, Thomas PR. Pediatric radiotherapy: An overview. Radiol Clin North Am. 1997;35:1265–1280. [PubMed] [Google Scholar]
- 23.Donaldson SS. Pediatric Hodgkin's disease: Up, up, and beyond. Int J Radiat Oncol Biol Phys. 2002;54:1–8. doi: 10.1016/s0360-3016(02)02903-6. [DOI] [PubMed] [Google Scholar]